 |
 |
- Search
| Neurospine > Volume 18(1); 2021 > Article |
|
|
Abstract
Objective
Vertebral aspergillosis is quite rare conditions, often misdiagnosed, that requires long-term antibiotic therapy, and sometimes, surgical treatments. The present investigations were aimed to investigate the epidemiology, clinical-radiological aspects, treatment protocols, and outcomes of Aspergillus-mediated vertebral osteomyelitis.
Methods
A systematic review of the pertinent English literature according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines was performed. The research was conducted on Cochrane library, MEDLINE, PubMed, and Scopus using as search-terms “Aspergillus,” “vertebral osteomyelitis,” “spondylodiscitis,” “spine infection.” A case of vertebral aspergillosis conservatively managed was also reported.
Results
Eighty-nine articles were included in our systematic review. Including the reported case, our analysis covered 112 cases of vertebral aspergillosis. Aspergillus fumigatus was isolated in 68 cases (61.2%), Aspergillus flavus in 14 (12.6%), Aspergillus terreus in 4 (3.6%), Aspergillus nidulans in 2 (1.8%). Seventy-three patients (65.7%) completely recovered at the last follow-up evaluation; in 7 patients (6.3%) radiological signs of chronic infection were reported, whereas 32 patients (28.8%) died during the follow-up.
Vertebral mycoses are subtypes of vertebral osteomyelitis (VO) that represent rare and severe life-threatening conditions, requiring long-term pharmacological therapy and often surgical treatments [1,2]. Aspergillus species are ubiquitous fungi. They are commonly found in the upper human respiratory tract, and rarely determine infectious diseases in immunocompetent patients. On the other hand, these infections may be associated with high mortality rates in immunocompromised patients [1]. Vertebral aspergillosis (VA) represents the most frequent extrapulmonary aspergillosis localization [3]. VA may determine osteolysis, intervertebral disc involvement, and epidural space collections, resulting in painful conditions neurological symptoms, and deformities. Early diagnosis, by blood cultures or direct sampling, and a proper medical therapy represent the key points in VA management [3,4]. Few cases of VA were reported, and a consensus on diagnostic criteria and the most effective medical treatment are still missing. Since fungal infections may be undiagnosed using standard laboratory tests and cultures, specific radiological or clinical signs suspicious for Aspergillus osteomyelitis would be helpful.
We present a case of spontaneous VA, sustained by Aspergillus fumigatus, and report the results of a systematic review of the international medical literature for epidemiology, clinical-radiological aspects, treatment protocols, and outcomes of Aspergillus-mediated VO.
The present investigation consists of a case report and a systematic review of the literature conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.
English written papers on spine infections sustained by Aspergillus, reporting demographical and clinical data, diagnostic work-flow, treatment protocol, complications, and prognosis were considered for eligibility. Exclusion criteria were: surgical technique reports, expert opinions, studies on animals, unpublished reports, cadaver or in vitro investigations, book chapters, abstracts from scientific meetings, articles describing postsurgical or postinjection VA.
The systematic review of the literature was conducted on 4 different online medical databases (Cochrane library, MEDLINE, PubMed, Scopus), using as search-terms “Aspergillus,” “vertebral osteomyelitis,” “spondylodiscitis,” “spine infection” [MeSH], combined with the Boolean operators “AND,” “OR,” and “NOT.” The search was reiterated until 20th March 2020. The search strategy is summarized in Fig. 1.
Abstracts and full-texts were independently screened by 2 authors (AP and LR), and any discordance was solved by consensus with a third senior author (LP). The full-texts of eligible papers and their references lists (forward search) were evaluated for inclusion.
A 76-year-old man was admitted to our Emergency Department on March 2019, reporting acute low back pain, rated as 9 on a 10-point itemized visual analogue scale (VAS), and lower extremity numbness started 2 months earlier, without fever. Patient medical background showed chronic lymphocytic leukemia (CLL) in treatment with ibrutinib, atrial fibrillation, and severe aortic stenosis; no alcohol or drugs abuse, smoking status, dental procedures, or recurrent infections were reported. The physical examination showed upper lumbar localized pain, worsened by the axial load and thoracic flexion; bilateral lower limbs paresthesia without motor deficits was observed.
Laboratory blood tests showed increased values of C-reactive protein (CRP: 66.2 mg/L; normal value, < 5), erythrocyte sedimentation rate (ESR: 45 mm/hr; normal value, <30), white blood cells (WBC) count (8,580 elements per mm3), and negative blood cultures for bacterial. QuantiFERON-Tb Gold and human immunodeficiency virus serology were negative.
Lumbar spine computed tomography (CT) scan and magnetic resonance imaging (MRI) revealed a spondylodiscitis on L2–3 disc (Fig. 2). Imaging of the chest, skull, and paranasal sinuses did not show any significant finding. A CT-guided needle biopsy was performed on L2–3 and the collected samples were cultured for bacterial and fungal species. The following media were used for mycotic culturing: Candida BCG agar (Meus, Italy) and Saboraud Dextrose Agar+CAF tube (Meus). Filamentous mycelium was found on both media was observed within 72 hours. Matrix-assisted laser desorption/ionization-time of flight mass spectrometry technology, directly from colonies grown on culture plates [5], identified A. fumigatus. Nonculture-based assays, that the patient had for CLL, showed low levels of 1,3-beta-D-glucan (BDG Fungitell assay) on 3 consecutive serum samples (186, 253, and 145 pg/mL), respectively 12, 10, and 6 weeks before the diagnosis. At the time of the diagnosis serum, 1,3-beta-D-glucan value was negative (< 80 pg/mL). The galactomannan serum Index (GM Platelia assay) was also negative. Intravenous administration of liposomal amphotericin B was promptly started, and the patient was immobilized in a rigid fiberglass brace.
After 30 days of medical treatment and immobilization, CRP and ESR values and the WBC count reported a decreasing trend, and back pain and lower extremities numbness improved as well; therefore, oral voriconazole and a rigid bracing were prescribed, then the patient was discharged. At the 3 months follow-up visit, the patient reported minimal low back pain (VAS 2); the MRI showed an improvement of the radiological scenario the local inflammatory pattern. After 6 months, the MRI and the x-rays showed a further improvement, with no longer signs of active inflammatory process or neurological compression and no signs of segmental or spinal deformity (Fig. 3). Accordingly, voriconazole oral therapy was stopped. No antibiotic therapy-side effects were recorded. The patient was pain free and able to walk for up to 400 m, hence he was advised to progressively weaning the brace. The patient signed a specific consensus for scientific purposes, according to the institutional guidelines.
After duplication removal 191 papers were considered for eligibility in the present systematic review. Eighty-seven papers were excluded for the following reason according to the exclusion criteria: non-English written papers (27), cases of Aspergillosis with no spinal involvement (44), non-Aspergillosis mediated spinal infections (16). Therefore, 104 full-text papers were evaluated. Fifteen cases of postsurgical or postinjection VA were also reported and excluded according to our inclusion criteria. Finally, 89 papers reporting 112 cases, including our case report, of spontaneous VA were included in the present review [3,6-94]. The mean age of included patients was 41.4 ±18.9 years, 16 (14.4%) were pediatric (< 18 years), and the male/female ratio was 2.6:1. Demographic and clinical data are summarized in Table 1.
VA seems to be slightly prevalent in the thoracic spine (46.8%), followed by the lumbar (42.3%) and cervical (10.9%) segments. Single localizations were found in 76 patients (67.6%), while multiple localization in 36 (32.4%), comprising 11 cases (9.9%) of skipped locations. Abscesses were reported in 79 cases (70.3%): epidural abscess in 35 (31.2%), paravertebral in 34 (30.3%), and both paravertebral and epidural in 10 (8.9%). The presenting symptoms were: back pain (57%) in 64 patients, lower extremity weakness in 35 (31.2%), and fever in 14 (12.5%). The authors reported as risk factors were: pulmonary aspergillosis (22.5%), hematological malignancies (17.1%), immunosuppressive therapy after organ transplantation (13.5%), and chronic granulomatosis disease (9%) [95].
The diagnostic sample was obtained by open biopsy in 78 cases (79.2%), needle biopsy in 32 (28.8%), blood culture confirmed by open biopsy in 1 (0.9%), and postmortem investigations in 1 (0.9%).
The etiological diagnosis was made by culturing samples in 61 cases (54.9%), histological examination confirmed by cultures in 36 (32.4%), histological examination only in 11 (9.9%), polymerase chain reaction (PCR) in 3 (2.7%), and cytological examination in 1 (0.9%). Isolated species was A. fumigatus in 68 cases (61.2%), Aspergillus flavus in 14 (12.6%), Aspergillus terreus in 4 (3.6%), Aspergillus nidulans in 2 (1.8%), Aspergillus niger in 1 (0.9%), and Aspergillus udagawae in 1 (0.9%). In 22 cases (20.8%), Aspergillus specie was not specified.
Medical treatment alone was administered in 23 patients (20.7%), while in 89 (80.1%) adjuvant surgery was reported: posterior or anterior decompression and local debridement was performed in 49 patients (44.1%), anterior decompression and fusion with autologous bone graft in 20 (18%), decompression and circumferential fusion in 13 (11.7%), and posterior decompression and fusion in 7 (6.3%).
Medical treatments consisted in (1) single drug in 54 cases (48.6%): amphotericin B in 31 patients (27.9%), voriconazole in 12 (10.8%), itraconazole in 10 (9%), and fluconazole in 1 (0.9%); (2) combination of 2 or more drugs in 50 patients (45%): amphotericin B+flucytosine in 10 cases (9%), amphotericin B+itraconazole in 11 (9.9%), amphotericin B+voriconazole in 4 (3.6%), amphotericin B+flucytosine+itraconazole in 4 (3.6%), and amphotericin B+fluconazole in 2 (1.8%).
The mean duration of therapy was 140±247.9 days.
Seventy-three patients (65.7%) completely recovered at the last follow-up evaluation; radiological signs of chronic infection were reported in 7 patients (6.3%), whereas 32 patients (28.8%) died during the follow-up. The complication rate was as high as 36%. Neurological sequelae were reported in 10 cases (9%), recurrence of the infection in 9 (8.1%), spine deformity in 8 (7.2%), sepsis in 6 (5.4%), and respiratory failure in 5 (4.5%). Regarding medical therapy-associated complications, liver and renal dysfunction occurred in 10 (9%) and 6 cases (5.4%), respectively.
Aspergillosis represents a rare disease, and its incidence is estimated of 12 cases per year/1,000,000 people [60]; on the other hand, it has been frequently reported in immunodeficient patients [1]. VA is the most common Aspergillus-mediated osteomyelitis [3], and 20 different species of Aspergillus can cause infections in humans [96]. The most involved species is A. fumigatus [96], whereas A. flavus, A. niger, and A. nidulans are rarely isolated.
Three different pathogenetic mechanisms have been proposed: direct invasion by contiguous pulmonary foci, hematogenous diffusion, and iatrogenic or traumatic inoculation. Pulmonary aspergillosis represents the most relevant risk factor for VA, reported in 22% of the cases [95].
Diagnosis of spondylodiscitis sustained by Aspergillus is currently based on cultures and histological examination [79,96]. The role of serum galactomannan in VA is still debated [79], whereas the PCR on bone samples has not been validated yet, although it could be useful in the case of nondiagnostic culture and histology. The clinical presentation does not recognize any pathognomonic symptom or sign, as well as in pyogenic spondylodiscitis [97,98]. Back pain and neurological deficits are commonly reported by patients at the time of hospitalization, affecting the chances for a correct diagnosis [99]. Spinal cord and/or radicular compression can be determined by VO, epidural abscess, Aspergillus granuloma, vertebral fractures, and postinfectious deformities [60]. Gadolinium-enhanced MRI sequences may help in identifying infectious collections, abscesses, and granulomas [100]; therefore, neuroimaging should be carefully evaluated by experienced radiologists.
A targeted medical antifungal therapy may result in increased chances for better clinical outcomes in VA, thus the isolation of the involved pathogen is strongly recommended. Percutaneous sampling under radiological guidance may represent a useful alternative to open biopsy [2]. However, worsening neurological symptoms and/or poor response to medical therapies may lead to surgical decompression [2]. Surgery may be useful to achieve a solid fusion and deformity correction in case of spinal instability or vertebral collapse and obtain a microbiological sample to reach the diagnosis in case of negative needle biopsy [100-102].
Medical treatment should be customized for each patient. Vertebral instability, neurological compression, and local progression in course of the antifungal therapy should be carefully considered as criteria for surgery [79].
Up to date, either amphotericin or voriconazole is the recommended antifungal agents for the treatment of Aspergillus osteomyelitis according to The Infectious Diseases Society of America guidelines [103]. Voriconazole has been reported as the first choice, because of its high bioavailability and tissue penetration, even though its pharmacological interactions, the necessity of therapeutic drug monitoring (TDM), liver toxicity, and photosensitivity may influence its role in prolonged therapies. Liposomal amphotericin B is an alternative to be considered, although it is available only for intravenous usage, and has been associated with nephrotoxicity [103]. However, the British Infection Society recommends the use of liposomal amphotericin B in combination with flucytosine as the first choice for the treatment of Aspergillus osteomyelitis [1]. The use of itraconazole should be reserved for the treatment of stable patients. In fact, it can be effective in osteoarticular aspergillosis reporting a toxicity profile similar to voriconazole, and a lower bioavailability. Isavuconazole has a higher bioavailability and safety profile compared with voriconazole, and does not require TDM; however, it has been anecdotally used in VA. Posaconazole was proposed in 2 case reports only [104,105].
There is no consensus on the optimal antifungal therapy duration for Aspergillus osteomyelitis. Infectious Diseases Society of America guidelines for the treatment of Aspergillus infections suggested a minimum of 8 weeks, although longer administrations (> 6 months) are often needed [103]. Furthermore, these recommendations do not consider the role of surgery and its influence on medical treatment duration.
There some limitations we need to report for proper data interpretation. All the included studies are case report or case series, reporting a low level of evidence (IV-V). The publication time range of the included papers is longer than 50 years. Data have been reported in a nonhomogeneous way among the included papers, thus not allowing the authors to conduct any data pooling for statistical analysis.
In conclusion, VA is a rare condition, although its real incidence could have been underestimated due to diagnostic limitations. Fungal species should be routinely investigated in culture-negative spondylodiscitis, especially in immunocompromised patients. Since VA has a poor prognosis, an early diagnosis for targeted medical therapy should be always pursued. This systematic review summarized the state of the art on VA, retrieving data on clinical features, diagnostic criteria, and current limitations, treatment alternatives, and their outcomes, along with future perspectives for the clinical management. Further clinical studies are needed to better investigate this challenging topic.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow-chart. *The names of the authors who performed the review.
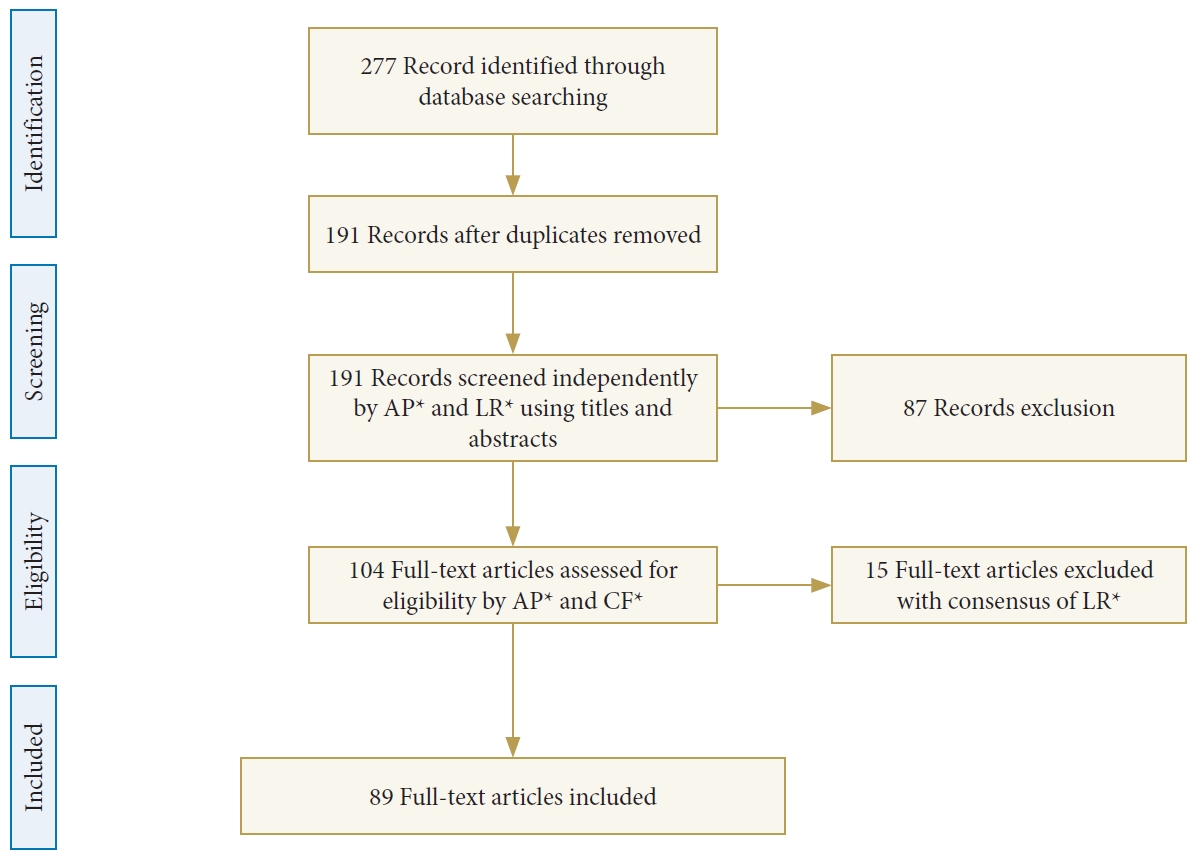
Fig. 2.
(A, B) TC images showing enlargement of the L2–3 disc space with erosion of the adjacent endplates with no evidences of epidural or paravertebral abscess. (C) Magnetic resonance images showing a T2 hyperintense signal on the L2–3 disc space with edema on the adjacent vertebral endplates, and partial obliteration of the anterior epidural space suggestive for L2–3 spondylodiscitis.
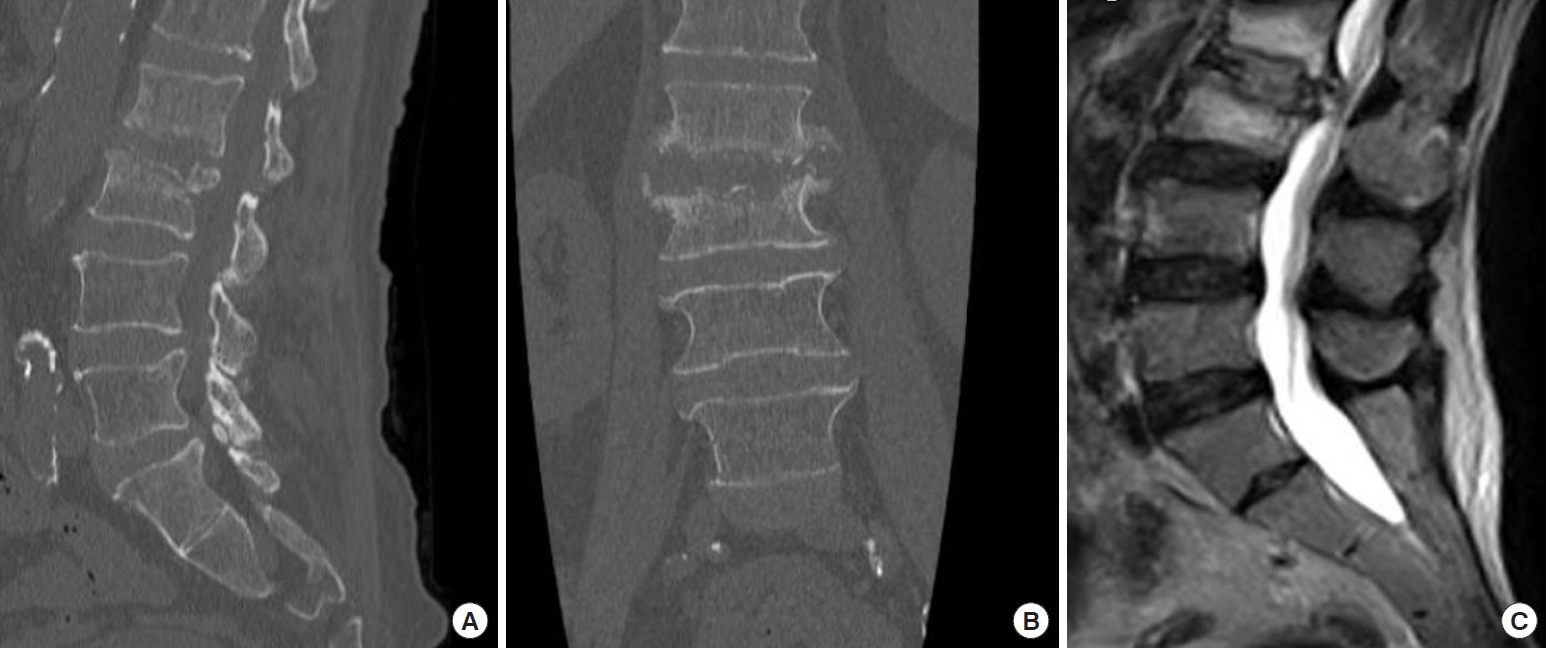
Fig. 3.
(A) Magnetic resonance images at 6 months of follow-up showing the reduction of hyperintense signal on the L2–3 space and the reduction of the epidural abscess. (B, C) Radiograph at 6-month follow-up.
Table 1.
Summary of clinical features describing patients with vertebral aspergillosis
Values are presented as mean±standard deviation or number (%).
TTBC, tubercolosis; IV, intravenous; AmB, amphotericin B; VOR, voriconazole; ITR, itraconazole; FLU, fluconazole; ABLC, amphotericin B lipid complex; 5-FC, 5-flucytosine; CAS, caspofungin; POS, posaconazole; KET, ketoconazole; RIF, rifampicin; MIC, micafungin.
REFERENCES
1. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008 46:1813-21.



2. Perna A, Ricciardi L, Sturiale CL, et al. Skipped vertebral spontaneous spondylodiscitis caused by granulicatella adiacens: case report and a systematic literature review. J Clin Orthop Trauma 2020 11:937-41.


3. van Ooij A, Beckers JM, Herpers MJ, et al. Surgical treatment of aspergillus spondylodiscitis. Eur Spine J 2000 9:75-9.



4. Groll AH, Shah PM, Mentzel C, et al. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect 1996 33:23-32.


5. De Carolis E, Posteraro B, Lass-Flörl C, et al. Species identification of Aspergillus, Fusarium and Mucorales with direct surface analysis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Microbiol Infect 2012 18:475-84.


9. Tack KJ, Rhame FS, Brown B, et al. Aspergillus osteomyelitis. Report of four cases and review of the literature. Am J Med 1982 73:295-300.

10. Chee YC, Poh SC. Aspergillus epidural abscess in a patient with obstructive airway disease. Postgrad Med J 1983 59:43-5.


11. Mawk JR, Erickson DL, Chou SN, et al. Aspergillus infections of the lumbar disc spaces. Report of three cases. J Neurosurg 1983 58:270-4.

12. McKee DF, Barr WM, Bryan CS, et al. Primary aspergillosis of the spine mimicking Pott's paraplegia. J Bone Joint Surg Am 1984 66:1481-3.


13. Nasca RJ, McElvein RB. Aspergillus fumigatus osteomyelitis of the thoracic spine treated by excision and interbody fusion. Spine (Phila Pa 1976) 1985 10:848-50.


14. Barnwell PA, Jelsma LF, Raff MJ. Aspergillus osteomyelitis. Report of a case and review of the literature. Diagn Microbiol Infect Dis 1985 3:515-9.


15. Ferris B, Jones C. Paraplegia due to aspergillosis. Successful conservative treatment of two cases. J Bone Joint Surg Br 1985 67:800-3.


16. Wagner DK, Varkey B, Sheth NK, et al. Epidural abscess, vertebral destruction, and paraplegia caused by extending infection from an aspergilloma. Am J Med 1985 78:518-22.


17. Brown DL, Musher DM, Taffet GE. Hematogenously acquired Aspergillus vertebral osteomyelitis in seemingly immunocompetent drug addicts. West J Med 1987 147:84-5.


18. White CJ, Kwon-Chung KJ, Gallin JI. Chronic granulomatous disease of childhood. An unusual case of infection with Aspergillus nidulans var. echinulatus. Am J Clin Pathol 1988 90:312-6.


19. Holmes PF, Osterman DW, Tullos HS. Aspergillus discitis. Report of two cases and review of the literature. Clin Orthop Relat Res 1988 226:240-6.
20. Bridwell KH, Campbell JW, Barenkamp SJ. Surgical treatment of hematogenous vertebral Aspergillus osteomyelitis. Spine (Phila Pa 1976) 1990 15:281-5.


21. Morgenlander JC, Rossitch E Jr, Rawlings CE 3rd. Aspergillus disc space infection: case report and review of the literature. Neurosurgery 1989 25:126-9.


22. Castelli C, Benazzo F, Minoli L, et al. Aspergillus infection of the L3-L4 disc space in an immunosuppressed heart transplant patient. Spine (Phila Pa 1976) 1990 15:1369-73.


23. Govender S, Rajoo R, Goga IE, et al. Aspergillus osteomyelitis of the spine. Spine (Phila Pa 1976) 1991 16:746-9.


24. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 11-1991. A 65-year-old man with a vertebral and disk lesion after a pulmonary operation for Aspergillus infection. N Engl J Med 1991 324:754-63.


25. Hendrix WC, Arruda LK, Platts-Mills TA, et al. Aspergillus epidural abscess and cord compression in a patient with aspergilloma and empyema. Survival and response to high dose systemic amphotericin therapy. Am Rev Respir Dis 1992 145:1483-6.


26. McGregor A, McNicol D, Collignon P. Aspergillus-induced discitis. A role for itraconazole in therapy? Spine (Phila Pa 1976) 1992 17:1512-4.

27. Hummel M, Schüler S, Weber U, et al. Aspergillosis with Aspergillus osteomyelitis and diskitis after heart transplantation: surgical and medical management. J Heart Lung Transplant 1993 12:599-603.

28. Cortet B, Richard R, Deprez X, et al. Aspergillus spondylodiscitis: successful conservative treatment in 9 cases. J Rheumatol 1994 21:1287-91.

29. Kline MW, Bocobo FC, Paul ME, et al. Successful medical therapy of Aspergillus osteomyelitis of the spine in an 11-year-old boy with chronic granulomatous disease. Pediatrics 1994 93:830-5.



30. Assaad W, Nuchikat PS, Cohen L, et al. Aspergillus discitis with acute disc abscess. Spine (Phila Pa 1976) 1994 19:2226-9.


31. Korovessis P, Repanti M, Katsardis T, et al. Anterior decompression and fusion for Aspergillus osteomyelitis of the lumbar spine associated with paraparesis. Spine (Phila Pa 1976) 1994 19:2715-8.


32. Collier J, Wolfe R, Lerner R, et al. Spinal aspergillus abscess in a patient with bronchocentric granulomatosis. J Intensive Care Med 1995 10:45-8.


33. Pfausler B, Kampfl A, Berek K, et al. Syndrome of the anterior spinal artery as the primary manifestation of aspergillosis. Infection 1995 23:240-2.


34. Lin MB, Chee SG. Aspergillus infection of the neck with an extradural component: an unusual presentation. Singapore Med J 1995 36:678-81.

35. Jones RS, Barman A, Byungse S, et al. Successful treatment of Aspergillus vertebral osteomyelitis with amphotericin B lipid complex. Inf Dis in Clin Pract 1995 4:237-9.

36. Dubbeld P, van Oostenbrugge RJ, Twinjstra A, et al. Spinal epidural abscess due to Aspergillus infection of the vertebrae: report of 3 cases. Neth J Med 1996 48:18-23.


37. Fan Y, Xie T, Pang Y, et al. Percutaneous transforaminal endoscopic discectomy for the treatment of lateral recess stenosis secondary occurred the discal fungus infection. BMC Musculoskelet Disord 2020 21:175.



38. Witzig RS, Greer DL, Hyslop NE Jr. Aspergillus flavus mycetoma and epidural abscess successfully treated with itraconazole. J Med Vet Mycol 1996 34:133-7.


39. Delmas Y, Merville P, Dousset V, et al. A renal transplant recipient with acute paraparesis due to an Aspergillus epidural abscess. Nephrol Dial Transplant 1997 12:2185-7.


40. Williams RL, Fukui MB, Meltzer CC, et al. Fungal spinal osteomyelitis in the immunocompromised patient: MR findings in three cases. AJNR Am J Neuroradiol 1999 20:381-5.


42. Martinez M, Lee AS, Hellinger WC, et al. Vertebral Aspergillus osteomyelitis and acute diskitis in patients with chronic obstructive pulmonary disease. Mayo Clin Proc 1999 74:579-83.


43. Tang TJ, Janssen HL, van der Vlies CH, et al. Aspergillus osteomyelitis after liver transplantation: conservative or surgical treatment? Eur J Gastroenterol Hepatol 2000 12:123-6.

44. Ur-Rahman N, Jamjoom ZA, Jamjoom A. Spinal aspergillosis in nonimmunocompromised host mimicking Pott’s paraplegia. Neurosurg Rev 2000 23:107-11.



45. Grandière-Perez L, Asfar P, Foussard C, et al. Spondylodiscitis due to Aspergillus terreus during an efficient treatment against invasive pulmonary aspergillosis. Intensive Care Med 2000 26:1010-1.


46. Abu Jawdeh L, Haidar R, Bitar F, et al. Aspergillus vertebral osteomyelitis in a child with a primary monocyte killing defect: response to GM-CSF therapy. J Infect 2000 41:97-100.


47. Auletta JJ, John CC. Spinal epidural abscesses in children: a 15-year experience and review of the literature. Clin Infect Dis 2001 32:9-16.


48. Park KU, Lee HS, Kim CJ, et al. Fungal discitis due to Aspergillus terreus in a patient with acute lymphoblastic leukemia. J Korean Med Sci 2000 15:704-7.



49. Govender S, Kumar KP. Aspergillus spondylitis in immunocompetent patients. Int Orthop 2001 25:74-6.



50. Gupta PK, Mahapatra AK, Gaind R, et al. Aspergillus spinal epidural abscess. Pediatr Neurosurg 2001 35:18-23.



51. Beckers EA, Strack van Schijndel RJ. Aspergillus spondylodiskitis in a patient with chronic obstructive pulmonary disease. Eur J Intern Med 2002 13:139-42.


52. Takagi K, Yoshida A, Yamauchi T, et al. Successful treatment of Aspergillus spondylodiscitis with high-dose itraconazole in a patient with acute myelogenous leukemia. Leukemia 2001 15:1670-1.


53. Chi CY, Fung CP, Liu CY. Aspergillus flavus epidural abscess and osteomyelitis in a diabetic patient. J Microbiol Immunol Infect 2003 36:145-8.

54. Stratov I, Korman TM, Johnson PD. Management of Aspergillus osteomyelitis: report of failure of liposomal amphotericin B and response to voriconazole in an immunocompetent host and literature review. Eur J Clin Microbiol Infect Dis 2003 22:277-83.


55. Salvalaggio PR, Bassetti M, Lorber MI, et al. Aspergillus vertebral osteomyelitis after simultaneous kidney-pancreas transplantation. Transpl Infect Dis 2003 5:187-90.


56. Farhoudi A, Siadati A, Atarod L, et al. Para vertebral abscess and rib osteomyelitis due to Aspergillous Fumigatus in a patient with chronic granulomatous disease. Iran J Allergy Asthma Immunol 2003 2:13-5.

57. Park SB, Kang MJ, Whang EA, et al. A case of fungal sepsis due to aspergillus spondylitis followed by cytomegalovirus infection in a renal transplant recipient. Transplant Proc 2004 36:2154-5.


58. Salloum A, Rao S, Havasi A, et al. Aspergillus rib and vertebral osteomyelitis in a former intravenous drug user. Am J Med 2004 116:208-9.


59. Santos AB, Llamas P, Gadea I, et al. Aspergillus fumigatus: a rare cause of vertebral osteomyelitis. Haematologica 2004 89:ECR10.

60. Vaishya S, Sharma MS. Spinal Aspergillus vertebral osteomyelitis with extradural abscess: case report and review of literature. Surg Neurol 2004 61:551-5.


61. Tendolkar U, Sharma A, Mathur M, et al. Epidural mass due to aspergillus flavus causing spinal cord compression--a case report and brief update. Indian J Med Microbiol 2005 23:200-3.


62. Son JM, Jee WH, Jung CK, et al. Aspergillus spondylitis involving the cervico-thoraco-lumbar spine in an immunocompromised patient: a case report. Korean J Radiol 2007 8:448-51.



63. Wéclawiak H, Garrouste C, Kamar N, et al. Aspergillus fumigatus-related spondylodiscitis in a heart transplant patient successfully treated with voriconazole. Transplant Proc 2007 39:2627-8.


64. Comacle P, Le Govic Y, Hoche-Delchet C, et al. Spondylodiscitis due to Aspergillus terreus in an immunocompetent host: case report and literature review. Mycopathologia 2016 181:575-81.


65. Dayan L, Sprecher H, Hananni A, et al. Aspergillus vertebral osteomyelitis in chronic leukocyte leukemia patient diagnosed by a novel panfungal polymerase chain reaction method. Spine J 2007 7:615-7.


66. Karapinar B, Yilmaz D, Asar G, et al. Disseminated invasive vertebral aspergillosis in an immunocompetent girl with a 7 year latent period. Pediatr Int 2007 49:516-8.


67. Andaluz N, Zuccarello M. Multidrug-resistant, progressive, invasive diffuse spinal aspergillosis: case report and review of the literature. J Neurosurg Sci 2008 52:49-53.

68. Beluffi G, Bernardo ME, Meloni G, et al. Spinal osteomyelitis due to Aspergillus flavus in a child: a rare complication after haematopoietic stem cell transplantation. Pediatr Radiol 2008 38:709-12.


69. Nandeesh BN, Kini U, Alexander B. Vertebral osteomyelitis with a rare etiology diagnosed by fine-needle aspiration cytology. Diagn Cytopathol 2010 38:360-3.


70. Gerlach UA, Kohler S, Sauer IM, et al. Aspergillus spondylodiscitis after multivisceral transplantation. Ann Transplant 2009 14:52-7.
71. Ho HH, Lin MC, Yu KH, et al. Pulmonary tuberculosis and disease-related pulmonary apical fibrosis in ankylosing spondylitis. J Rheumatol 2009 36:355-60.


72. Tew CW, Han FC, Jureen R, et al. Aspergillus vertebral osteomyelitis and epidural abscess. Singapore Med J 2009 50:e151-4.

73. Batra S, Arora S, Meshram H, et al. A rare etiology of cauda equina syndrome. J Infect Dev Ctries 2011 5:79-82.


74. Al-Tawfiq JA, Al-Abdely HM. Vertebral osteomyelitis due to Aspergillus fumigatus in a patient with chronic granulomatous disease successfully treated with antifungal agents and interferon-gamma. Med Mycol 2010 48:537-41.


75. Karthik K, Shetty AP, Rajasekaran S. Spontaneous cord transection due to invasive aspergillus spondylitis in an immunocompetent child. Eur Spine J 2011 20:S188-92.


76. Winterstein AR, Bohndorf K, Vollert K, et al. Invasive aspergillosis osteomyelitis in children--a case report and review of the literature. Skeletal Radiol 2010 39:827-31.


77. Ranjan R, Mishra S, Ranjan S. Aspergillus vertebral osteomyelitis in an immunocompetent person. Neurol India 2010 58:806-8.


78. Rossouw I, Goedhals D, van der Merwe J, et al. A rare, fatal case of invasive spinal aspergillosis in an antiretroviral-naive, HIV-infected man with pre-existing lung colonization. J Med Microbiol 2011 60:1534-8.

79. Studemeister A, Stevens DA. Aspergillus vertebral osteomyelitis in immunocompetent hosts: role of triazole antifungal therapy. Clin Infect Dis 2011 52:e1-6.


80. Ersoy A, Dizdar OS, Koc AO, et al. Aspergillus fumigatus spondylodiskitis in renal transplant patient: voriconazole experience. Exp Clin Transplant 2011 9:265-9.

81. Zhu LP, Chen XS, Wu JQ, et al. Aspergillus vertebral osteomyelitis and ureteral obstruction after liver transplantation. Transpl Infect Dis 2011 13:192-9.


82. Chang HM, Yu HH, Yang YH, et al. Successful treatment of Aspergillus flavus spondylodiscitis with epidural abscess in a patient with chronic granulomatous disease. Pediatr Infect Dis J 2012 31:100-1.


83. Sethi S, Siraj F, Kalra K, et al. Aspergillus vertebral osteomyelitis in immunocompetent patients. Indian J Orthop 2012 46:246-50.



84. Li XF, Liu ZD, Xia Q, et al. Aspergillus spondylodiscitis in solid organ transplant recipients. Transplant Proc 2010 42:4513-7.


85. Jiang Z, Wang Y, Jiang Y, et al. Vertebral osteomyelitis and epidural abscess due to Aspergillus nidulans resulting in spinal cord compression: case report and literature review. J Int Med Res 2013 41:502-10.


86. Nicolle A, de la Blanchardière A, Bonhomme J, et al. Aspergillus vertebral osteomyelitis in immunocompetent subjects: case report and review of the literature. Infection 2013 41:833-40.


87. Raj KA, Srinivasamurthy BC, Nagarajan K, et al. A rare case of spontaneous Aspergillus spondylodiscitis with epidural abscess in a 45-year-old immunocompetent female. J Craniovertebr Junction Spine 2013 4:82-4.



88. Ma H, Lv G, Wang B. Does surgery influence the outcome of Aspergillus osteomyelitis? Clin Microbiol Infect 2014 20:O788.

89. McCaslin AF, Lall RR, Wong AP, et al. Thoracic spinal cord intramedullary aspergillus invasion and abscess. J Clin Neurosci 2015 22:404-6.


90. Soule DDO, Bearman G. Invasive Aspergillosis manifested as lumbar vertebral osteomyelitis in an immunocompetent man. Inf Dis in Clin Pract 2016 24:234-6.

91. Sohn YJ, Yun JH, Yun KW, et al. Aspergillus terreus spondylodiscitis in an immunocompromised child. Pediatr Infect Dis J 2019 38:161-3.


92. Senosain-Leon V, Hidalgo-Benites A, Arriola-Montenegro J, et al. Invasive pulmonary aspergillosis with Aspergillus vertebral osteomyelitis in an HIV-infected adult: a case report. Int J STD AIDS 2019 30:1140-2.


93. Sabo MC, Blain M, McCulloch D, et al. back pain in a 23-year-old male with x-linked chronic granulomatous disease. Open Forum Infect Dis 2019 6:ofz449.



94. Yang H, Shah AA, Nelson SB, et al. Fungal spinal epidural abscess: a case series of nine patients. Spine J 2019 19:516-22.


95. Gamaletsou MN, Rammaert B, Bueno MA, et al. Aspergillus osteomyelitis: epidemiology, clinical manifestations, management, and outcome. J Infect 2014 68:478-93.


96. Gabrielli E, Fothergill AW, Brescini L, et al. Osteomyelitis caused by Aspergillus species: a review of 310 reported cases. Clin Microbiol Infect 2014 20:559-65.


97. Fantoni M, Trecarichi EM, Rossi B, et al. Epidemiological and clinical features of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci 2012 16:2-7.

98. Pola E, Taccari F, Autore G, et al. Multidisciplinary management of pyogenic spondylodiscitis: epidemiological and clinical features, prognostic factors and long-term outcomes in 207 patients. Eur Spine J 2018 27:229-36.


99. Tamburrelli FC, Meluzio MC, Masci G, et al. Etiopathogenesis of traumatic spinal epidural hematoma. Neurospine 2018 15:101-7.



100. Proietti L, Ricciardi L, Noia G, et al. Extensive spinal epidural abscesses resolved with minimally invasive surgery: two case reports and review of the recent literature. Acta Neurochir Suppl 2019 125:345-53.


101. Tamburrelli FC, Perna A, Proietti L, et al. The feasibility of long-segment fluoroscopy-guided percutaneous thoracic spine pedicle screw fixation, and the outcome at two-year follow-up. Malays Orthop J 2019 13:39-44.



102. De Vitis R, Passiatore M, Perna A, et al. Modified MattiRusse technique using a “butterfly bone graft” for treatment of scaphoid non-union. J Orthop 2019 19:63-6.



103. Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016 15:63:e1-60.





























