 |
 |
- Search
| Neurospine > Volume 19(1); 2022 > Article |
|
|
Abstract
Objective
Intramedullary spinal cord metastasis from lung cancer (ISCM-LC) are increasing in prevalence. We aim to investigate its clinical features, treatments and prognosis.
Methods
We reported 6 ISCM-LC cases and conducted a systematic review. Descriptive summarization, survival analysis, and multivariate Cox regression analysis were performed to comprehensively study the disease.
Results
All 6 patients had surgery. One used chemotherapy and the other had targeted drugs. Two patients died of ISCM-LC, 1 died of pulmonary embolism, 1 was alive, and 2 were lost to follow-up. We identified 197 ISCM-LC cases in literature with a mean age of 58 years and male preponderance. Small cell lung cancer accounted for 39.1%. The median interval from lung cancer to ISCM-LC was 7 months. Limb weakness was the most common symptom, and 45% cases progressed rapidly. Concomitant brain, leptomeningeal, and vertebral metastasis occurred to 55.8%, 20%, and 19.5%, respectively. Peritumoral edema appeared in 83.3%. Through survival analysis, we found sex, extraspinal metastasis, pathology, and improved symptoms affected the overall survival. Additionally, gross total resection (GTR) shared similar effectiveness with non-GTR, and other treatments following surgery hardly added extra effect. Surgery, improved symptoms, and sex were 3 independent prognostic factors after adjusting for confounding. The estimated median survival time was 5 months.
Intramedullary spinal cord metastases (ISCM) are a rare but devastating issue of systematic malignancy. They constitute 4.2-8.5% of neuraxial metastasis and 1-3% of intramedullary neoplasms [1]. Among them, ISCM from lung cancer (ISCM-LC) were estimated to be the predominant type, accounting for 42.4%–67.21% [2-4]. As cancer patients survived longer over decades, the incidence of ISCM has reportedly increased [5]. However, the sporadic cases and inconsistent conclusions have still rendered ISCM understudied [1,4].
The occurrence of ISCM usually heralds severe neurological deficits and shortened life expectancy [2,4-6]. ISCM rising from lung cancer appeared to fare worse than breast cancer, suggesting the strata based on primary pathologies were necessary [5,6]. The dismal prognosis was partly due to controversial treatments. Though clinicians reported experience in surgery [2,4,7,8], radiotherapy [9], chemotherapy [10,11], and targeted drugs [12], no treatment paradigm has been established yet. Herein, we reported 6 cases of ISCM-LC and pooled cases from literature, aiming to explore the clinical features as well as treatment modalities, and lastly investigate the prognostic factors for the overall survival (OS).
From December 2012 to February 2020, a total of 6 patients diagnosed as ISCM-LC at our center were retrospectively analyzed. The inclusion criteria were as follows: (1) age over 18 years; (2) intramedullary lesions were histologically consistent with lung cancer; (3) primary pathology was identified in lung; (4) no other malignancy was found through full body computed tomography (CT) or positron emission tomography (PET)-CT scan. We recommended surgical intervention in the presence of unknown solitary intramedullary mass and rapid clinical deterioration. Intraoperative neurophysiological monitoring was applied in all surgical cases. Study parameters include demographics, smoking history, primary pathology, duration of symptoms, interval from lung cancer to ISCM, location and segments of lesion, other concomitant metastasis, pre- and postoperative neurological statuses, treatments, cause of death, and OS. We define OS as the duration from the diagnosis of ISCM to the death of patients or last follow-ups, and 1 month equals to 30 days. Modified McCormick Classification (MMC) was applied to assess neurological statuses [13]. Karnofsky Performance Score (KPS) at diagnosis and discharge were also used to assess patient performance status [14]. The last follow-up date was July 11th, 2021. All 6 patients and their kin consented to the use of their medical records for research purposes. This retrospective study was approved by the Institutional Review Board (IRB) of Beijing Tiantan Hospital, Capital Medical University (IRB No. KY2021-017-01).
We also performed a systematic search on PubMed and Embase for ISCM-LC cases from inception till October 1st, 2020 (Fig. 1). We incorporated keywords, MeSH terms, and alternative spellings such as “spinal cord tumor,” “spinal cord neoplasms,” “spinal cord metastasis,” “intramedullary spinal cord metastasis,” “lung cancer,” “lung carcinomas” into our search strategy. References of the included articles were also searched for relevant cases. We strictly adhered to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocol along the search [15]. The protocol of this study was registered on PROSPERO-International Prospective Register of Systematic Reviews on April 28, 2020 (CRD42020166779).
Title and abstract screening, full-text eligibility assessment, and data extraction were conducted by 2 reviewers independently and in duplicate. A third reviewer was consulted if there was disagreement unresolved. Case inclusion criteria were as follows: (1) identified spinal cord mass, (2) histopathology consistent with lung cancer, (3) primary lesion found in lung, and (4) no other identified malignancy. Exclusion criteria were (1) ambiguous definitions of the lesion, (2) no primary lesion identified in lung, (3) a second or more malignancies found, (4) lesions affecting epidural space only, and (5) case series without specific case illustration. The selected study parameters for data extraction were the same as ours. Emails were sent for requesting the missing raw data.
The risk of bias assessment was performed using the JoannaBriggs Institute critical appraisal tool for case reports and case series [16,17]. Reporting bias of articles was carefully addressed through comparing authors and baseline information of patients. This excluded duplication. The attrition bias was also observed due to incomplete outcome data. The literature cases with excessive missing data, especially those without endpoint status or survival time were excluded from statistical analysis. Baseline information was kept as possible for descriptive analysis.
Subgroup analyses of ISCM-LC were done via Mann-Whitney test, Pearson chi-square test, Fisher exact test, and Log-rank test, respectively. Kaplan-Meier curves were generated, and Log-rank tests were performed to evaluate the association between categorical variables and OS. Mortality caused by ISCM-LC was defined as endpoint event while others were considered censored, including cases which we failed to acquire from authors. Multivariate Cox regression analysis was done to investigate prognostic factors. The proportional hazard assumption was examined and met by plotting the log minus log survival curves. Statistical significance was defined as p<0.05. All statistical analyses were performed using both IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA) and GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Three males and 3 females with a median age of 60 years (range, 58–67 years) were included in our study (Table 1). Case 2 smoked 2 packs of cigarettes per day for 40 years, and case 4 smoked the same amount for 20 years. Others never smoked. Limb weakness was noticed in 5 patients, sensory deficits in 5, pain in 4, and sphincter dysfunction in 2. The duration of symptoms ranged from 0.4 to 12 months. The median interval from lung cancer to ISCM was 18 months (range, 0–72 months). Specially, 2 patients presented with ISCM primarily without lung cancer history. Four metastases occurred at cervical level while 2 were located at thoracic cord. The lesions measured 2 segments long mostly with edema extending a bit longer. Generally, the lesion demonstrated iso-intensity on T1-weighted imaging (WI), iso- or hyperintensity on T2WI, and irregular enhancement with contrast (Fig. 2).
Gross total resection (GTR) was achieved in 3 patients. Another 3 patients underwent subtotal resection (STR) due to the compromised intraoperative neurophysiologic monitoring. We recommended Radiotherapy Department and Tumor Clinics to all patients for further consultation, though only case 6 accepted targeted drugs and case 3 was administered chemotherapy in local hospital. Adenocarcinoma was histologically confirmed in 5 patients, and squamous carcinoma in 1 patient.
We completed the last follow-up on July 1st, 2021. Two patients were lost to follow-up on their 21st day (case 1) and 13th day (case 2) since the primary diagnosis. At discharge, the neurological statuses of case 1 and case 2 were graded MMC IV and MMC III, respectively. Unfortunately, case 1 experienced declining status after surgery, while the KPS of case 2 remained 80. Case 4 died of acute pulmonary embolism on his 6th day since the diagnosis. Additionally, 2 patients died of cancer-caused cachexia after 25 months (case 3) and 2 months (case 5), respectively. These 2 patients experienced no postoperative complications, and their KPS at discharge remained 40 at the time. Case 6 was still fighting cancer 18 months later, and her neurological status was graded MMC III and scored KPS 60, revealing slight improvement. At initial diagnosis, we only found brain and multiple lymph nodes metastases in case 4. However, case 6 developed brain metastasis 10 months later and was put on osimertinib.
We eligibly identified 89 studies, among which, 197 cases of ISCM-LC were carefully assessed by 2 experienced neurosurgeons. With a mean age of 58 years, patients diagnosed with ISCM-LC had a male to female ratio of 2.22:1 (Table 2). The most common symptom was limb weakness (76.4%, n=161), and then came paresthesia (61.5%), pain (49.7%), sphincter dysfunction (48.4%), and dysreflexia (18.0%). Around 6.2% cases were asymptomatic and accidentally found. The median duration of symptoms was 0.7 month (range, 0–12 months), and the median interval from lung cancer diagnosis to ISCM was 7 months (range, 0–144 months). Specially, about 31.2% (n=141) patients had no lung cancer history.
Around 32.2% (n=180) ISCM-LC occurred to thoracic cord, followed by 28.3% to cervical, 23.9% to conus medullaris and below, 4.4% to cervicothoracic junction, and 11.1% involved multiple sections. In addition, 66% (n=147) ISCM-LC measured 1 to 2 segments long. Extraspinal metastasis were found in 76.6% (n=141) patients, concomitant brain metastasis in 55.8% (n=138), leptomeningeal involvement in 20% (n=125), and vertebral metastasis in 19.5% (n=118). Besides, edema surrounding ISCM was noticed in 83.3% (n=48) cases. Lung cancer subtypes varied in proportions. Of 179 ISCM-LC cases, small cell lung cancer (SCLC) accounted for 39.1%, followed by adenocarcinoma for 25.1%, and squamous carcinoma for 10.6%.
No treatment paradigm was established yet. Among the dataset, 26 cases recorded no therapeutic strategies, and 35 (20.5%, n=171) adopted only the basic supportive care (Fig. 3). Radiotherapy alone was performed in 29.2% cases, surgery alone in 12.3%, chemotherapy alone in 2.3%. The multimodal treatments accounted for 16.4%. Through multivariate Cox regression analysis, we found surgery was a protective factor (hazard ratio [HR], 0.379; 95% confidence interval [CI]; 0.164–0.877; p=0.023) for OS, reducing mortality risk by 62.1% (Fig. 4). However, we failed to conclude radiotherapy (HR, 0.692; 95% CI, 0.323–1.482; p=0.343) or chemotherapy (HR, 0.624; 95% CI, 0.294–1.325; p=0.220) could increase OS adjusted by age and gender. Using survival analysis, we noticed surgery followed by other treatments hardly favored a better survival than surgery alone (p=0.406) (Fig. 5). In addition, GTR shared similar effects on OS with non-GTR procedure (p=0.611).
Univariate analysis through Kaplan-Meier method was conducted, and female (p=0.046), absence of limb weakness (p=0.034), non-small cell lung cancer (NSCLC) (p=0.014), absence of extraspinal metastasis (p=0.025), and improved symptoms (p=0.046) were significantly correlated with better survival. Multivariate Cox regression analysis was thereby performed, and some prognostic factors were identified. Surgery was found an effective intervention as mentioned (p=0.023). And improved symptoms after interventions were associated with better OS than those with worsened symptoms, reducing mortality risk by 78.8% (HR, 0.212; 95% CI, 0.099–0.454; p<0.001). Additionally, we identified male was a risk factor, and the risk of male dying from ISCM-LC was around twice the risk in female (HR, 2.016; 95% CI, 1.013–4.013; p=0.046).
No difference between ISCM rising from SCLC and NSCLC was observed in age (p=0.797), sex (p=0.672), smoking (p=0.626), concomitant brain metastasis (p=0.098), and location of lesions (p=0.188). In addition, the performances of radiotherapy (p=0.290), and chemotherapy (p=0.446) were not different between the 2 groups, while therapies such as surgery (p=0.019), targeted drugs (p=0.046), and intratumoral 125I implant (p=0.043) were mainly used in NSCLC group (Table 3). ISCM from SCLC might involve over 3 segments (p=0.030), affect sphincter function (p=0.012), and compromise OS (p=0.014) more frequently than those from NSCLC.
ISCM are a rare disease across the whole course of malignancy [2,3,18]. However, as therapeutic modalities evolved over decades, survival from primary malignancy has improved considerably, and the reports of ISCM were more frequent [5]. The Cancer Statistics for 2020 demonstrated lung cancer to be the leading cause of cancer mortality and the second leading cause of new cancer cases [19]. ISCM-LC is therefore a focus for systematic management of lung cancer patients.
Comparing our dataset with other published series, we found the mean age of 58 years comparable [4,5]. We identified the male to female ratio of 2.22:1, which was consistent with previous results that males were most frequently affected [1]. Nicotine was found to promote brain metastasis [20]. We noticed 84.6% (n=26) ISCM-LC patients smoked, and similar explanation of nicotine fostering ISCM seemed reasonable but required further proofs. Concerning localization of ISCM lesions, there seemed to be no tendency for ISCM to affect particular sections, which was consistent with a large series [1]. Conversely, some single institutes drew distinctive conclusions, which possibly reflected the selection bias [5,21]. ISCM frequently occurred with surrounding edema in our dataset (83.3%, n=48), distorting the spinal cord and causing symptoms [1]. First came limb weakness followed by paresthesia, pain and so [1,2,4]. However, there were also some asymptomatic cases (6.2%, n=161). Considering the insidious feature of lung cancer and densely distributed functional areas of spinal cord, ISCM as the primary emerge (31.2%, n=141) might be understandable. Some deterioration was rapid, and we noticed 45% (n=80) cases worsened within half a month. Some investigators, therefore, advised to differentiate ISCM from other primary spinal neoplasms using this characteristic [1,22]. Limited by case numbers, most studies focused on ISCM from all malignancies [2,4,7]. One large series revealed SCLC was the most common subtype, followed by adenocarcinoma and squamous carcinoma. This proportion was roughly reflected in our dataset.
Concomitant metastasis was frequently observed in published cases [23]. We identified concomitant brain metastasis in 55.8% (n=138), leptomeningeal metastasis in 20% (n=125), and vertebral metastasis in 19.5% (n=118) ISCM-LC cases. The concomitant brain metastasis was comparable to the percentages given by Sung et al. [1] and Goyal et al. [4] Interestingly, Hashii et al. [9] concluded that occurrence of brain metastasis was around sixfold higher in ISCM patients than those in vertebral metastasis cases.
Accurate recognition is a tough challenge. Magnetic resonance imaging (MRI) remains first-line diagnostic tool [24]. Before MRI came into use, most ISCM-LC cases were diagnosed only upon autopsy [25,26]. Only 5% were diagnosed before death [27]. PET-CT was considered a promising technique for metastasis localization [28]. Spinal cord provided a favorable tumor-to-background with fluorine-18 fluorodeoxyglucose contrast, and ISCM, therefore, turned more amenable for detection on whole-body scan [28,29]. This was noted to have 96% sensitivity and 50% specificity for ISCM [29].
Optimal treatment modalities remain unestablished. Surgical resection was proposed by neurosurgeons in the presence of good preoperative KPS, or rapidly declining neurological deficits, or indolent and solitary spinal cord lesion without brain metastasis, or radiation failure, or the necessity to locate the primary malignancy [4,5,8,21,30]. Conversely, the presence of leptomeningeal carcinomatosis generally portends grim prognosis, surgical removal of ISCM should be considered cautiously [30]. Some investigators also argued surgery should not be recommended when patients were experiencing complete paraplegia or in asymptomatic status [21]. We noticed surgery was performed less in patients with ISCM from SCLC than NSCLC. Though, we lacked information on preoperative neurological statuses for individual cases and left this unexplained. Surgery was a protective factor, reducing mortality risk by 62.1%. This conclusion that surgery favors better OS in selected cases was consistent with many studies [4,21,30]. We believe the benefits of surgery were attributed to decompression, reducing tumor load, and improving symptoms. The correlation of surgery and better symptom improvement (Pearson chi-square test, p=0.013) might have indicated better local control. However, the necessity of GTR was not proved, and radical resection might lead to irreversible deficits [7]. Similarly, we failed to conclude patients undergoing GTR achieved better OS than non-GTR receiver (Log-rank test, p=0.611). We believe radical resection could cause extra damage to the spinal cord, and thus add no extra benefit to OS. Moreover, the investigator also concluded that STR followed by adjuvant therapy seemed a valid option [7]. Interestingly, we hardly found significance in surgical adjunct with respect to achieving better OS than surgery alone through multivariate Cox analysis.
Radiotherapy and chemotherapy were controversial in improving OS. ISCM patients undergoing radiotherapy alone attained an objective response rate of 61.9% and local control rate of 90.48% [2]. Another study concluded prompt radiotherapy was required to maintain quality of life [9]. We also found the correlation of adjuvant radiotherapy and better symptom improvement (Pearson chi-square test, p<0.001). However, no evidence revealed radiotherapy could improve OS and our dataset drew identical conclusion. One series reported though 2 patients showed a transient neurological improvement after local radiotherapy, their motor deficits became worse eventually [6]. This indicated radiotherapy could improve neurological deficits to certain extent rather than increase OS. In Fig. 4, we noticed the trend for efficacy of radiotherapy after conducting multivariate Cox regression analysis, but this was not statistically significant. We believe this was due to the heterogenous radiation dose, various clinical practices, and the very special limitation of retrospective study-reporting bias. Concerning radiation dose, Sung et al. set the range from 20 Gy to 30 Gy, which was the same with Dam-Hieu et al. and Hashii et al. [6,9,21]. Chemotherapy was trialed mainly together with radiotherapy [31]. Similarly, multivariate Cox regression analysis revealed no positive effect of chemotherapy on OS. However, current standard chemotherapies were not generally applied previously. In advanced NSCLC, cisplatin plus pemetrexed was the first-line treatment with good tolerability and convenient administration [32]. Whereas in extensive stage SCLC patients, durvalumab with platinum-etoposide strategy was recommended as first-line treatment [33]. And we knew little about the efficacy of them in ISCM-LC patients.
Some alternative treatments seemed promising choices. When a radical surgical resection meant compromised neurological status, intratumoral 125I implant might be trialed given its effectiveness and good toleration [34]. The seed could be applied surgically or percutaneously under CT-guidance. And the implanted seed number could be calculated through treatment planning system, providing peripheral radiation dose. Besides, some targeted drugs were reported for efficacy [12,35-39]. Three options were trialed in the treatment of ISCM rising from NSCLC, and they respectively targeted epidermal growth factor receptor (EGFR) mutants [12,38], anaplastic lymphoma kinase (ALK) [35,39], and the immune checkpoint (PD-1L) [36]. Osimertinib, afatinib, and gefitinib targeting EGFR mutants all achieved good response in treating ISCM from NSCLC [12,23,38]. In our series, case 6 was given gefinitib and osimertinib in succession and attained good maintenance. ALK inhibitors, such as ceritinib and lorlatinib could penetrate blood-brain barrier easily and achieved good response, whereas crizotinib, also an ALK inhibitor, worked less effectively and left brain susceptible to metastasis [35,39]. Another alternative drug was nivolumab, a checkpoint inhibitor, whose clinical results also proved itself a potential option for patients with asymptomatic small solitary ISCM from NSCLC [36].
The OS of ISCM-LC patients was dismal, and the estimated median survival was only 5 months. Hashii et al. [9] concluded that the OS of ISCM was worse than vertebral metastasis. We noticed extraspinal metastasis could affect OS using Kaplan-Meier method. However, after adjusting confounding factors, we hardly found it significant, which was consistent with results from Goyal et al. [4,5]. In addition, limb weakness was also not a significant indicator after multivariate analysis. We thought complete paraplegia severely compromised the life quality and thus reasonably harmed survival. Nevertheless, this dataset provided little information on the prognostic value of limb weakness in OS. Through multivariate Cox regression analysis, we found male was a risk factor. Males had twice the mortality risk once they developed ISCM-LC. Study had it that women were more likely to have adenocarcinoma and stage 1A lung cancer, generally favoring a better survival [40]. We thereby suspected the predominant pathological subtypes in males and females might play a role in this risk elevation. However, we failed to find the gender ratio difference between SCLC group and NSCLC group in dataset. Another prognostic indicator for OS was improvement of symptoms. After any intervention, improved symptoms meant the risk of mortality from ISCM was reduced by 78.8% in comparison with worsened symptoms. Though there were heterogeneities in this covariate, including uneven recording time and ambiguity of improvement level, we still believed and concluded that this alteration of neurological status was a meaningful indicator for assessing OS under limited information.
Our study has limitations. There was attrition bias due to incomplete values and some authors might have reported selected information. Moreover, some critical prognostic parameters such as KPS could not be collected from most retrospective cases, which might have biased the multivariate analysis. However, ISCM were a rare malignancy, and a comprehensive analysis of the pooled data was maybe the best we could offer to explore the disease.
NOTES
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) chart depicting the systematic search strategy.
Fig. 2.
Illustration of case 1. Preoperative sagittal magnetic resonance imaging demonstrated iso-intensity on T1-weighted imaging (WI) (A), extended hyperintensity on T2WI (B), and slightly irregular enhancement with contrast (C). (D) Histopathology of intramedullary metastasis from lung adenocarcinoma (hematoxylin and eosin, × 200).
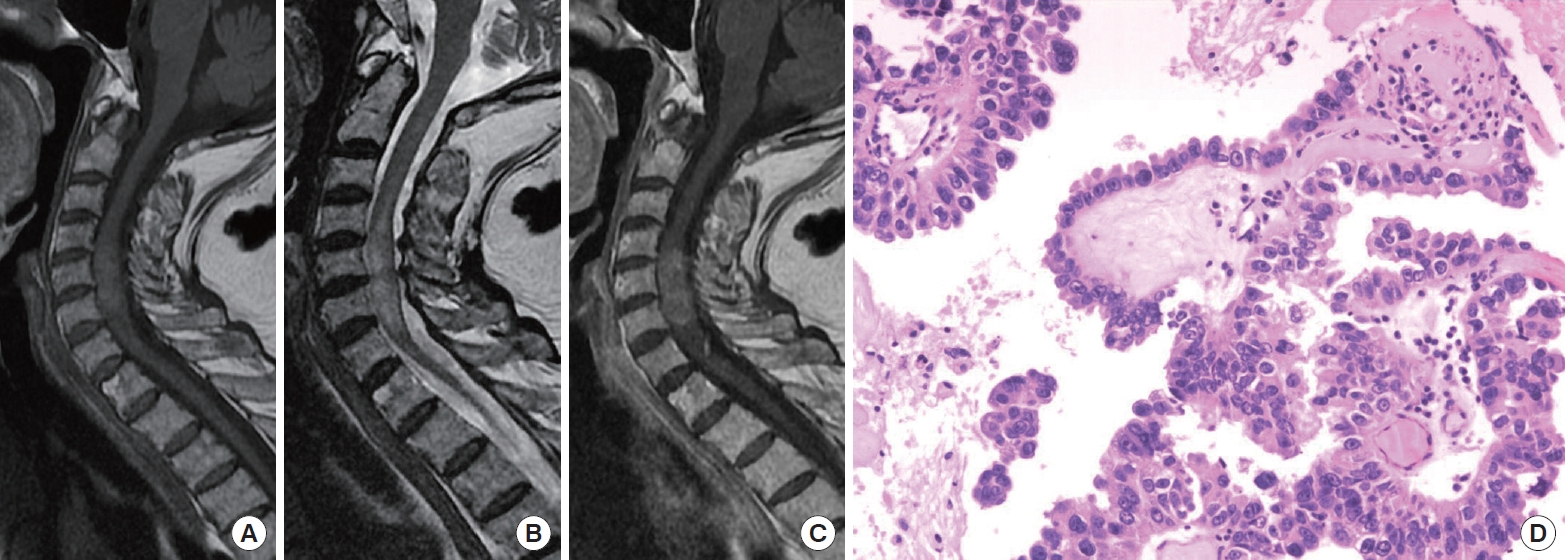
Fig. 3.
Conventional treatment modalities (A) and alternative treatment modalities (B). Chemo, chemotherapy; NA, not available; RT, radiotherapy.
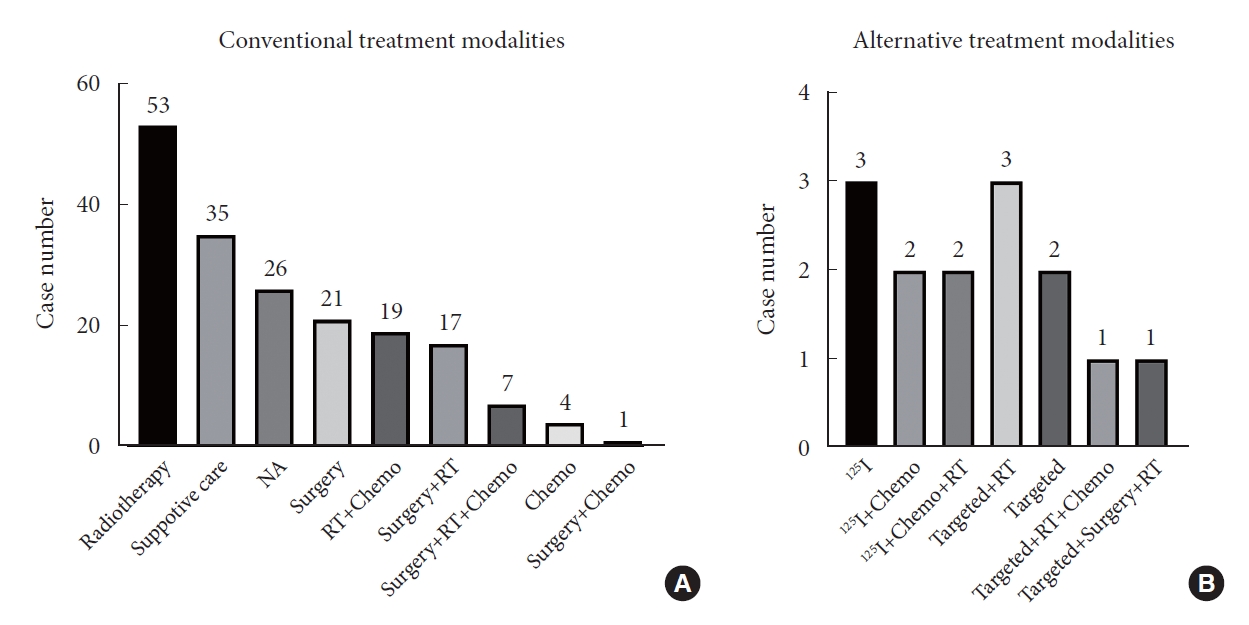
Fig. 4.
Multivariate Cox regression analysis was employed to evaluate the prognostic factors for overall survival. Black diamonds indicated the hazard ratio, error bars revealed the 95% confidence interval (CI), and asterisks represented p<0.05. ISCM, intramedullary spinal cord metastasis.
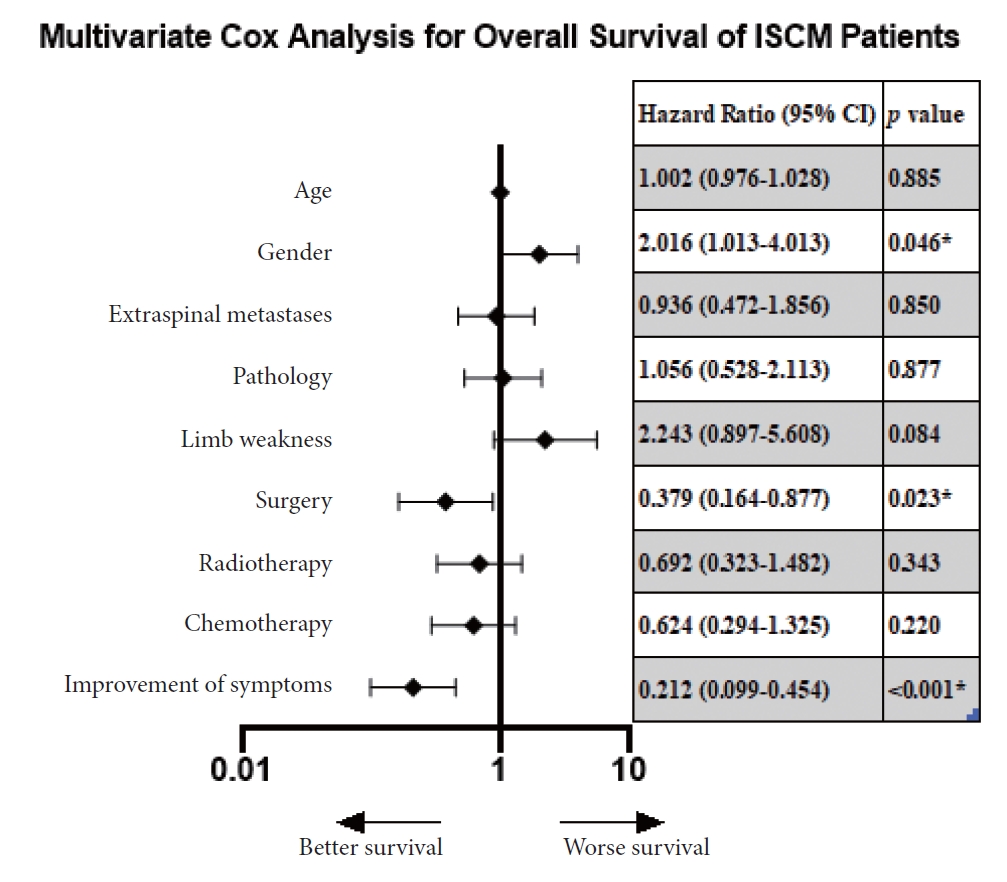
Fig. 5.
Kaplan-Meier curves depicted the overall survival of all literature cases (A), and the effects of some categorical variables on overall survival, including sex (B), pathology (C), improvement of symptoms (D), gross total resection (GTR) (E), and surgery followed by other treatments (F). SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer.
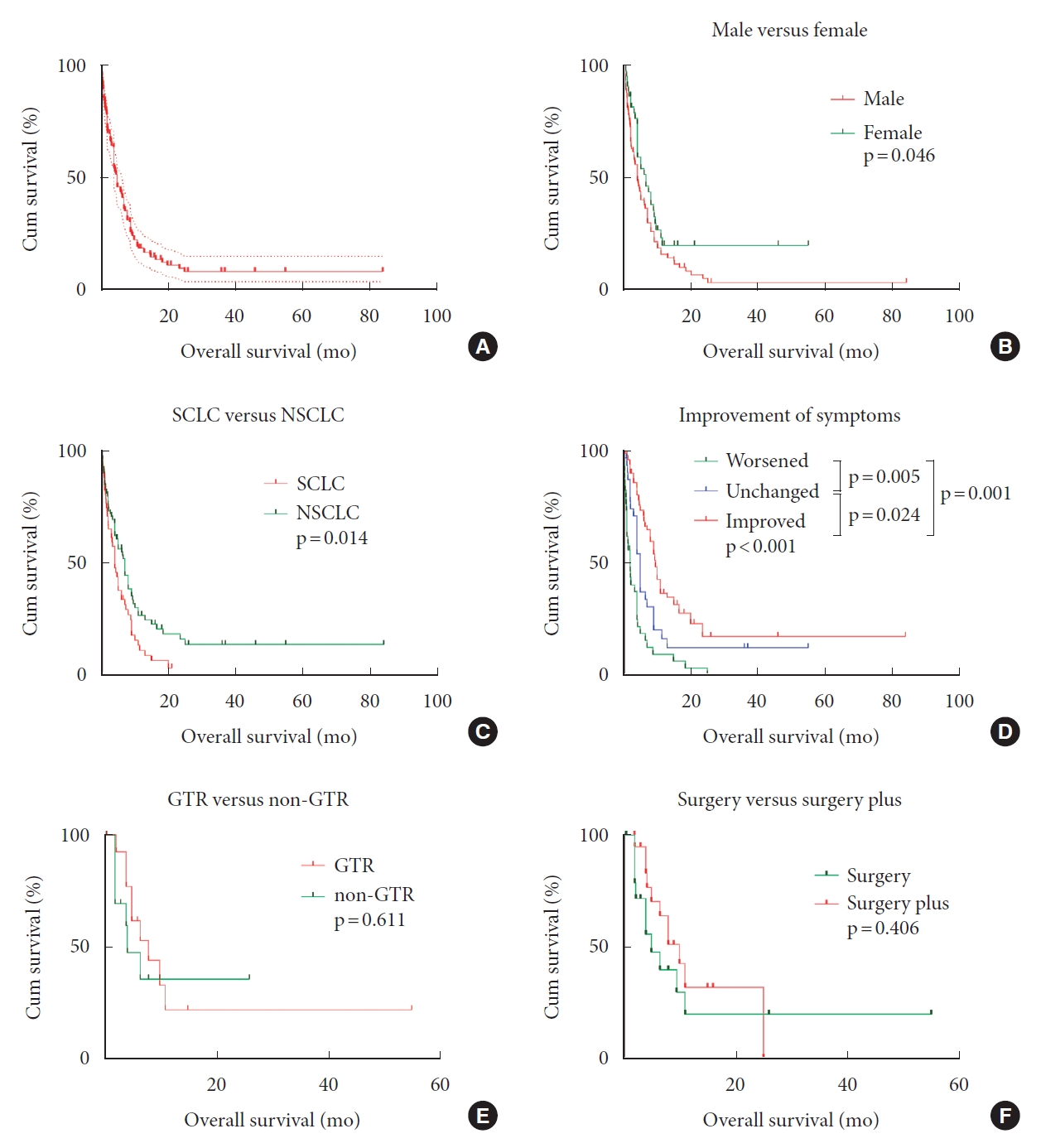
Table 1.
Summary of 6 patients with ISCM from lung cancers
Table 2.
Descriptive analyses of ISCM-LC cases – demographics, characteristics, and outcomes
Table 3.
Comparisons of demographics, treatments and outcomes between SCLC and NSCLC
| Variable | SCLC (n = 70) | NSCLC (n = 109) | p-value | |
|---|---|---|---|---|
| Age (yr), median (range) | 59 (38–79) | 59 (27–80) (n = 102) | 0.797† | |
| Male sex | 48 | 73 (n = 102) | 0.672‡ | |
| Smoke | 9 (n = 10) | 12 (n = 15) | 0.626§ | |
| Concomitant brain metastasis | 38 (n = 59) | 36 (n = 72) | 0.098‡ | |
| Location | n = 64 | n = 99 | 0.188‡ | |
| Cervical | 13 | 33 | ||
| Cervicothoracic | 4 | 3 | ||
| Thoracic | 19 | 33 | ||
| Lumbar | 19 | 19 | ||
| Multiple | 7 | 11 | ||
| Over 3 segments | 16 (n = 50) | 14 (n = 87) | 0.030*,‡ | |
| Sphincter dysfunction | 36 (n = 59) | 36 (n = 90) | 0.012*,‡ | |
| Surgery | 9 (n = 62) | 30 (n = 97) | 0.019*,‡ | |
| GTR | 4 (n = 7) | 11 (n = 21) | 0.827*,‡ | |
| Radiotherapy | 41 (n = 62) | 56 (n = 97) | 0.290*,‡ | |
| Chemotherapy | 16 (n = 62) | 20 (n = 97) | 0.446*,‡ | |
| Targeted drugs | 0 (n = 62) | 7 (n = 97) | 0.046*,§ | |
| 125I Implants | 0 (n = 62) | 7 (n = 97) | 0.043*,§ | |
| Improvement | n = 51 | n = 80 | 0.471*,‡ | |
| Improved | 16 | 42 | ||
| Unchanged | 13 | 20 | ||
| Worsened | 16 | 18 | ||
| EMST (95% CI) (mo) | 4 (2.670–5.330) | 7 (5.969–8.031) | 0.014*,II | |
| 3-Month survival | 65.40% | 70.90% | ||
| 6-Month survival | 38.10% | 56.60% | ||
| 1-Year survival | 11.40% | 26.90% | ||
REFERENCES
1. Sung WS, Sung MJ, Chan JH, et al. Intramedullary spinal cord metastases: a 20-year institutional experience with a comprehensive literature review. World Neurosurg 2013;79:576-84.


2. Lv J, Liu B, Quan X, et al. Intramedullary spinal cord metastasis in malignancies: an institutional analysis and review. Onco Targets Ther 2019;12:4741-53.


3. O'Neill AH, Phung TB, Lai LT. Intramedullary spinal cord metastasis from thyroid carcinoma: case report and a systematic pooled analysis of the literature. J Clin Neurosci 2018;49:7-15.


4. Goyal A, Yolcu Y, Kerezoudis P, et al. Intramedullary spinal cord metastases: an institutional review of survival and outcomes. J Neurooncol 2019;142:347-54.


5. Payer S, Mende KC, Westphal M, et al. Intramedullary spinal cord metastases: an increasingly common diagnosis. Neurosurg Focus 2015;39:E15.

6. Lee SS, Kim MK, Sym SJ, et al. Intramedullary spinal cord metastases: a single-institution experience. J Neurooncol 2007;84:85-9.


7. Gazzeri R, Telera S, Galarza M, et al. Surgical treatment of intramedullary spinal cord metastases: functional outcome and complications-a multicenter study. Neurosurg Rev 2021;44:3267-75.


8. Callovini GM, Bolognini A, Giordano M, et al. Surgical considerations for intramedullary conus medullaris metastatic tumors with origin from primary lung lesions: a review of the literature. Neurol India 2017;65:211-4.


9. Hashii H, Mizumoto M, Kanemoto A, et al. Radiotherapy for patients with symptomatic intramedullary spinal cord metastasis. J Radiat Res 2011;52:641-5.


10. Miura S, Kaira K, Kaira R, et al. The efficacy of amrubicin on central nervous system metastases originating from small-cell lung cancer: a case series of eight patients. Invest New Drugs 2015;33:755-60.


11. Shimada T, Iwami E, Kuroda A, et al. A patient with small cell lung cancer presenting with paralysis and intramedullary metastasis successfully treated with chemotherapy. Gan To Kagaku Ryoho 2020;47:1217-9.

12. Horiuchi K, Asakura T, Sakaguchi S, et al. Successful osimertinib treatment in a patient who exhibited intramedullary spinal cord metastases of lung adenocarcinoma with an acquired EGFR T790M mutation. BMJ Case Rep 2019;12:e229310.



13. Aghakhani N, David P, Parker F, et al. Intramedullary spinal ependymomas: analysis of a consecutive series of 82 adult cases with particular attention to patients with no preoperative neurological deficit. Neurosurgery 2008;62:1279-85. discussion 85-6.


14. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187-93.


15. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12.


16. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth 2020;18:2127-33.


17. Moola S, Munn Z, Tufanaru C, et al. Chapter 7. Systematic reviews of etiology and risk. In: Aromataris E, Munn Zet al., editors. JBI manual for evidence synthesis. Adelaide (Australia): Joanna Briggs Institute; 2020.
18. Diehn FE, Rykken JB, Wald JT, et al. Intramedullary spinal cord metastases: prognostic value of MRI and clinical features from a 13-year institutional case series. AJNR Am J Neuroradiol 2015;36:587-93.



20. Wu SY, Xing F, Sharma S, et al. Nicotine promotes brain metastasis by polarizing microglia and suppressing innate immune function. J Exp Med 2020;217:e20191131.



21. Dam-Hieu P, Seizeur R, Mineo JF, et al. Retrospective study of 19 patients with intramedullary spinal cord metastasis. Clin Neurol Neurosurg 2009;111:10-7.


22. Connolly ES Jr, Winfree CJ, McCormick PC, et al. Intramedullary spinal cord metastasis: report of three cases and review of the literature. Surg Neurol 1996;46:329-37. discussion 37-8.


23. Majmundar N, Shao B, Assina R. Lung adenocarcinoma presenting as intramedullary spinal cord metastasis: case report and review of literature. J Clin Neurosci 2018;52:124-31.


24. Loughrey GJ, Collins CD, Todd SM, et al. Magnetic resonance imaging in the management of suspected spinal canal disease in patients with known malignancy. Clin Radiol 2000;55:849-55.


25. Hashizume Y, Hirano A. Intramedullary spinal cord metastasis. Pathologic findings in five autopsy cases. Acta Neuropathol 1983;61:214-8.



26. Edelson RN, Deck MD, Posner JB. Intramedullary spinal cord metastases. Clinical and radiographic findings in nine cases. Neurology 1972;22:1222-31.


27. Okamoto H, Shinkai T, Matsuno Y, et al. Intradural parenchymal involvement in the spinal subarachnoid space associated with primary lung cancer. Cancer 1993;72:2583-8.


28. Sari O, Kaya B, Kara Gedik G, et al. Intramedullary metastasis detected with 18F FDG-PET/CT. Rev Esp Med Nucl Imagen Mol 2012;31:299-300.


29. Laufer I, Lis E, Pisinski L, et al. The accuracy of [(18)F]fluorodeoxyglucose positron emission tomography as confirmed by biopsy in the diagnosis of spine metastases in a cancer population. Neurosurgery 2009;64:107-13. discussion 13-4.


30. Strickland BA, McCutcheon IE, Chakrabarti I, et al. The surgical treatment of metastatic spine tumors within the intramedullary compartment. J Neurosurg Spine 2018;28:79-87.


31. Potti A, Abdel-Raheem M, Levitt R, et al. Intramedullary spinal cord metastases (ISCM) and non-small cell lung carcinoma (NSCLC): clinical patterns, diagnosis and therapeutic considerations. Lung Cancer 2001;31:319-23.


32. Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51.


33. Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51-65.

34. Wang J, Yuan H, Ma Q, et al. Interstitial 125I seeds implantation to treat spinal metastatic and primary paraspinal malignancies. Med Oncol 2010;27:319-26.


35. Pellerino A, Buffoni L, Ruda R, et al. Complete response of spinal metastases from non-small cell lung cancer with ALK inhibitors. Neurology 2019;93:217-9.


36. Phillips KA, Gaughan E, Gru A, et al. Regression of an intramedullary spinal cord metastasis with a checkpoint inhibitor: a case report. CNS Oncol 2017;6:275-80.



37. Arreola KN, Ying J, Hughes R, et al. Intramedullary spinal cord hemorrhage after treatment with bevacizumab in a long-term survivor with metastatic non-small-cell lung cancer. J Thorac Oncol 2014;9:e60-1.


38. Hata Y, Takai Y, Takahashi H, et al. Complete response of 7 years’ duration after chemoradiotherapy followed by gefitinib in a patient with intramedullary spinal cord metastasis from lung adenocarcinoma. J Thorac Dis 2013;5:E65-7.






























