 |
 |
- Search
|
|
||
Abstract
Objective
Lumbar lateral interbody fusion (LLIF) allows placement of large interbody cages while preserving ligamentous structures important for stability. Multiple clinical and biomechanical studies have demonstrated the feasibility of stand-alone LLIF in single-level fusion. We sought to compare the stability of 4-level stand-alone LLIF utilizing wide (26 mm) cages with bilateral pedicle screw and rod fixation.
Methods
Eight human cadaveric specimens of L1–5 were included. Specimens were attached to a universal testing machine (MTS 30/G). Flexion, extension, and lateral bending were attained by applying a 200 N load at a rate of 2 mm/sec. Axial rotation of ±8° of the specimen was performed at 2°/sec. Three-dimensional specimen motion was recorded using an optical motion-tracking device. Specimens were tested in 4 conditions: (1) intact, (2) bilateral pedicle screws and rods, (3) 26-mm stand-alone LLIF, (4) 26-mm LLIF with bilateral pedicle screws and rods.
Results
Compared to the stand-alone LLIF, bilateral pedicle screws and rods had 47% less range of motion in flexion-extension (p < 0.001), 21% less in lateral bending (p < 0.05), and 20% less in axial rotation (p = 0.1). The addition of bilateral posterior instrumentation to the stand-alone LLIF resulted in decreases of all 3 planes of motion: 61% in flexion-extension (p < 0.001), 57% in lateral bending (p < 0.001), 22% in axial rotation (p = 0.002).
Lateral lumbar interbody fusion (LLIF) is one minimally invasive operative technique used to create fusion in patients with degenerative lumbar spinal diseases [1]. Surgical advantages of LLIF as compared to anterior or posterior lumbar fusion include excellent visualization, preservation of ligamentous and bony structures contributing to spine stability, access for discectomy, and decreased mobilization of neurologic structures and vasculature [2-4]. The lateral approach allows for implantation of interbody cages of large sizes, which facilitates restoration of disc height, correction of deformity, and dispersion of axial loads across the endplate [4-6]. As a minimally invasive technique, LLIF has demonstrated less tissue trauma, low intraoperative blood loss, and lower infection rates than open surgery [7-9]. In addition, its efficacy has been shown in multiple clinical studies utilizing validated patient-reported outcome measures [6,10-12].
In practice, LLIF is usually combined with supplemental posterior fixation to enhance segmental stability, reduce subsidence, and thereby increase rates of instrumented fusion [13,14]. Multiple level lateral interbody fusion combined with percutaneous pedicle screw fixation is a minimally invasive surgical strategy with high fusion rates and favorable radiographic and clinical results [14]. The placement of supplemental posterior fixation represents an additional step of surgery and often repositioning of the patient, although single-position surgery has been attempted [15].
If stand-alone lateral interbody fusion can confer adequate biomechanical stability to promote fusion, this would be important knowledge because it could potentially obviate the need for posterior instrumentation. The literature suggests that a standalone lateral approach is sufficient in some situations. A recent systematic review of 22 studies by Manzur et al. [16] found that stand-alone LLIF of various cage widths achieved pooled clinical fusion rate that was not statistically different from combined LLIF and posterior (circumferential) fusion. Biomechanically, single-level stand-alone LLIF has been reported to reduce the segmental range of motion (ROM) more than transforaminal lumbar interbody fusion (TLIF) or stand-alone anterior lumbar interbody fusion (ALIF) [17].
Cage width may also have an important role in the feasibility of stand-alone lateral interbody fusion. In a cadaveric study of extra wide (26 mm) cages, Pimenta et al. [18] found that increasing the width of the cages from 18 mm to 26 mm resulted in a more significant reduction of ROM. In addition, they reported that the stand-alone 26-mm LLIF was more rigid than TLIF with bilateral pedicle screws. Clinically, Lang et al. [19] reported that 26-mm wide cages decreased subsidence compared to 22-mm and 18-mm wide cages.
Considering the previous works supporting stand-alone LLIF for single-level surgery and the favorable biomechanical profile of 26-mm cages, we sought to investigate a stand-alone LLIF approach to multilevel fusion. The biomechanical stability of multilevel stand-alone LLIF utilizing extra wide cages has not been characterized. This study compared the biomechanical stability of stand-alone LLIF using 26-mm cages for long multilevel fusion (L1–5, 4 levels) to posterior bilateral pedicle screws and rods. We chose bilateral pedicle screws and rods because this surgical strategy is widely accepted as providing adequate stability to promote spinal fusion. We hypothesized that the multilevel stand-alone LLIF will provide comparable stability as traditional pedicle screw fixation due to the use of extra wide cages.
Eight fresh-frozen human cadaveric specimens of L1–5 were included. Specimens were prepared by cleaning surrounding soft tissue and muscle and preserving the discs and spinal ligaments (supraspinous, interspinous, facet capsules, posterior longitudinal ligament, anterior longitudinal ligament). The mean specimen age was 66.5±11.5 years. There were 7 male and 1 female specimens. The average body mass index was 31.1±7.32 kg/m2. All specimens were visually inspected to confirm no fracture, deformity, previous surgery, or severe spondylosis.
A computed tomography (CT) scan (GE Brightspeed, Boston, MA, USA) was performed on all specimens (120 kV, 20 mA, 0.62-mm resolution) to investigate the bone quality and produce measurements to plan optimal implant size. Nondestructive testing was performed for all the conditions in flexion/extension, lateral bending, and axial rotation.
Lateral interbody cages were implanted with the specimen in the lateral decubitus position utilizing the LLIF surgical technique and instrumentation specific to this technique (eXtreme Lateral Interbody Fusion, Nuvasive, San Diego, CA, USA). All interbody cages were 26 mm in width (anteriorposterior dimension) and polyetheretherketone material (CoRoent, Nuvasive, San Diego, CA, USA). Each implant’s height (superiorinferior) and length (medial-lateral) were determined by CT scan and adjusted when necessary.
Pedicle screws (Armada, Nuvasive, San Diego, CA, USA) were placed with the specimen in the prone position. Screws were implanted bilaterally at every level from L1–5 utilizing standard freehand technique with anatomic landmarks. Screw size was determined by CT scan and adjusted if necessary. Pilot holes were tapped and probed, in addition to visual inspection of the specimens, to detect any breach. Rod size was 5.5-mm titanium and placed bilaterally for the conditions that required posterior instrumentation. The specimens were tested with screws in place but without rods for the intact and stand-alone conditions.
Specimens were attached to a universal testing machine (MTS 30/G) using specially designed holding jigs. Flexion, extension, and lateral bending were attained by applying a 200 N load at a rate of 2 mm/sec to the loading arm connecting the cup containing the thoracic end of the spine while the cup with the sacral end was fixed to the base of the loading frame (Fig. 1A). Axial rotation of ±8° of the specimen was achieved by coupling the thoracic end to a servo motor rotating at 2°/sec with the sacral end fixed (Fig. 1B). A 50N preload (follower load) was applied from L1 to L5. During all testing, 3-dimensional specimen motion was recorded using an optical motion-tracking device (Optotrak, Northern Digital Inc., Waterloo, ON, Canada). The apparatus was designed to apply compressive follower preload representing the physiologic preload acting in the lumbar spine and maintaining the spine alignment. This was applied using bilateral cables passing freely through guides anchored to each vertebra. Additional load for flexion and extension was applied with a compressive force that varied between 200–300 N with a lever arm of 1.5 cm and allowed for a combined moment of 4.5–6 Nm. Most of the reported experiments using the follower method reported a pure moment load between 4–8 Nm.
Specimens were tested in 4 conditions: (1) intact, (2) bilateral pedicle screws and rods L1–5 (posterior-only). Following testing for posterior-only, lateral interbody cages were implanted as described above, and the experiment continued for (3) 26-mm lateral interbody cages L1–5 without rods (stand-alone LLIF), (4) 26-mm lateral interbody cages with bilateral pedicle screws and rods L1–5 (LLIF+posterior).
Descriptive statistics for continuous variables are reported as mean±standard deviation. Change in ROM after instrumentation was reported as percentage decrease from the intact specimen. Paired t-test was used to compare ROM between instrumentation conditions, which mitigates the confounding effect of differences among specimens, such as in bone quality. Statistical analyses were performed using Microsoft Excel Version 2013. Significance was set as p < 0.05.
Bone quality was determined by CT scan utilizing a previously described technique [20]. The mean Hounsfield unit (HU) was 143±29.4 (range, 84–169.4). Only one specimen was below the suggested threshold for osteoporosis of less than 110 HU [21].
Lateral interbody cages were 26 mm in width and ranged from 8 to 14 mm. The most common heights were 10 mm (n = 13). Length ranged from 45 to 60 mm. The most common length was 55 mm (n = 14). Pedicle screw diameters ranged from 6.5 mm to 8.5 mm in diameter and 40 to 60 mm in length.
Axial rotation was measured in all 8 specimens. In addition, flexion/extension and lateral bending are presented for 7 specimens because of a change in methodology that excluded one specimen.
In the intact specimen, each disc space’s mean flexion-extension ROM was 4.95°±1.18°. With stand-alone LLIF, flexion-extension decreased by 55% to 2.23°±1.07° (p < 0.001). Mean lateral bending was 3.65°±1.62° in the intact condition. With standalone LLIF, lateral bending decreased by 18% to 3.01° ±1.70° (p = 0.3). For axial rotation, the intact specimen mean ROM was 1.4°±0.62°. With stand-alone LLIF, axial rotation decreased 15% to 1.19°±0.67° (p = 0.2). While a decrease in all 3 planes of motion was observed, only flexion-extension was statistically significant.
Comparing stand-alone LLIF with bilateral pedicle screws and rods, posterior-only had 47% less ROM in flexion-extension, 21% less ROM in lateral bending, and 20% less ROM in axial rotation (Table 1). The differences were statistically significant for flexion-extension and lateral bending (p ≤ 0.03). However, the difference did not reach statistical significance for axial rotation (p = 0.1).
When comparing bilateral pedicle screws and rods with or without LLIF, the LLIF+posterior group had 27% less ROM in flexion-extension and 45% less ROM in lateral bending, both differences being statistically significant (p ≤ 0.02) (Table 2). However, axial rotation was similar between the 2 groups, with a mean reduction of 0.02° (p = 0.4).
The addition of bilateral posterior instrumentation to the stand-alone condition resulted in statistically significant decreases in all 3 planes of motion (Table 3): 61% decrease in flexion-extension (p < 0.001), 57% decrease in lateral bending (p < 0.001), 22% decrease in axial rotation (p = 0.002).
This study sought to characterize the biomechanical stability of multilevel stand-alone LLIF utilizing extra wide cages compared to posterior-only bilateral pedicle and rod screw fixation. Our results indicate that, although the 4-level stand-alone condition reduced ROM in all tested planes, this reduction was statistically significant for only flexion-extension. Our study’s assessment of stability is limited to angular ROM and does not include other possible measures such as endplate stress, translation, or cage motion. The stand-alone construct did not provide stability, defined here as a reduction in ROM, that was equivalent to bilateral pedicle screws and rods. Bilateral pedicle screw fixation provided greater stability, which was statistically significant in flexion-extension and lateral bending. The addition of pedicle screw and rod instrumentation to LLIF provides substantially higher stability than the stand-alone condition, even with the use of 26-mm cages.
The degree of mechanical stability required for spine fusion is unknown [22,23]. In a cadaveric study, Harris et al. [23] reported that TLIF with bilateral pedicle screws, a procedure currently in broad practice, showed flexibility not significantly different from intact specimens. It is generally accepted that greater stability is associated with higher fusion rates, with stability influenced by the bone quality, implant choice, and surgical approach [24,25]. We used pedicle screws and rods as the comparison group because this is an accepted technique with high reported rates of fusion [24,26]. Open surgery for placement of posterior instrumentation requires extensive intraoperative soft tissue trauma and dissection [27,28], leading to an interest in less invasive alternatives to create stability and fusion. LLIF is an interbody fusion technique that utilizes a lateral retroperitoneal approach to access the disc space via a transpsoas or anterior to psoas trajectory. This preserves the anterior and posterior longitudinal ligaments and posterior facet joints. As a minimally invasive technique, LLIF has demonstrated reduced blood loss, shorter operative times, and shorter hospital lengths of stay [28].
Multilevel LLIF has been reported to have favorable outcomes compared to posterior-only surgery for adult spinal deformity [29]. Strom et al. [30] reviewed 92 adult deformity operations with 5 or more levels, comparing the deformity correction and morbidity of open posterior-only surgery versus combined LLIF and open posterior surgery. The LLIF group had lower total blood loss, fewer intensive care unit days, similar hospital length of stay, and less need for inpatient rehabilitation services, despite undergoing 2 procedures. The LLIF cohort also reported greater improvement in visual analogue scale (VAS) and Oswestry Disability Index (ODI) scores. Radiographically, the LLIF group had greater Cobb angle correction and lumbar lordosis restoration than the open posterior-only group. Matsukura et al. [31] performed a propensity-matched comparison between 21 pairs of patients undergoing LLIF or posterior lumbar interbody fusion (PLIF)/TLIF. Both groups received subsequent posterior instrumentation. While both techniques resulted in similar radiographic improvements postoperatively, LLIF resulted in lower intraoperative blood loss than PLIF/TLIF. Bae et al. [32] compared outcomes of 221 adult deformity patients who underwent a posterior spinal fixation (PSF) only approach LLIF+PSF, or ALIF+ PSF. At a mean follow-up time of 34.5 months, patients in the LLIF+PSF group had similar radiographic parameters as those in the PSF only or ALIF+PSF groups, with a lower incidence of proximal junctional kyphosis, lower ODI scores, and the most improvement in pain scores. The benefits of the less invasive LLIF approach could potentially be amplified if stand-alone LLIF for fusion of multiple segments is also feasible.
There are few reports describing the clinical results of standalone LLIF, and none that include 26-mm wide cages to our knowledge. Previously suggested indications for stand-alone LLIF include degenerative disc disease, adjacent segment disease, degenerative scoliosis, spinal stenosis, and spondylolisthesis [6,9,13,19,33,34]. Aichmair et al. [13] followed 52 patients after singlelevel LLIF for adjacent segment disease. There was a difference between fusion rates between stand-alone LLIF (54%) and circumferential fusion (88%) that was not statistically significant, and cage width was not specified. Malham et al. [6] described an algorithm to identify patients suitable for stand-alone LLIF. Prospectively applied to 21 patients who underwent stand-alone LLIF, all cages being 18 or 22 mm wide, they observed no differences in clinical outcomes from patients with supplemental posterior fixation, as well as a 95% fusion rate in the stand-alone cohort. Castro et al. [33] reported the clinical outcomes of 35 adult degenerative scoliosis patients receiving an average of 3.1 levels of stand-alone LLIF, demonstrating a 57% improvement in VAS scores for leg pain and 74% symptom resolution, and 56% improvement in ODI scores. Ten patients experienced cage subsidence. The cage width was 18 mm, and the authors suggested that larger cages may reduce the subsidence rate. In further exploring the impact of cage width, Lang et al. [19] reviewed patients undergoing LLIF with and without supplementation fixation, finding that radiographic subsistence rates decreased nearly linearly when implanting 18-mm to 22-mm to 26-mm wide cages.
The biomechanical characteristics of LLIF lends credence to the idea that a stand-alone approach may be sufficient [4-6,16,29,35]. Results of in vitro studies appear to support this. Cappuccino et al. [17] demonstrated that stand-alone LLIF of L4–5 using an 18-mm wide cage reduced the flexion-extension ROM and lateral bending ROM to 31.6% and 32.5% of the intact spine ROM. They concluded that stand-alone LLIF improved stability more than stand-alone ALIF and TLIF. Fogel et al. [36] found that, across 10 cadaveric specimens, insertion of a single L3–4 LLIF 18-mm wide cage reduced ROM to 32% of the flexion-extension, 33% of the lateral bending, and 69% of the axial rotation of the intact state. Kretzer et al. [37] compared the reduction of ROM at L2–3 and L4–5 in 4 conditions—stand-alone LLIF, bilateral pedicle screw fixation, and 2 facet screw systems—and concluded that all instrumentation decreased ROM compared to the intact spine, with no differences detected among the fixation techniques. In a model of adjacent segment disease, Chioffe et al. [38] found in 6 cadaveric specimens that L3–4 stand-alone LLIF decreased adjacent segment motion by 56%. Finally, in a study that highlights the role of cage width, Pimenta et al. [18] found that a stand-alone 26-mm wide cage provided similar stability to bilateral pedicle screws and rods and greater stability than TLIF with bilateral pedicle screws, as well as 18-mm wide LLIF with unilateral pedicle screws. These results formed the rationale for the current investigation of stand-alone LLIF of multiple levels.
Our results found that stand-alone LLIF from L1 to L5 decreased the mean motion segment flexion-extension ROM to 45% of the intact spine, with smaller decreases observed in lateral bending and axial rotation, to 83% and 85%, respectively. Compared to stand-alone LLIF, posterior bilateral pedicle screws and rods showed more considerable reductions in ROM in all tested planes of motion, statistically significant for flexion-extension and lateral rotation. These results contrast with some of the previous studies described above and highlight the pitfalls of extrapolating the results of single-level studies to the multilevel fusion environment. Despite the biomechanical advantages associated with the lateral approach and 26-mm wide cages, stand-alone LLIF for 4-level fusion is not equivalent to pedicle screws and rods. It should be noted that the stand-alone LLIF included pedicle screws without rods, rather than lateral interbody cages only, to avoid the confounding effect of removal and replacement of screws. Screws were carefully placed extra-articular to the facet joints, there was no visualized contact of screws with adjacent segments or screws during testing, and the tested ranges of motion were low. Nevertheless, the presence of screws is a potential confounder and therefore a limitation of the study.
In a study similar to this investigation, Lai et al. [35] examined intersegmental and overall ROM after L2–5 stand-alone LLIF with 17-mm wide cages. They found that, although overall ROM was decreased by 55% in flexion-extension, 54% in lateral bending, and 31% in axial rotation, addition of either unilateral or bilateral pedicle screws further reduced flexion-extension and axial rotation motion. The reported reductions in ROM in the above studies are more significant than seen in our results, which may be attributable to the long fusion (4-level) model, differences in experimental technique, and lower baseline ROM of our intact specimens.
Our results are also consistent with the conclusions of a finite element analysis that multiple level stand-alone LLIF with a 22-mm wide cages did not provide sufficient stability [39]. The consistency of in vitro and finite element results strengthens their validity [40]. In addition to ROM, the finite element analysis allowed measurement of endplate stress, in which the stand-alone condition exceeded supplemental screws and rods in all planes of motion, exceeding 170% in lateral bending. Endplate stress is an important factor in the risk of cage subsidence, and that our analysis of stability was limited to ROM represents a weakness of this study. In addition, there is emerging literature supporting the necessity of posterior instrumentation to prevent subsidence. A recently completed systematic review found that LLIF without posterior fixation had distinguishably higher rates of subsidence than LLIF with posterior fixation [41]. Although bone mineral density has been shown to correlate with the risk of subsidence [42], Jones et al. [43] found that the use of stand-alone technique was a stronger risk factor for subsidence after LLIF than endplate volumetric bone mineral density.
In conclusion, because stand-alone multilevel LLIF provided less stability than bilateral pedicle screw and rod instrumentation, surgeons may not consider it an acceptable alternative, save for exceptional cases where the probability of fusion is already high. However, the addition of bilateral pedicle screws and rods to multilevel LLIF provided significantly higher stability than posterior-only instrumentation. Therefore, in the presence of risk factors for nonunion or cage subsidence, 26-mm cages with pedicle screw and rod fixation may be a good strategy for surgeons seeking to maximize biomechanical stability.
NOTES
Conflict of Interest
Drs. James Mok and Farid Amirouche disclose in-kind product donation by Nuvasive. Dr. James Mok discloses that he receives consulting fees and royalties from MiRus. The other authors have nothing to disclose.
Fig. 1.
Experimental setup demonstrating the load cell applied to the cadaveric spine specimen in flexion/extension (A) and axial rotation (B). Optical motion sensors were placed from L1 to L5 to track segmental motion.
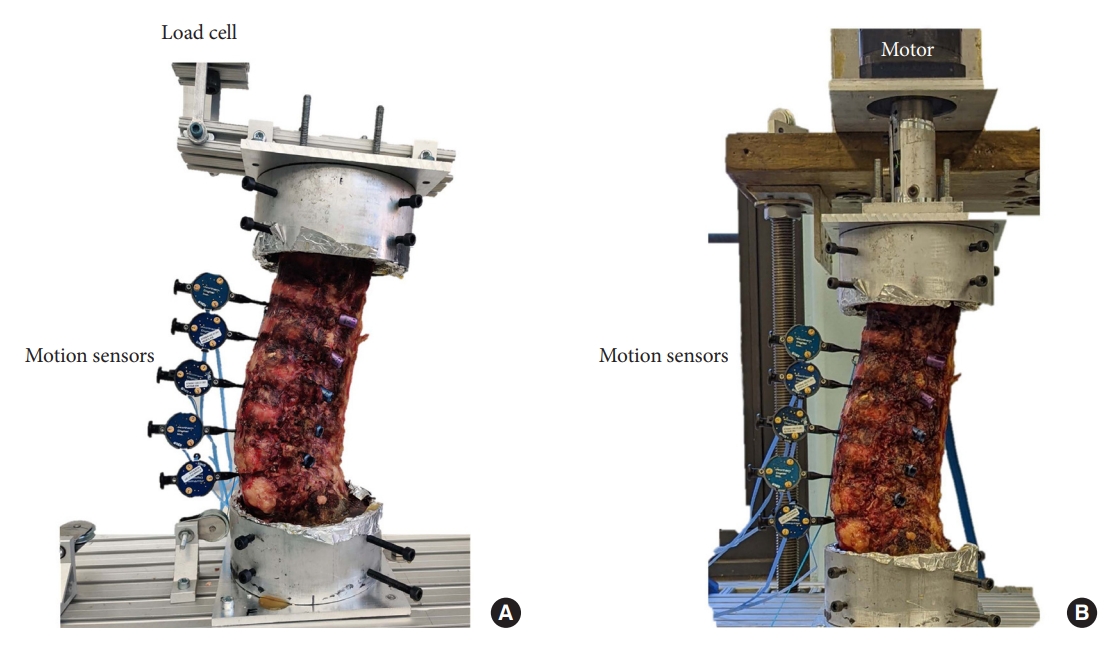
Table 1.
Comparing range of motion after stand-alone lateral lumbar interbody fusion (LLIF) versus posterior fixation only
| Level |
Flexion-extension (°) |
Lateral bending (°) |
Axial rotation (°) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Stand-alone LLIF | Posterior fixation only | p-value† | Stand-alone LLIF | Posterior fixation only | p-value† | Stand-alone LLIF | Posterior fixation only | p-value† | |
| L1–2 | 1.90 ± 0.69 | 1.34 ± 0.44 | 0.053 | 2.39 ± 1.02 | 2.05 ± 1.48 | 0.362 | 1.10 ± 0.96 | 0.72 ± 0.50 | 0.239 |
| L2–3 | 2.45 ± 1.16 | 0.92 ± 0.55 | 0.011* | 1.77 ± 1.39 | 1.54 ± 1.54 | 0.399 | 1.27 ± 0.42 | 1.00 ± 0.33 | 0.153 |
| L3–4 | 2.33 ± 1.27 | 0.77 ± 0.17 | 0.011* | 2.49 ± 2.59 | 2.13 ± 2.62 | 0.226 | 0.94 ± 0.35 | 0.80 ± 0.30 | 0.235 |
| L4–5 | 2.25 ± 1.15 | 1.72 ± 1.17 | 0.232 | 5.41 ± 1.78 | 3.75 ± 2.18 | 0.023* | 1.45 ± 0.93 | 1.28 ± 0.93 | 0.388 |
| Combined value (N = 28) | 2.23 ± 1.07 | 1.19 ± 0.58 | < 0.001* | 3.01 ± 1.70 | 2.37 ± 1.96 | 0.035* | 1.19 ± 0.67 | 0.95 ± 0.51 | 0.112 |
| % Decrease from stand-alone | 47% | 21% | 20% | ||||||
Table 2.
Comparing range of motion after posterior fixation with and without lateral lumbar interbody fusion (LLIF)
| Level |
Flexion-extension (°) |
Lateral bending (°) |
Axial rotation (°) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Posterior fixation only | LLIF+posterior fixation | p-value† | Posterior fixation only | LLIF+posterior fixation | p-value† | Posterior fixation only | LLIF+posterior fixation | p-value† | |
| L1–2 | 1.34 ± 0.44 | 0.8 ± 0.39 | 0.005* | 2.05 ± 1.48 | 0.97 ± 0.77 | 0.079 | 0.72 ± 0.5 | 1.07 ± 0.88 | 0.183 |
| L2–3 | 0.92 ± 0.55 | 0.42 ± 0.25 | 0.071 | 1.54 ± 1.54 | 0.47 ± 0.27 | 0.066 | 1 ± 0.33 | 0.84 ± 0.25 | 0.227 |
| L3–4 | 0.77 ± 0.17 | 0.71 ± 0.66 | 0.479 | 2.13 ± 2.62 | 1.13 ± 0.88 | 0.145 | 0.8 ± 0.3 | 0.61 ± 0.25 | 0.060 |
| L4–5 | 1.72 ± 1.17 | 1.52 ± 1.18 | 0.342 | 3.75 ± 2.18 | 2.64 ± 1.52 | 0.110 | 1.28 ± 0.93 | 1.2 ± 0.82 | 0.469 |
| Combined value (N = 28) | 1.19 ± 0.58 | 0.86 ± 0.62 | 0.024* | 2.37 ± 1.96 | 1.3 ± 0.86 | 0.003* | 0.95 ± 0.51 | 0.93 ± 0.55 | 0.432 |
| % Decrease from bilateral screws | 27% | 45% | 3% | ||||||
Table 3.
Comparing range of motion after stand-alone lateral lumbar interbody fusion (LLIF) versus LLIF with posterior fixation
| Level |
Flexion-extension (°) |
Lateral bending (°) |
Axial rotation (°) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Stand-alone LLIF | LLIF+posterior fixation | p-value† | Stand-alone LLIF | LLIF+posterior fixation | p-value† | Stand-alone LLIF | LLIF+posterior fixation | p-value† | |
| L1–2 | 1.9 ± 0.69 | 0.8 ± 0.39 | 0.002* | 2.39 ± 1.02 | 0.97 ± 0.77 | 0.001* | 1.1 ± 0.96 | 1.07 ± 0.88 | 0.439 |
| L2–3 | 2.45 ± 1.16 | 0.42 ± 0.25 | 0.001* | 1.77 ± 1.39 | 0.47 ± 0.27 | 0.022* | 1.27 ± 0.42 | 0.84 ± 0.25 | 0.003* |
| L3–4 | 2.33 ± 1.27 | 0.71 ± 0.66 | 0.001* | 2.49 ± 2.59 | 1.13 ± 0.88 | 0.064 | 0.94 ± 0.35 | 0.61 ± 0.25 | 0.002* |
| L4–5 | 2.25 ± 1.15 | 1.52 ± 1.18 | 0.137 | 5.41 ± 1.78 | 2.64 ± 1.52 | 0.002* | 1.45 ± 0.93 | 1.2 ± 0.82 | 0.182 |
| Combined value (N = 28) | 2.23 ± 1.07 | 0.86 ± 0.62 | < 0.001* | 3.01 ± 1.7 | 1.3 ± 0.86 | < 0.001* | 1.19 ± 0.67 | 0.93 ± 0.55 | 0.002* |
| % Decrease from stand-alone LLIF | 61% | 57% | 22% | ||||||
REFERENCES
1. Shah M, Kolb B, Yilmaz E, et al. Comparison of lumbar laminectomy alone, lumbar laminectomy and fusion, stand-alone anterior lumbar interbody fusion, and stand-alone lateral lumbar interbody fusion for treatment of lumbar spinal stenosis: a review of the literature. Cureus 2019;11:e5691.



2. Watkins R 4th, Watkins R 3rd, Hanna R. Non-union rate with stand-alone lateral lumbar interbody fusion. Medicine (Baltimore) 2014;93:e275.



3. Winder MJ, Gambhir S. Comparison of ALIF vs. XLIF for L4/5 interbody fusion: pros, cons, and literature review. J Spine Surg 2016;2:2-8.



4. Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43.


5. Agarwal N, White MD, Zhang X, et al. Impact of endplateimplant area mismatch on rates and grades of subsidence following stand-alone lateral lumbar interbody fusion: an analysis of 623 levels. J Neurosurg Spine 2020 Mar 6:1-5. doi: 10.3171/2020.1.SPINE19776. [Epub].


6. Malham GM, Ellis NJ, Parker RM, et al. Maintenance of segmental lordosis and disk height in stand-alone and instrumented extreme lateral interbody fusion (XLIF). Clin Spine Surg 2017;30:E90-8.


7. Bocahut N, Audureau E, Poignard A, et al. Incidence and impact of implant subsidence after stand-alone lateral lumbar interbody fusion. Orthop Traumatol Surg Res 2018;104:405-10.


8. O’Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine 2009;11:471-6.


9. Li H, Li J, Tao Y, et al. Is stand-alone lateral lumbar interbody fusion superior to instrumented lateral lumbar interbody fusion for the treatment of single-level, low-grade, lumbar spondylolisthesis? J Clin Neurosci 2021;85:84-91.


10. Marchi L, Abdala N, Oliveira L, et al. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal 2012;2012:456346.




11. Ahmadian A, Bach K, Bolinger B, et al. Stand-alone minimally invasive lateral lumbar interbody fusion: multicenter clinical outcomes. J Clin Neurosci 2015;22:740-6.


12. Screven R, Pressman E, Rao G, et al. The safety and efficacy of stand-alone lateral lumbar interbody fusion for adjacent segment disease in a cohort of 44 patients. World Neurosurg 2021;149:e225-30.


13. Aichmair A, Alimi M, Hughes AP, et al. Single-level lateral lumbar interbody fusion for the treatment of adjacent segment disease: a retrospective two-center study. Spine (Phila Pa 1976) 2017;42:E515-22.


14. Katz AD, Singh H, Greenwood M, et al. Clinical and radiographic evaluation of multilevel lateral lumbar interbody fusion in adult degenerative scoliosis. Clin Spine Surg 2019;32:E386-96.


15. Hiyama A, Katoh H, Sakai D, et al. Accuracy of percutaneous pedicle screw placement after single-position versus dual-position insertion for lateral interbody fusion and pedicle screw fixation using fluoroscopy. Asian Spine J 2022;16:20-7.



16. Manzur MK, Steinhaus ME, Virk SS, et al. Fusion rate for stand-alone lateral lumbar interbody fusion: a systematic review. Spine J 2020;20:1816-25.


17. Cappuccino A, Cornwall GB, Turner AW, et al. Biomechanical analysis and review of lateral lumbar fusion constructs. Spine (Phila Pa 1976) 2010;35(26 Suppl):S361-7.


18. Pimenta L, Turner AW, Dooley ZA, et al. Biomechanics of lateral interbody spacers: going wider for going stiffer. ScientificWorldJournal 2012;2012:381814.




19. Lang G, Navarro-Ramirez R, Gandevia L, et al. Elimination of subsidence with 26-mm-wide cages in extreme lateral interbody fusion. World Neurosurg 2017;104:644-52.


20. Anderson PA, Polly DW, Binkley NC, et al. Clinical use of opportunistic computed tomography screening for osteoporosis. J Bone Joint Surg Am 2018;100:2073-81.


21. Lee SJ, Binkley N, Lubner MG, et al. Opportunistic screening for osteoporosis using the sagittal reconstruction from routine abdominal CT for combined assessment of vertebral fractures and density. Osteoporos Int 2016;27:1131-6.



22. Krijnen MR, Mensch D, van Dieen JH, et al. Primary spinal segment stability with a stand-alone cage: in vitro evaluation of a successful goat model. Acta Orthop 2006;77:454-61.


23. Harris BM, Hilibrand AS, Savas PE, et al. Transforaminal lumbar interbody fusion: the effect of various instrumentation techniques on the flexibility of the lumbar spine. Spine (Phila Pa 1976) 2004;29:E65-70.

24. Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976) 1997;22:2807-12.

25. Park SB, Chung CK. Strategies of spinal fusion on osteoporotic spine. J Korean Neurosurg Soc 2011;49:317-22.



26. Eismont FJ, Norton RP, Hirsch BP. Surgical management of lumbar degenerative spondylolisthesis. J Am Acad Orthop Surg 2014;22:203-13.


27. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. Part 2: Histologic and histochemical analyses in humans. Spine (Phila Pa 1976) 1994;19:2598-602.

28. Arnold PM, Anderson KK, McGuire RA Jr. The lateral transpsoas approach to the lumbar and thoracic spine: a review. Surg Neurol Int 2012;3(Suppl 3):S198-215.



29. Acosta FL, Liu J, Slimack N, et al. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults: a radiographic study. J Neurosurg Spine 2011;15:92-6.


30. Strom RG, Bae J, Mizutani J, et al. Lateral interbody fusion combined with open posterior surgery for adult spinal deformity. J Neurosurg Spine 2016;25:697-705.


31. Matsukura Y, Yoshii T, Morishita S, et al. Comparison of lateral lumbar interbody fusion and posterior lumbar interbody fusion as corrective surgery for patients with adult spinal deformity-a propensity score matching analysis. J Clin Med 2021;10:4737.



32. Bae J, Theologis AA, Strom R, et al. Comparative analysis of 3 surgical strategies for adult spinal deformity with mild to moderate sagittal imbalance. J Neurosurg Spine 2018;28:40-9.


33. Castro C, Oliveira L, Amaral R, et al. Is the lateral transpsoas approach feasible for the treatment of adult degenerative scoliosis? Clin Orthop Relat Res 2014;472:1776-83.


34. Louie PK, Haws BE, Khan JM, et al. Comparison of standalone lateral lumbar interbody fusion versus open laminectomy and posterolateral instrumented fusion in the treatment of adjacent segment disease following previous lumbar fusion surgery. Spine (Phila Pa 1976) 2019;44:E1461-9.


35. Lai O, Chen Y, Chen Q, et al. Cadaveric biomechanical analysis of multilevel lateral lumbar interbody fusion with and without supplemental instrumentation. BMC Musculoskelet Disord 2021;22:280.




36. Fogel GR, Parikh RD, Ryu SI, et al. Biomechanics of lateral lumbar interbody fusion constructs with lateral and posterior plate fixation: laboratory investigation. J Neurosurg Spine 2014;20:291-7.

37. Kretzer RM, Molina C, Hu N, et al. A comparative biomechanical analysis of stand alone versus facet screw and pedicle screw augmented lateral interbody arthrodesis: an in vitro human cadaveric model. Clin Spine Surg 2016;29:E336-43.

38. Chioffe M, McCarthy M, Swiatek PR, et al. Biomechanical analysis of stand-alone lateral lumbar interbody fusion for lumbar adjacent segment disease. Cureus 2019;11:e6208.



39. Liu X, Ma J, Park P, et al. Biomechanical comparison of multilevel lateral interbody fusion with and without supplementary instrumentation: a three-dimensional finite element study. BMC Musculoskelet Disord 2017;18:63.




40. Bohn T, Lang SAJ, Roll S, et al. Meta-analyses comparing spine simulators with cadavers and finite element models by analysing range-of-motion data before and after lumbar total disc replacement. J Adv Res 2020;26:29-41.



41. Parisien A, Wai EK, ElSayed MSA, et al. Subsidence of spinal fusion cages: a systematic review. Int J Spine Surg 2022;16:1103-18.































