|
 |
- Search
|
|
||
Abstract
Objective
The impact of adjuvant radiotherapy on overall survival (OS) and progression-free survival (PFS) of patients with grade II spinal cord astrocytomas remains controversial. Additionally, the relationship between progression and clinical deterioration after radiotherapy has not been well investigated.
Methods
This study included 53 patients with grade II intramedullary spinal cord astrocytomas treated by either subtotal, partial resection or open biopsy. Their clinical performance status was assessed immediately before operation and 1, 6, 12, 24, and 60 months after surgery by Karnofsky Performance Scale (KPS). Patients with and without adjuvant radiotherapy were compared.
Results
The groups with and without radiation comprised 23 and 30 patients with a mean age of 50.3 ±22.6 years (range, 2–88 years). The mean overall disease progression rate was 47.1% during a mean follow-up period of 48.4 ±39.8 months (range, 2.5–144.5 months). In the radiation group, 11 patients (47.8%) presented with progressive disease, whereas 14 patients (46.7%) presented with progressive disease in the group without radiation. There were no significant differences in OS or PFS among patients with or without adjuvant radiotherapy. KPS in both groups, especially radiation group, gradually decreased after operation and deteriorated before the confirmation of disease progression.
Intramedullary spinal cord tumors are rare, accounting for 2%–4% of all central nervous system tumors; spinal cord astrocytoma is the most common spinal cord tumor in children (39%) and the second most common in adults (24%) [1-3]. Adjuvant radiotherapy is generally recommended (1) when complete resection is abandoned due to risk of postoperative neurological deficits or (2) for high-grade intramedullary invasive spinal cord astrocytomas that are not amenable to gross total resection. The role of radiation therapy in the management of grade II spinal cord astrocytoma is controversial, as the standard therapeutic dose of 45 to 50 Gy with conventional fractionation of 1.8 to 2 Gy/day is well tolerated with low risk of toxicity, its influence in patient outcome is unknown. The aim of this study was to analyze progression free survival and clinical performance status in patients treated with and without adjuvant radiotherapy after incomplete resection of grade II spinal cord astrocytomas.
Data for this study was obtained from a multicenter cohort study authorized by the Neurological Society of Japan between 2009 and 2020. This database collects the clinical course and surgical outcomes of intramedullary spinal cord tumors from 58 neurosurgical centers across Japan [4].
In total, 168 patients with surgically treated intramedullary spinal cord astrocytomas were included. Of these patients, 56 patients with histologically confirmed grade II intramedullary spinal cord astrocytomas were identified and retrospectively reviewed. Although the updated 2021 World Health Organization (WHO) classification includes various diagnostic genes, molecules, pathways, and histological findings for diagnosis, this study was based on conventional histopathological grading. The extent of resection was defined as macroscopic gross total resection (100%), subtotal resection (> 90%), partial resection (< 90%), or open biopsy. In the present study, 3 patients with an attempt of gross total resection presented neurological decline related with the surgical treatment and were excluded from the analysis. Finally, 53 patients with grade II spinal cord astrocytomas with either subtotal, partial resection or open biopsy were enrolled.
Clinical characteristics including age, sex, Karnofsky Performance Scale (KPS), radiological data from magnetic resonance imaging (including tumor levels and lesion lengths), and pathological diagnoses (based on pathohistological diagnoses) were anonymously extracted from the database. Increase enhancement in axial and sagittal T1-weighted magnetic resonance imaging, or development of diffuse meningeal spread were considered to have disease progression. The patients’ clinical performance status following surgery with or without adjuvant radiotherapy was analyzed, comparing the subgroups with and without disease progression.
The KPS enables the quantification of a patient’s overall state and quality of life [5]. The score ranges from 0 to 100, with 100 indicating that the patient has normal physical abilities with no signs of disease, and 0 indicating that the patient is dead. The clinical functional status was assessed immediately before operation and 1, 6, 12, 24, and 60 months after surgery.
The survival period, defined as the number of months from surgery to death, was censored at the last available follow-up or cutoff study date (December 31, 2020) for those who were still alive. Progression-free survival (PFS) was estimated using the Kaplan-Meier method with associated log-rank tests for the entire cohort and survival in subgroups classified based on the presence/absence of adjuvant radiotherapy and disease progression. Statistical analyses were performed using JMP statistical software ver. 13 (SAS Institute Inc., Cary, NC, USA). The chi-square test was used for continuous and binary values. For non-parametric tests, the Mann-Whitney U-test and Fisher exact probability test were used to compare subgroups. Significance of the obtained results was assessed at the 5% level.
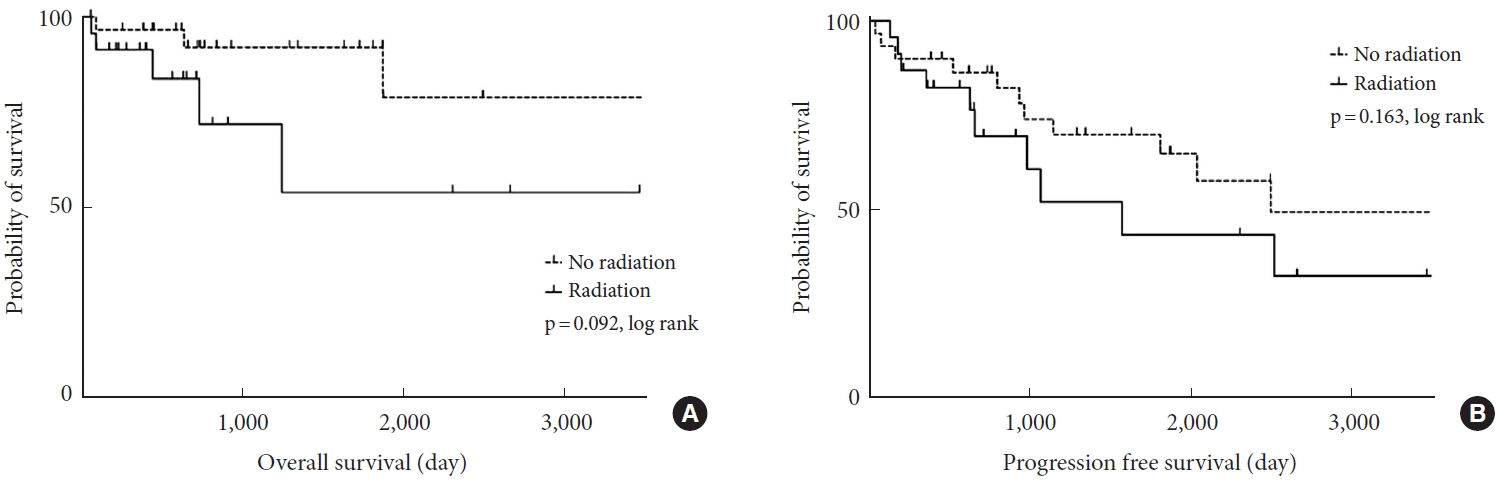
Patient demographics are described in Table 1. Patients included 32 men and 21 women with a mean age of 50.3±22.6 years (range, 2–88 years). The groups with and without radiation comprised 23 and 30 patients, respectively. The lesions were cervical (n= 13), cervicothoracic (n= 14), thoracic (n= 14), thoracolumbar (n= 3), and lumbosacral (n= 9). The mean overall disease progression rate (the number of progressions/the total of 53 patients) was 47.1% and mortality rate was 15.1% (8 of 53) during a mean follow-up period of 48.4±39.8 months (range, 2.5–144.5 months). Seven out of 8 patients had death related with tumor burden and 1 patient died of a cause other than tumor. Overall survival (OS) and PFS were depicted with Kaplan-Meier curves (log rank, p= 0.092, p= 0.163) (Fig. 1). Tumors located in the cervical spine and with high MIB-1 index were more likely to receive post-operative radiation treatment (Table 2).
In the radiation group (n= 23), 3 patients (13%) underwent subtotal resection, 9 (39.2%) underwent partial resection, and 11 (47.8%) underwent open biopsy. These 23 patients underwent adjuvant radiotherapy 28 days (range, 15–91 days) after surgery. The radiation dose given to grade II spinal cord astrocytoma ranged between 45 and 50.4 Gy delivered in a conventional dose per fraction of 1.8–2.0 Gy. During the 36.8 months follow-up period, 11 patients (47.8%) showed clinical deterioration, exhibiting either local recurrence/progression (n = 6), intracranial dissemination (n= 1), or combined local progression and diffuse meningeal spread (n= 4). Treatments for local progression were reoperation (n= 1), temozolomide administration (n= 4), and palliative therapy (n= 1). Pathohistological findings of the reoperation for local progression revealed grade II glioblastoma, which suggested malignant transformation. The transition of KPS is shown in Fig. 2. KPS in subgroups without disease progression stayed flat, whereas KPS in the disease progression subgroup continued to decrease before adjuvant radiotherapy and was aggravated after the confirmation of progressive disease. In the radiation group, disease progression was more common in younger than older patients (Table 3).
In the group without radiation (n = 30), 5 patients (16.7%) underwent subtotal resection, 15 (50%) underwent partial resection, and 10 (33.3%) underwent open biopsy. In patients treated by only biopsy, close observation was made without any adjuvant treatment due to concern of radiation induced myelopathy and malignant transformation. During the 57.3±39.8-month follow-up period, 14 patients (46.7%) presented with progressive disease, exhibiting either local recurrence/progression (n= 12, 85.7%) or combined local recurrence/progression and diffuse meningeal spread (n= 2, 14.3%). Treatments for local recurrence/progression were reoperation (n= 5), radiotherapy after reoperation (n = 1), radiotherapy with/without temozolomide administration (n= 2), and palliative therapy (n= 4). The transition of KPS is shown in Fig. 2. KPS in the subgroup without disease progression stayed flat with a slight up and down, whereas KPS in the disease progression subgroup decreased gradually just after operation and then deteriorated. There were no significant differences regarding age, lesion length, initial KPS, the percentage of cervical lesions, the extent of resection, or the MIB-1 index (Table 4).
Due to the rarity of spinal cord gliomas, no consensus has been reached in the literature regarding the role of adjuvant radiotherapy for grade II spinal cord astrocytoma [6-11]. Diaz-Aguilar et al. [12] report that younger age, gross total resection and absence of radiotherapy positively influenced survival factors in a series of 561 patients with low-grade spinal cord astrocytoma. A systematic review by Hamilton et al. [13] found a negative correlation between radiation therapy and survival for low-grade spinal cord tumors (hazard ratio for OS, 5.20; p<0.01). Lastly, Abdel-Wahab et al. [14] report a multivariate analysis in 40 patients indicating that adjuvant radiotherapy enhanced disease progression. However, the extent of tumor resection has not been clearly shown in these studies, and the impact and therapeutic effect of radiotherapy have not been clarified.
Meticulous microsurgical technique and use of intraoperative neuromonitoring is the standard of care to maximize safe surgical resection in diffuse spinal cord astrocytomas, however, the lack of a separation plane and changes in sensory and motor evoked potential correlating with severe postoperative neurological dysfunction usually precludes a gross total resection of these lesions. This study investigated the impact of radiotherapy on clinical performance status by KPS, focusing on patients with either subtotal, partial resection or biopsy. The tolerance dose for the spinal cord has been reported to be 45–50 Gy with conventional fractionation schedules of 1.8–2 Gy/day, and the upper dose in the present study was under this level [15]. The actual incidence of myelopathy with these conventionally fractionated doses is less than 0.2%–0.5% after 50 Gy and 1%–5% after 60 Gy [16]. Additionally, patients who did not presented disease progression presented improvement in the KPS regardless of the radiation status. This observation can correlate with the decompressive effect of surgery as result of laminectomy and duraplasty during the partial resection or biopsy. Furthermore, regardless of adjuvant radiotherapy, KPS in both disease progression groups, especially radiation group, decreased gradually before the confirmation of disease progression.
The present study demonstrate that disease progression was more frequent in younger than older patients who received post-operative radiation therapy. This observation goes along with increase evidence younger age has been increasingly identified as a surrogate of aggressive behavior in adolescents and young adults (AYAs, age 15–39 years) [17]. It is considered that AYAs have polymorphisms or genomic properties that differ from older people with respect to cancer susceptibility and treatment. For example, melanomas with BRAF mutations are more prevalent in the AYA population and thus are more likely to respond to a BRAF inhibitor [18]. Less favorably, triple negative breast cancer is more prevalent in patients under 40 years and is associated with increased mortality partly due to fewer treatment options [19]. Similarly, spinal cord astrocytoma among AYAs may have unique genetic and epigenetic differences compared with older population.
Extent of resection is one of the most contentious and intriguing aspects of intramedullary spinal cord tumors, which had a significantly greater factor in patients with grade II spinal cord astrocytoma with adjuvant radiotherapy in our study. A systematic review by Hamilton et al. [13] showed patients undergoing GTR had a lower mortality rate at 2.5 and 10-year follow-up than patients with lesser resection volumes. Cytological reduction by greater extent of resection could have a positive effect on disease progression. Also, patients with adjuvant radiotherapy had higher MIB-1 index than those without adjuvant radiotherapy. In the patients with adjuvant radiotherapy, KPS in the disease progression subgroup continued to decrease. It may suggest that the tumor classified according to classical pathohistological diagnosis included more malignant properties. The updated 2021 WHO classification includes various diagnostic genes, molecules, pathways, and pathohistological findings for diagnosis [20]. Dubbink et al. [21] reported that out of 123 patients with anaplastic oligodendroglioma according to classical pathohistological classification, 55 patients exhibited intracranial glioblastoma according to the molecular classification. The genetic underpinnings of spinal cord tumors remain less well understood than those of their intracranial counterparts due to their rarity. This study suggested that conventional classical classification for spinal cord astrocytoma did not reflect disease progression and clinical outcome well.
In the present study, one patient showed pathohistological malignant transformation, which was confirmed in the specimen of the reoperation; however, the mechanisms underlying such transformations are still unknown. In the majority of published case reports regarding malignant transformation, patients had received previous radio- or chemotherapy [22,23]. The incidence of malignant transformation in patients with intracranial low-grade glioma ranges between 23% and 72% [22,24,25]. On the other hand, the incidence and the latency period of malignant transformation in patients with spinal cord low-grade glioma is unknown, therefore meticulous close observation is essential.
This study has several limitations. First, the indication of adjuvant radiotherapy varies by institution because the role is still debatable. This study is retrospective multi-institutional study and the indication of adjuvant radiotherapy may not be consistent, which could be one of the limitations. Second, this study was retrospective in nature, and the sample size was small and thus had limited statistical power. Despite these limitations, we believe that our study provides important information regarding the impact of adjuvant radiotherapy on the clinical performance status of patients with grade II intramedullary spinal cord astrocytoma.
NOTES
ACKNOWLEDGEMENTS
Investigators of Intramedullary Spinal Cord Tumors in the Neurospinal Society of Japan -- Masahito Hara and Masahiro Aoyama: Aichi Medical University. Taku Sugawara: Akita Cerebrospinal and Cardiovascular Center. Hiroaki Shimizu: Akita University. Kotaro Ogihara: Iwakuni Clinical Center. Atsushi Sugawara: Iwate Medical University. Phyo Kim and Kazushige Itoki: Utsunomiya Brain and Spinal Cord Center. Seiji Matsui and Seiji Shigekawa: Ehime University. Noritsugu Kunihiro: Osaka City General Hospital. Kentaro Naito: Osaka City University. Shinji Yamamoto: Ohnishi Neurological Center. Takao Yasuhara: Okayama University. Motoyuki Iwasaki: Otaru City Hospital. Yasuyuki Miyoshi: Kawasaki Medical School. Hideki Hayashi: Kitano Hospital. Nakayama Noriyuki and Toru Iwama: Gifu University. Daisuke Umebayashi: Kyoto Prefectural University of Medicine. Hiroshi Nakagawa and Manabu Sumiyoshi: Kushiro Kojinkai Memorial Hospital. Yasukazu Hijikata: Spine and Low Back Pain Center, Kitasuma Hospital. Hisaaki Uchikado: Kurume University. Hitoshi Fukuda: Kochi University. Tomoaki Nakai and Takashi Sasayama: Kobe University. Kazuhiko Mishima: Saitama Medical University, International Medical Center. Tomoo Inoue: Saitama Red Cross Hospital. Shunsuke Yano and Toru Sasamori: Sapporo Azabu Neurosurgical Hospital. Nobuhiro Mikuni and Yukinori Akiyama: Sapporo Medical University. Tsuyoshi Hara: Juntendo University. Gakuji Gondo: Shonan Kamakura General Hospital. Mitsuhiro Yoshida: Yokkaichi Municipal Hospital. Shigeo Ueda and Minoru Hoshimaru: Shin-Aikai Spine Center. Hideki Komatani and Yuichi Takahashi: Shin Komonji Hospital. Kiyoshi Ito: Shinshu University. Hisaharu Goto and Node Yasuhiro: Shin-Yurigaoka General Hospital. Mizuki Watanabe: Seirei Hamamatsu General Hospital. Yasunobu Ito: Tokyo General Hospital. Yoshitaka Hirano: Southern Tohoku Research Institute for Neuroscience. Teiji Tominaga: Tohoku University. Hirokazu Takami: Tokyo University. Jun Karakama: Tokyo Medical and Dental University. Hiroki Ohashi: The Jikei University School of Medicine. Naoyuki Harada: Toho University. Ryu Kurokawa: Tetsuro Shingo and Satoshi Kawajiri, Dokkyo Medical University. Tomohiro Yamauchi: Tomakomai City Hospital. Tetsuji Uno: Tottori University. So Fujimoto and Keisuke Takai: Tokyo Metropolitan Neurological Hospital. Yasufumi Otake: Nakamura Memorial Hospital. Yasuhiro Takeshima and Hiroyuki Nakase: Nara Medical University. Akihiko Saito: Niigata City Hospital. Daijiro Morimoto and Kyongsong Kim: Nippon Medical School. Tatsuya Ohtonari: Brain Attack Center, Ota Memorial Hospital. Takafumi Mitsuhara: Hiroshima University. Yosuke Kuromi: Fukushima Medical University. Toshiyuki Takahashi and Ryo Kanematsu: Fujieda Heisei Memorial Hospital. Tatsushi Inoue: Fujita Health University. Toshitaka Seki and Kazuyoshi Yamazaki: Hokkaido University. Izumi Koyanagi: Hokkaido Neurosurgical Memorial Hospital. Kazuhisa Yoshifuji: Hokkaido Medical Center for Child Health and Rehabilitation. Masashi Fujimoto: Mie University. Misao Nishikawa: Moriguchi-Ikuno Memorial Hospital. Takashi Yagi and Hiroyuki Kinouchi: University of Yamanashi. Hidetoshi Murata: Yokohama City University. Mari Kitayama: Wakayama Medical University.
Fig. 2.
The transition of Karnofsky Performance scale in adjuvant radiotherapy and no adjuvant radiotherapy group with or without disease progression. OP, operation; 6M, 6 months; 12M, 12 months; 24M, 24 months; 60M, 60 months.
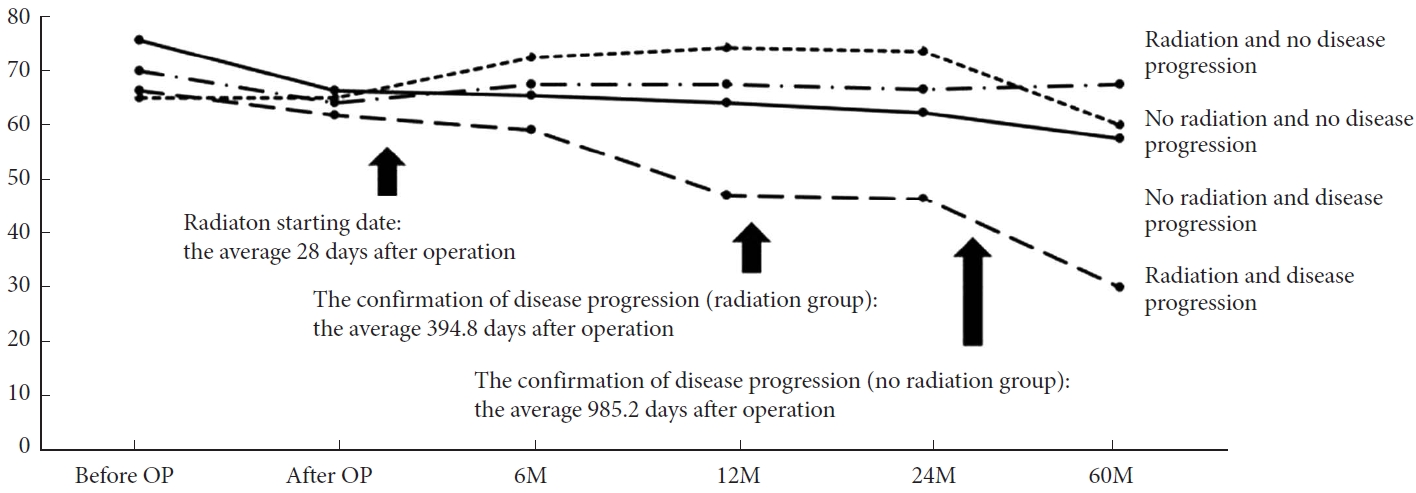
Table 1.
Patients’ characteristics
Table 2.
Comparison of 53 grade II spinal cord astrocytoma patients with or without adjuvant radiotherapy
Table 3.
Characteristics of 23 grade II spinal cord astrocytoma patients with adjuvant radiotherapy
Table 4.
Characteristics of 30 grade II spinal cord astrocytoma patients without adjuvant radiotherapy
REFERENCES
1. Adams H, Avendaño J, Raza SM, et al. Prognostic factors and survival in primary malignant astrocytomas of the spinal cord: a population-based analysis from 1973 to 2007. Spine (Phila Pa 1976) 2012;37(12):E727-35.

2. Fakhreddine MH, Mahajan A, Penas-Prado M, et al. Treatment, prognostic factors, and outcomes in spinal cord astrocytomas. Neuro Oncol 2013;15:406-12.



3. Miller DC. Surgical pathology of intramedullary spinal cord neoplasms. J Neurooncol 2000;47:189-94.

4. Endo T, Inoue T, Mizuno M, et al. Current trends in the surgical management of intramedullary tumors: a multicenter study of 1,033 patients by the Neurospinal Society of Japan. Neurospine 2022;19:441-52.




5. Schei S, Solheim O, Jakola AS, et al. Perioperative fatigue in patients with diffuse glioma. J Neurooncol 2020;147:97-107.




6. Sandalcioglu IE, Gasser T, Asgari S, et al. Functional outcome after surgical treatment of intramedullary spinal cord tumors: experience with 78 patients. Spinal Cord 2005;43:34-41.



7. Kim MS, Chung CK, Choe G, et al. Intramedullary spinal cord astrocytoma in adults: postoperative outcome. J Neurooncol 2001;52:85-94.

8. O’Sullivan C, Jenkin RD, Doherty MA, et al. Spinal cord tumors in children: long-term results of combined surgical and radiation treatment. J Neurosurg 1994;81:507-12.


9. Rodrigues GB, Waldron JN, Wong CS, et al. A retrospective analysis of 52 cases of spinal cord glioma managed with radiation therapy. Int J Radiat Oncol Biol Phys 2000;48:837-42.


10. Shirato H, Kamada T, Hida K, et al. The role of radiotherapy in the management of spinal cord glioma. Int J Radiat Oncol Biol Phys 1995;33:323-8.


11. Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: a review. Curr Neurol Neurosci Rep 2011;11:320-8.



12. Diaz-Aguilar D, ReFaey K, Clifton W, et al. Prognostic factors and survival in low grade gliomas of the spinal cord: a population-based analysis from 2006 to 2012. J Clin Neurosci 2019;61:14-21.


13. Hamilton KR, Lee SS, Urquhart JC, et al. A systematic review of outcome in intramedullary ependymoma and astrocytoma. J Clin Neurosci 2019;63:168-75.


14. Abdel-Wahab M, Etuk B, Palermo J, et al. Spinal cord gliomas: a multi-institutional retrospective analysis. Int J Radiat Oncol Biol Phys 2006;64:1060-71.


15. Linstadt DE, Wara WM, Leibel SA, et al. Postoperative radiotherapy of primary spinal cord tumors. Int J Radiat Oncol Biol Phys 1989;16:1397-403.


16. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22.


17. Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer 2008;8:288-98.



18. Menzies AM, Haydu LE, Visintin L, et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res 2012;18:3242-9.



19. Partridge AH, Hughes ME, Warner ET, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol 2016;34:3308-14.


20. Nagashima Y, Nishimura Y, Eguchi K, et al. Recent molecular and genetic findings in intramedullary spinal cord tumors. Neurospine 2022;19:262-71.




21. Dubbink HJ, Atmodimedjo PN, Kros JM, et al. Molecular classification of anaplastic oligodendroglioma using nextgeneration sequencing: a report of the prospective randomized EORTC Brain Tumor Group 26951 phase III trial. Neuro Oncol 2016;18:388-400.


22. Murphy ES, Leyrer CM, Parsons M, et al. Risk factors for malignant transformation of low-grade glioma. Int J Radiat Oncol Biol Phys 2018;100:965-71.


23. Ryu SJ, Kim JY, Kim KH, et al. A retrospective observational study on the treatment outcomes of 26 patients with spinal cord astrocytoma including two cases of malignant transformation. Eur Spine J 2016;25:4067-79.




- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2