 |
 |
- Search
|
|
||
Abstract
Objective
A preliminary report from a single institution, noninferiority, prospective randomized controlled trial is conducted to determine the effectiveness of interlaminar endoscopic lumbar discectomy (IELD) versus microscopic lumbar discectomy (MLD) for the treatment of L5–S1 lumbar disc herniation (LDH).
Methods
This prospective, noncrossover, randomized controlled trials was conducted at a single neurosurgical center. Patients with symptomatic radiculopathy or intermittent neurogenic claudication caused by LDH were enrolled from July 2016 to July 2021. The study compared the effectiveness of microscopic and full-endoscopic discectomy procedures. Outcome measures included visual analogue scale (VAS) scores for back and leg pain, Oswestry Disability Index scores, radiologic measurements, endurance time of walking, and satisfaction rate.
Results
Of 37 assessed patients, both IELD and MLD groups demonstrated significant improvements in VAS scores for pain over time, with no significant difference between them. For secondary outcomes, the IELD group had a shorter hospital stay and reduced blood loss but a longer operation time than the MLD group. Radiographic evaluations showed no change compared to preoperative data. Patient satisfaction and recovery rates were slightly higher for the MLD group, but both groups were comparable in most evaluations, with complications being minimal.
Conclusion
The IELD was noninferior in improving the intensity of back and leg pain and functional disability, compared to the MLD. Additionally, the IELD showed no difference in clinical outcomes for patients in terms of radiographic results and patient satisfaction rates. The results of this research preliminarily demonstrate that the IELD could be considered an effective alternative to MLD for L5–S1 central or paracentral LDH.
Lumbar disc herniation (LDH) at the L5–S1 level is a prevalent spinal condition frequently resulting in lower back pain, sciatica, and other debilitating symptoms [1]. The L5–S1 junction serves as a crucial shock absorber for the entire spinal alignment, providing an essential foundation for maintaining flexibility and stability [2-4]. The high mechanical load on the L5–S1 intervertebral disc renders it the second most common site for LDH, surpassed only by the L4–5 level. Consequently, surgical intervention is typically considered when conservative treatments fail to provide adequate relief [5]. It is vital to select the appropriate decompression surgery that preserves spinal structures while effectively alleviating compression.
Over the past 2 decades, minimally invasive surgery (MIS) techniques have gradually emerged as the preferred surgical modality for treating neural compression due to single-segment uncomplicated LDH [6-8]. MIS techniques reduce injury to paravertebral soft tissues and maintain the biomechanical stability of the normal spinal sequence, accelerating the recovery time and return to work [9,10]. Interlaminar endoscopic lumbar discectomy (IELD) and microscopic lumbar discectomy (MLD) are 2 prominent surgical techniques gaining traction in this context [11-13]. Recently, advancements in techniques and iterative improvements in instrumentation have facilitated the global adoption of MIS techniques, whether in microscopic or endoscopic surgery [14,15]. Innovations, such as enhanced display pixel resolution or the enhanced intraoperative navigation guidance system, have significantly increased the efficiency and effectiveness of surgery [16,17].
Randomized controlled trials (RCTs) are considered the gold standard for evaluating the effectiveness of medical interventions. A previously published noninferiority RCT demonstrated that full-endoscopic discectomy was noninferior to open microdiscectomy in leg pain control [18]. Nonetheless, the employment of IELD remains a subject of debate due to the scarcity of robust evidence demonstrating its safety and efficacy relative to MLD at the L5–S1 level [19-21]. In this paper, we present the first preliminary RCT results comparing IELD and MLD for L5–S1 LDH to assess the clinical outcomes, complications, and patient satisfaction rates associated with each surgical approach, providing an initial foundation for future research and guiding clinical decision-making.
This prospective, noncrossover, RCT was conducted at a single center of the neurosurgical department. The study aimed to evaluate the effectiveness of microscopic and full-endoscopic discectomy procedures in patients with sciatica caused by LDH. This research is an IIT (investigator-initiated trial), seeking to answer questions related to comparative benefits between mainstream MIS techniques in microscopic and full-endoscopic techniques. All authors contributed to the study design and data interpretation. The first and corresponding authors drafted the manuscript and underwent substantial revisions from all other authors. The research received approval and registration from the Institutional Review Board of The Catholic University of Korea Seoul St. Mary’s Hospital (No. KC15OISI0665). All patients provided written informed consent before enrolment in the trial. The trial adhered to the Declaration of Helsinki and the Good Clinical Practice guidelines, ensuring ethical conduct and scientific rigor throughout the study. The results will provide valuable insights into the effectiveness and safety of microscopic and full-endoscopic discectomy for the treatment of L5–S1 symptomatic LDH.
Patient enrolment was from July 2016 to July 2021. The inclusion criteria were patients who aged more than 18 years old with the complaint of symptomatic radiculopathy or intermittent neurogenic claudication for at least 6 months, with or without neurological deficit after failed conservation treatments, having clinical symptoms of L5–S1 nerve root compression which correlated with magnetic resonance imaging findings of LDH at this level [22-25]. The patients with foraminal/extraforaminal LDH, records of previous spine surgery at the same disc level, psychiatric disorders, or any spinal comorbidities and pathologies that may affect the outcomes of surgery, including isthmic or degenerative spondylolisthesis or spinal infection, or spinal oncologic diseases, were all excluded.
In this RCT, the whole process of blinding was unfeasible due to the nature of the intervention. The methodology employed for patient randomization and blinded allocation to the respective planned surgical groups was designed to minimize the potential bias in preoperative planning and patient care. Throughout the study, blinding was maintained for outcome assessors and data analysts to minimize biases in clinical outcome evaluation and data analysis. While patients were blinded initially, the distinction in surgical wounds between micro and endoscopic surgeries made it impossible to maintain this blinding postoperatively. The surgical interventions under investigation included IELD and MLD. The allocation of patients to the respective surgical groups was executed using a computer-generated randomization sequence. The allocation sequence was concealed using sequentially numbered, opaque, sealed envelopes prepared by an independently trained clinical research coordinator (CRC) who had no involvement in patient care or outcome assessment, thereby guaranteeing unbiased allocation. One day before the scheduled surgery, the sealed envelope containing the allocated surgical method was opened by the CRC, who subsequently informed the operating surgeon of the assignment. This approach ensured that the surgeon remained blinded to the patient’s assigned group until just before the procedure.
All surgeries were performed by a single senior spine surgeon with extensive experience in minimally invasive spine surgery, including both microscopic and full-endoscopic lumbar discectomy techniques. Patients in the MLD group underwent the procedure under general anesthesia. A standard MLD technique was employed, which involved making a small incision applying a tubular retractor system, and operating via a microscope for enhanced visualization during the surgery [26]. The herniated disc material was removed using a combination of forceps and a curette (Fig. 1A, B).
For IELD procedure, the patient was placed in prone position on a Jackson table with a Wilson frame and intubated under general anesthesia (Fig. 1C, D). The procedure was performed under intraoperative C-arm guidance, with the surgeon standing on the side corresponding to the herniation [27,28]. A 1-cm vertical skin incision is made medially for the working cannula in relation to the interlaminar window, with the craniocaudal localization depending on the pathological finding, with the beveled opening facing the medial side of the facet joint. The procedure proceeds under real-time visualization and irrigation with isotonic saline. The inferior edge of the L5 lamina is palpated using the bevel of the cannula, applying gentle traction on the soft tissue. Bone resection was performed with a diamond burr to improve access to the interlaminar working space to gain adequate access for decompression of the affected nerve root. Then, the ligamentum flavum was resected to expose the dura sac. Any adjacent fat and vascular tissues were coagulated. With neural structure being protected with the working cannula, the surgeon removed the disc fragment and explored annular defects. Then, the annuloplasty was performed. Adequate decompression was confirmed by the tension-free nerve root with pulsatile movement. The hemostasis was performed and drainage was placed. In our practice, we emphasize preserving as much healthy disc tissue and bone structure as possible. Therefore, we limit the resection to the herniated disc tissue and perform the annuloplasty to repair the damaged annulus fibrosus entrance, avoiding removal of the in situ disc tissue, it has the potential benefit of maintaining disc height and reducing adjacent segment degeneration in the target segment.
Eligible participants’ data were meticulously recorded, encompassing demographic information, intervention details, and outcome measures. An independent CRC assessed the outcomes during preoperative evaluations and subsequent postoperative follow-ups. The primary outcomes included improvements in visual analogue scale (VAS) scores (pain rating scales ranging from 0–10 for both back and leg pain, with higher values indicating higher pain intensity) and Oswestry Disability Index (ODI) scores (functional status questionnaires rated from 0–100, with higher values indicating greater disability) [29,30]. Secondary outcomes encompassed self-assessed satisfaction and recovery rates (measured on a scale from 0% to 100%), maximum walking once endurance/daily walking time (minutes), and radiologic measurements (disc height, segmental lordosis, and lumbar lordosis). Among of them, the walking distance was divided into 5 levels: A: more than 60 minutes; B: 30–60 minutes; C: 15–30 minutes; D: 5–15 minutes; E: less than 5 minutes. An independent spine clinical fellow assessed radiologic measurements at the last 12-month follow-up. All self-reported outcome measures were gathered through questionnaires sent to patients via email or phone calls. Patients visited the clinic for neurological examinations conducted by a clinical assistant.
All statistical analyses were conducted using STATA/MP 17 (Stata Corp LLC, College Station, TX, USA). The primary analysis was adhered to the intention-to-treat principle, and perprotocol analysis was performed as sensitivity analysis. We employed multiple imputation with chained equations approach, specifically predictive mean matching to handle missing variables. The predictor variables included in the imputation model were adjust by baseline variables. Descriptive statistics were employed to summarize patient demographics and clinical characteristics. Continuous variables were reported as means and standard deviations or medians, while categorical variables were expressed as frequencies or percentages. Fisher exact test and r by c chi-square tests were used to compare categorical variables.
A linear mixed-effects model was employed to evaluate the primary outcomes, while accounting for within-patient correlations and adjusting for the baseline. The primary outcome of the study was the mean difference in VAS and ODI scores between the IELD and MLD groups at 1, 3, 6, 12 months postoperatively, along with corresponding 95% confidence intervals (CIs). Other statistical calculations were evaluated with a 2-sided p-value of less than 0.05 considered statistically significant. Graphical representations of outcome trends were generated using GraphPad software (San Diego, CA, USA).
A total of 37 patients were assessed for eligibility in the study, which compared full endoscopic to microscopic techniques. Nine of these patients were excluded either because they did not meet the inclusion criteria or they declined to participate. As a result, 28 patients entered the randomized process. Of these, 13 patients were allocated to the full-endoscopic group and 15 to the microscopic group. No patients withdrew from the allocated interventions due to dissatisfaction with the process or discontinuation of the intervention process (Fig. 2). The baseline characteristics of the patients were comparable between the 2 groups, as presented in Table 1. The average age of the participants was 49.71 ± 15.83 years, with females comprising 53.57% of the cohort. Their average body mass index stood at 24.27 ± 4.03 kg/m2, and the mean follow-up period was 12.61 months. When evaluating disc herniation characteristics, both the microscopic and endoscopic groups displayed similar distributions in terms of herniation side (p = 1.000), axial herniation patterns (p = 0.445), and migration patterns (p = 1.000).
VAS scores for back and leg pain showed significant improvements over time in both IELD and MLD groups (Fig. 3). Our linear mixed-effects model analysis evaluated the effect of treatment (IELD vs. MLD) on VAS, and ODI scores over time, adjusting for age, sex (Table 2). The estimated marginal means revealed that both IELD and MLD groups experienced significant reductions in VAS scores and no difference with each other compare to the preoperative data. Specifically, the effect for the improvement on the VAS-leg pain scores at postoperative 1 month showed a mean difference of 1.97 (95% CI, -4.12 to 0.18); and at 12 months, the difference was 0.71 (95% CI, -2.54 to 1.12).
The results revealed significant differences between the MLD and IELD groups in terms of length of stay (5.47 ± 1.36 days vs. 3.69 ± 1.60 days, p = 0.003), operation time (95.53 ± 18.91 minutes vs. 134.77 ± 33.12 minutes, p = 0.001), and blood loss (44 ± 26.67 mL vs. 20 ± 20.99 mL, p = 0.009) (Table 3). There is no statistical difference in surgical time between the 2 groups for migrated disc herniation and nonmigrated disc herniation (p = 0.759). The IELD group showed a shorter length of stay and less blood loss, while the operation time was longer compared to the MLD group. At the preoperative stage, the disc height for the MLD and IELD groups were 9.40 ± 2.68 mm and 8.98 ± 2.03 mm, respectively, with a difference of -0.42 (95% CI, -2.01 to 1.17). At the last-time follow-up, the disc height values were 10.11 ± 2.82 mm for MLD and 9.93 ± 1.83 mm for IELD, resulting in a difference of -0.15 (95% CI, -1.66 to 1.36). For segmental lordosis, the preoperative values were 9.76° ± 5.47° for MLD and 9.51° ± 4.47° for IELD, with a difference of -0.74 (95% CI, -4.11 to 2.64). The segmental lordosis values were 11.18° ± 5.49° for MLD and 10.98° ± 5.30° for IELD, with a difference of -0.56 (95% CI, -3.91 to 2.80). Regarding lumbar lordosis, the preoperative values for MLD and IELD were 42.16° ± 8.37° and 36.39° ± 14.09°, respectively, with a difference of -7.00 (95% CI, -15.20 to 1.20). The lumbar lordosis values were 44.30° ± 7.69° for MLD and 44.25° ± 7.16° for IELD, resulting in a difference of -0.31 (95% CI, -5.92 to 5.30). All of the radiographic showed no difference compared to the preoperative data.
In terms of patient satisfaction, the last-time follow-up scores were 88.14% ± 10.14% for MLD and 83.85% ± 12.13% for IELD, with a difference of -6.27 (95% CI, -13.64 to 1.09). The recovery rate at the last-time follow-up was 81.98% ± 16.11% for MLD and 71.54% ± 18.36% for IELD, with a difference of -12.45 (95% CI, -24.91 to 0.02). The MacNab criteria comparisons between the 2 groups showed no significant difference, with a Fisher exact test p-value of 0.66. For walking ability assessment, there were no statistical difference in maximum walking once endurance or daily walking time. Furthermore, in both the IELD and MLD groups, one patient experienced recurrence at the same level. During the IELD procedure, one patient encountered a dural tear, necessitating a switch to the MLD surgery.
LDH most commonly afflicts the L5–S1 and L4–5 levels. Also, the large patient pool provides ideal case selection for novice surgeons. Particularly, the L5–S1 level is of unique interest due to its anatomical characteristics with notably wider interlaminar space than other levels, resulting in a more spacious surgical corridor, and facilitates easier access to the disc fragment [28,31]. This broader space offers enhanced visualization and easier maneuverability during surgery. Therefore, for novice surgeons beginning their journey with minimally invasive procedures, these anatomical features make the L5–S1 level an ideal starting point to practice and refine their first step spine surgical skills. Therefore, we reported this preliminary RCT aimed to compare the efficacy and safety of IELD and MLD in patients with LDH, specifically at the L5–S1 level (Figs. 4, 5). By employing robust statistical analysis of VAS and ODI from baseline to 12 months postoperatively for primary and secondary outcomes. Following the upper limit of the 95% CI for the mean difference in VAS and ODI scores, our intention-to-treat analysis demonstrated that IELD was considered noninferior to MLD in terms of expectations. Furthermore, the analysis revealed a significant improvement in leg pain VAS scores and ODI scores over time in both the IELD and MLD groups, indicating that both surgical techniques effectively alleviate pain and improve functional status in patients with L5–S1 LDH.
The primary finding of pain control and functional disability improvement is consistent with several previous non-specific L5–S1 segmental comparisons of noninferiority RCTs of percutaneous translaminar endoscopic discectomy versus open microdiscectomy in terms of pain control or disability index [18]. In terms of secondary outcomes, there were no significant differences between the IELD and MLD groups in self-rated satisfaction and recovery rates, maximum walking endurance, or radiological measurements. These findings suggest that both surgical techniques yielded comparable in preserving spine alignment and improvements in mobility. However, it is essential to consider that the similarity in bias present in postoperative clinical outcomes may also be due to all procedures being performed by a single institution and a single neurosurgeon, which may have reduced the variability in surgical technique and outcomes [32].
In our study, both the IELD and MLD groups exhibited a low complication rate, with no significant differences, and our finding is also consistent with previous studies compare the full-endoscopic lumbar discectomy with microdiscectomy [33,34]. Additionally, IELD demonstrated distinct advantages, such as reduced hospital stay and less intraoperative bleeding [21,35]. These advantages can be credited to the full-endoscopic system’s use of angled magnification and clear visualization under the continuous saline irrigation, enabling precise paraneural soft tissue stripping and thorough detection and manipulation of concealed areas around the circumferential dural sac, while further lowering the risk of biomechanical instability at the surgical segment. Moreover, the dilator set working channel, which create a centimeter level surgical access, allows less disruption of surrounding muscles and tissues. Technically, alternation employed between the applications of radiofrequency and a high-speed diamond burr properly facilitates precise and fast hemostasis, particularly at the junction between osseous and soft tissue interfaces. Such an approach considerably mitigates the potential for postoperative hematoma formation due to the suboptimal intraoperative hemostatic control [36]. However, a salient point to underscore is the comparative inflexibility with steep learning curve of instrument manipulation in colinear full-endoscopic technique when juxtaposed with the two-handed microscopic technique.
Although the IELD procedure have potential advantages in terms of a shorter length of stay and reduced blood loss, consistent with previous different approach study [37-40]. However, the operation time is longer compared to the MLD procedure. The difference operation time between the 2 procedures were attributed to the distinct nature of both surgical procedures, such as patient-specific factors, equipment used, or the skill and experience of the surgeon [41-43]. With miniaturized endoscopic tools, the operation can provide a clearer visualization and potentially decrease postoperative complications due to reduced tissue trauma. However, there’s a trade-off that smaller instruments may require more precise handling, meticulous maneuvers, and sometimes multiple instrument changes to achieve the desired outcome. Meanwhile, meticulously design the endoscopic entry angle and surgical approach preoperatively to reach the lesion boundaries is crucial to alleviate thoroughly nerve compression.
Rare evidence from meta-analyses have been conducted to compare the effectiveness of full-endoscopic spine surgery in the treatment of LDH. A recent meta-analysis of endoscopic discectomy with nonendoscopic discectomy for treatment of symptomatic LDH reported by Li et al. [44] found no significant differences in the improvement of MacNab criteria, and no difference in the rate of recurrence, but the complication rate of full-endoscopic surgery was lower than microscopic discectomy. Conversely, another moderate to low quality of evidence meta-analysis reported the comparable overall complication between full-endoscopic lumbar discectomy and conventional open or microscopic discectomy [34]. The complications of full-endoscopic discectomy focus on the higher risks of transient dysesthesia and residual fragment. The observed discrepancy in these meta-analyses results may be attributed to 2 factors. First, there is a difference in the time frame of the included studies; although the 2 meta-analyses were published only a year apart, the latter incorporated more recent evidence compared to the former. This could potentially lead to variations in the pooled estimates and conclusions drawn from the analyses. Second, the earlier meta-analysis did not categorize endoscopic techniques into dual-channel and single-channel subgroups for further analysis. This distinction may be relevant, as different endoscopic systems may yield varied outcomes, potentially contributing to the observed heterogeneity in results. Therefore, the lack of subgroup analysis in the former meta-analysis may have masked potential differences in outcomes based on full-endoscopic technique, leading to disparate findings between the 2 meta-analyses [45].
This study has several limitations that warrant consideration. Firstly, complete blinding of the experimental procedure was unattainable due to the nature of the intervention, which could introduce bias in evaluating the results [46]. Secondly, the investigation was conducted at a single center by one physician, potentially limiting the generalizability of the findings to other settings. Thirdly, the small sample size and relatively short-term follow-up may result in underpowered studies, thus affecting the reliability of the outcomes. While we endeavored to recruit a more expansive cohort over the duration of the study, the inherent challenges associated with enlisting patients with this specific diagnosis limited our sample. This constrained sample size holds implications for the statistical robustness of our findings. Meanwhile, there is an increased potential for type II error, whereby genuine differences or effects might be overlooked due to limited power. Moreover, the small sample might reduce the generalizability of our results. Fourthly, the trial exclusively included patients with single-level L5–S1 LDH, so the conclusions may not extend to patients with multilevel or other types of disc herniation. Despite the limitations, our study demonstrates several strengths, including the meticulous randomization and allocation concealment methods employed, which effectively minimized the risk of bias. It is important to note that this study focused on the initial outcomes of the first RCTs.
In our future research, we intend to expand the patient cohort to perform a more comprehensive comparison of the impacts on muscle damage and clinical outcomes between these 2 minimally invasive surgical techniques. Long-term follow-up, critical for evaluating potential differences in reoperation rates between the procedures, will be emphasized. We also plan to conduct multicenter RCTs to collect broader, longer-term follow-up data. Simultaneously, we acknowledge the necessity of further controlling for confounding factors to investigate the impact of IELD on postoperative clinical outcomes more accurately. We aim to incorporate health-related quality of life questionnaires, such as EuroQoL-5 dimension or SF-36 (36-item Short Form health survey, to gain deeper insights into patients’ health status and mental functional capacity following spine surgery). Additionally, forthcoming studies should include an economic evaluation aspect to determine the comparative cost-effectiveness of these techniques. This information will provide healthcare policymakers with the necessary data to make informed decisions about the optimal surgical approach for treating L5–S1 LDH.
This RCT substantiated that the IELD and MLD yielded comparable enhancements in pain intensity, disability index, and radiological assessments. Collectively, these findings position the IELD as a potential effective alternative to MLD in addressing L5–S1 central or paracentral LDH. However, given the preliminary nature of these outcomes, it is imperative to conduct large sample, multicenter RCT with prolonged follow-up periods to solidify this evidence.
NOTES
Conflict of Interest
JSK, the corresponding author, is a consultant of Richard Wolf, GmbH, and Elliquence, LLC. The other authors have no conflicts of interest to declare.
ACKNOWLEDGEMENTS
The authors express a special acknowledgment to Cho Rong Lee, BS, and Eun Kim, BS, for preparing the data and manuscript.
Fig. 1.
Two distinct minimally invasive spine surgery techniques are depicted. (A) Microscopic surgery being performed by 2 surgeons. (B) A surgeon’s intraoperative view of the ligamentum flavum. (C) Full-endoscopic surgery can be manipulated by a single surgeon. (D) Exposure of the neural structure under real-time visualization, provided by the fullendoscopic real-time magnification vision system.

Fig. 2.
Flowchart of study eligibility and enrolment at each time point. ITT, intention-to-treat; PP, per-protocol.

Fig. 3.
A graph shows the clinical outcome trend of VAS-back, VAS-leg, and ODI scores. VAS, visual analogue scale; ODI, Oswestry Disability Index; MLD, microscopic lumbar discectomy; IELD, interlaminar endoscopic lumbar discectomy; ITT, intention-to-treat; PP, per-protocol.
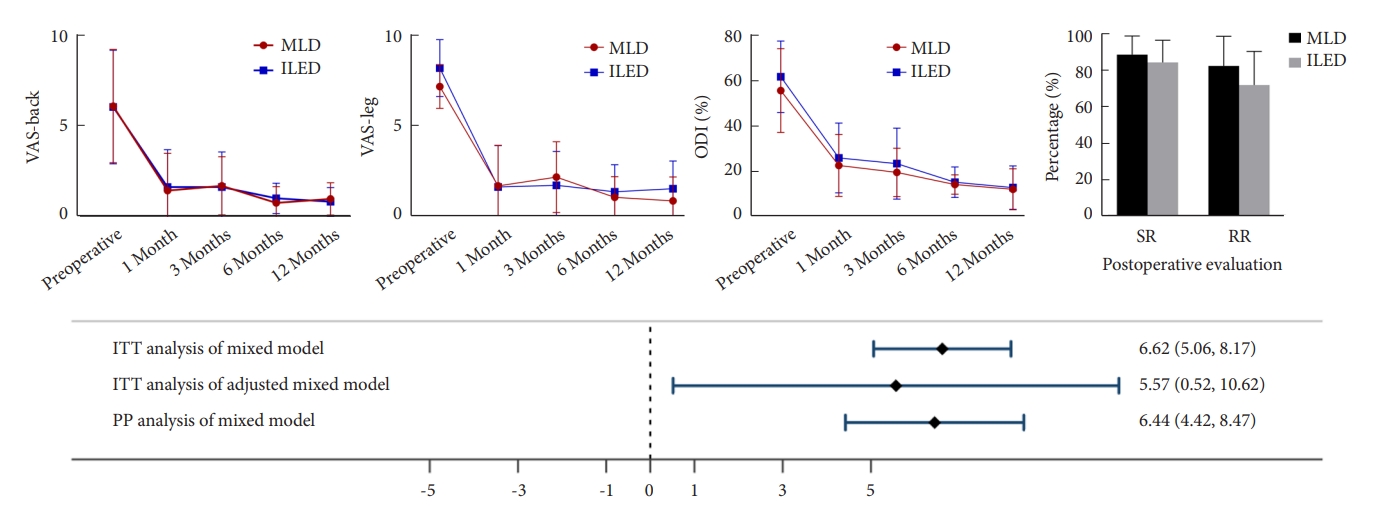
Fig. 4.
A 38-year-old male patient presented with left buttock pain and left leg radiating pain. (A) The patient’s lumbar plain radiographs show suspected left short-segmented postural scoliosis of the lumbosacral junction. (B) The lateral image shows the curvature of the spine appears normal without any signs of subluxation or misalignment. (C, D) The lateral, and axial magnetic resonance imaging (MRI) study confirmed the large left-sided paracentral disc herniation of the L5–S1 level, compressing the left S1 nerve root. (E) Following microscopic lumbar discectomy procedure, the postoperative lumbar plain radiographs demonstrate the abrupt decrease degree of the scoliosis. (F) The lateral image shows the properly alignment. (G) Postoperative lateral MRI reveal the well decompressed. (H) An increased cross-sectional area of the dural sac from axial image. The red dashed line indicates the preoperative lesion area, and the green dashed line area indicates successful decompression of the lesion area postoperatively.
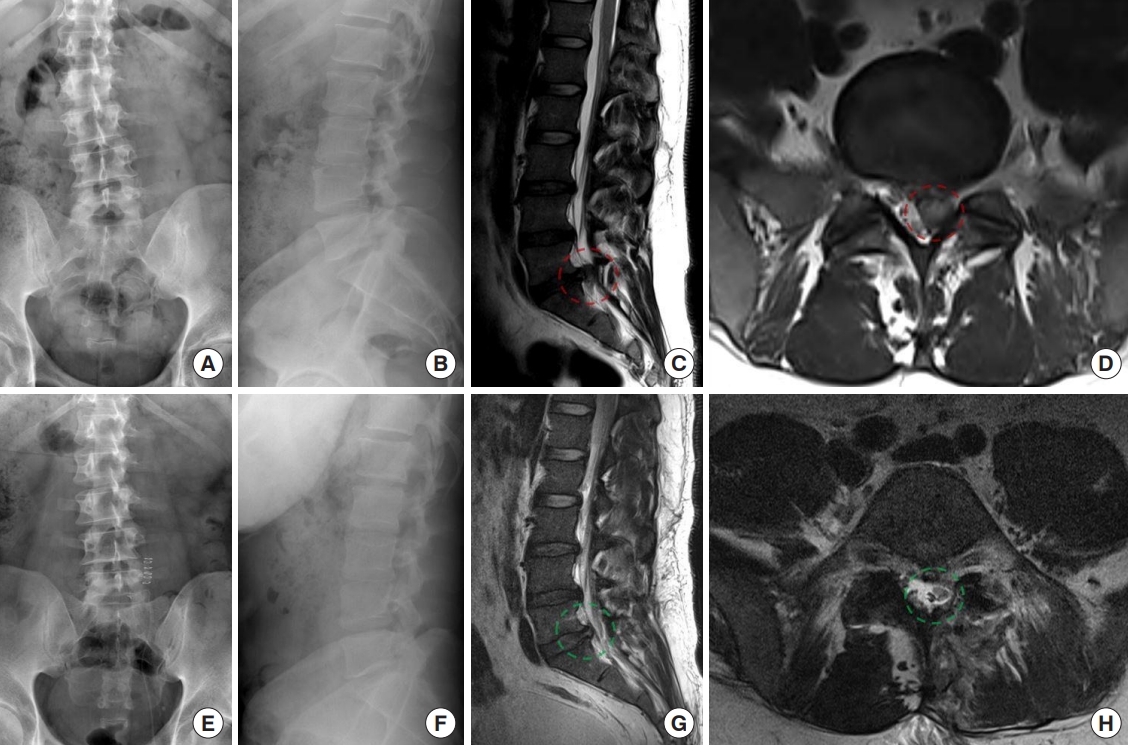
Fig. 5.
A 26-year-old male patient experienced lower back pain along with right buttock and right posterior leg radiating pain. (A) Preoperative plain radiographs of his lumbar spine revealed the loss of lumbar lordosis. (B) The lateral image reveals a reduced curvature in the lumbar lordosis. (C, D) The lateral, and axial magnetic resonance imaging (MRI) study confirmed the diagnosis of L5–S1 disc herniation from the right-sided paracentral to central area. After undergoing an interlaminar endoscopic lumbar discectomy procedure. Post-operative radiographs in the anteroposrerior (E) and lateral view (F) reveal that the disc height was still relatively maintain, the lumbar lordotic curvature had been restored. (G) Postoperative MRI imaging demonstrated the alleviation of dural, and (H) nerve root compression. The red dashed line indicates the preoperative lesion area, and the green dashed line area indicates successful decompression of the lesion area postoperatively.
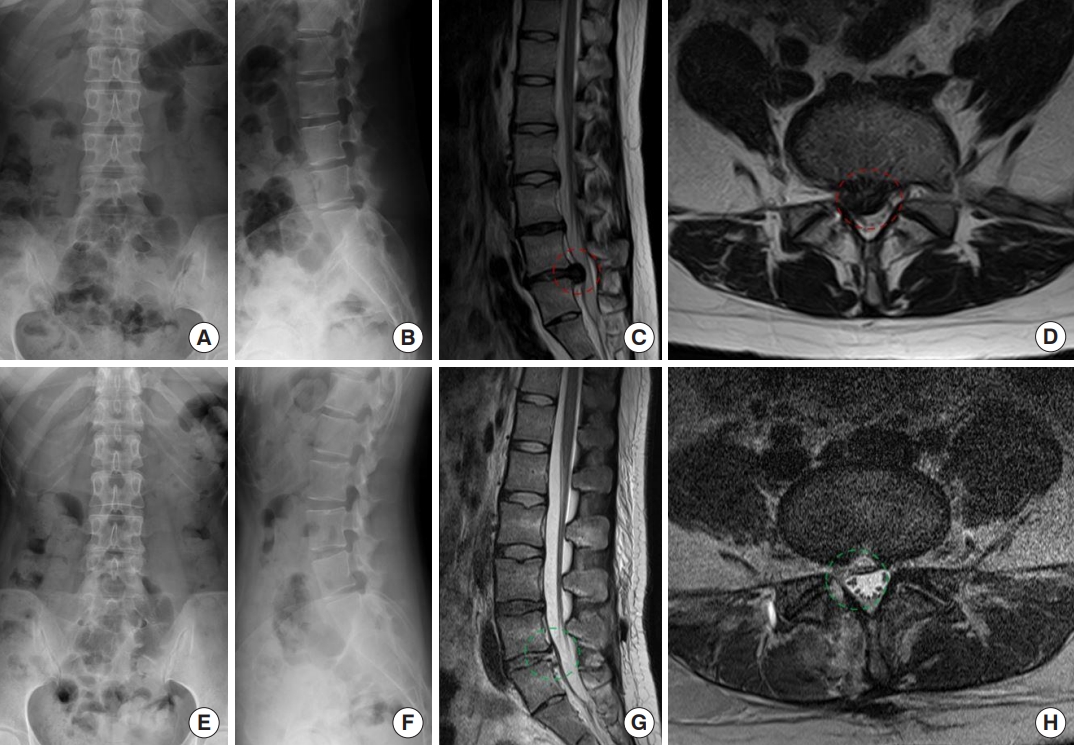
Table 1.
Baseline demographic and clinical characteristics of the participants by treatment group
| Variable | MLD (n = 15) | IELD (n = 13) | p-value |
|---|---|---|---|
| Age (yr) | 47.67 ± 14.30 | 52.08 ± 16.75 | 0.472 |
| Sex, female:male | 7:8 | 8:5 | 0.476 |
| BMI (kg/m2) | 25.16 ± 4.94 | 23.26 ± 1.98 | 0.219 |
| Predominant side (left) | 10 | 9 | 1.000† |
| Pathology location | |||
| Central:subarticular:both | 0:7:8 | 2:7:4 | 0.218 |
| Operation history, yes | 8 | 6 | 1.000† |
| VAS-back | 6.13 ± 3.12 | 6.08 ± 3.13 | 0.939 |
| VAS-leg | 7.13 ± 1.200 | 8.15 ± 1.56 | 0.070 |
| ODI | 55.43 ± 18.44 | 61.51 ± 15.76 | 0.194 |
| ASA PS classification grade | 1.000† | ||
| I/II | 14 | 13 | |
| III/IV | 1 | 0 | |
| Disc herniation | |||
| Side, right:left | 5:10 | 5:8 | 1.000† |
| Central:paracentral | 5:10 | 7:6 | 0.445 |
| Migration, superior:inferior | 1:7 | 2:5 | 1.000† |
| Length of stay (day) | 5.47 ± 1.36 | 3.69 ± 1.60 | 0.003* |
| Operation time (min) | 95.53 ± 18.91 | 134.77 ± 33.12 | 0.001* |
| Blood loss (mL) | 44 ± 26.67 | 20 ± 20.99 | 0.009* |
Table 2.
Comparison of primary outcomes following postsurgical treatment interventions
| Variable |
Postoperative 1 month |
Postoperative 3 months |
Postoperative 6 months |
Postoperative 12 months |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MLD | IELD | df (95% CI) | MLD | IELD | df (95% CI) | MLD | IELD | df (95% CI) | MLD | IELD | df (95% CI) | |
| VAS-back | 1.48 ± 2.05 | 1.67 ± 2.06 | -0.56 (-3.26 to 2.15) | 1.74 ± 1.60 | 1.67 ± 1.93 | -0.07 (-2.72 to 2.58) | 0.80 ± 0.90 | 1.05 ± 0.83 | -2.06 (-4.43 to 0.31) | 1.02 ± 0.89 | 0.87 ± 0.77 | 0.08 (-2.25 to 2.42) |
| t = -0.40 | p = 0.69 | t = -0.05 | p = 0.96 | t = -1.70 | p = 0.09 | t = 0.07 | p = 0.94 | |||||
| VAS-leg | 1.69 ± 2.23 | 1.63 ± 2.27 | -1.97 (-4.12 to 0.18) | 2.17 ± 1.94 | 1.72 ± 1.86 | -0.19 (-2.04 to 1.64) | 1.06 ± 1.14 | 1.37 ± 1.48 | -0.64 (-2.41 to 1.12) | 0.87 ± 1.31 | 1.54 ± 1.52 | -0.71 (-2.54 to 1.12) |
| t = -1.80 | p = 0.07 | t = -0.21 | p = 0.83 | t = -0.71 | p = 0.48 | t = -0.76 | p = 0.45 | |||||
| ODI | 22.44 ± 13.63 | 25.77 ± 15.38 | 2.53 (-7.19 to 12.25) | 19.38 ± 10.64 | 23.25 ± 15.66 | 3.58 (-6.22 to 13.38) | 14.08 ± 4.26 | 15.05 ± 6.73* | 7.98 (0.37 to 15.60) | 11.96 ± 9.04 | 12.71 ± 9.55 | 8.48 (-1.67 to 18.63) |
| t = 0.51 | p = 0.61 | t = 0.72 | p = 0.47 | t = 2.07 | p = 0.04 | t = 1.66 | p = 0.10 | |||||
Table 3.
Comparison of secondary outcomes following postsurgical treatment interventions
REFERENCES
1. Amin RM, Andrade NS, Neuman BJ. Lumbar disc herniation. Curr Rev Musculoskelet Med 2017;10:507-16.




2. El-Rich M, Aubin CE, Villemure I, et al. A biomechanical study of L5–S1 low-grade isthmic spondylolisthesis using a personalized finite element model. Stud Health Technol Inform 2006;123:431-4.

3. Lee BS, Walsh KM, Healy AT, et al. Biomechanics of L5/S1 in long thoracolumbosacral constructs: a cadaveric study. Global Spine J 2018;8:607-14.




4. Kuhns CA, Bridwell KH, Lenke LG, et al. Thoracolumbar deformity arthrodesis stopping at L5: fate of the L5–S1 disc, minimum 5-year follow-up. Spine (Phila Pa 1976) 2007;32:2771-6.

5. Gugliotta M, da Costa BR, Dabis E, et al. Surgical versus conservative treatment for lumbar disc herniation: a prospective cohort study. BMJ Open 2016;6:e012938.



6. Kanno H, Aizawa T, Hahimoto K, et al. Minimally invasive discectomy for lumbar disc herniation: current concepts, surgical techniques, and outcomes. Int Orthop 2019;43:917-22.



7. Yeung AT, Yeung CA. Minimally invasive techniques for the management of lumbar disc herniation. Orthop Clin North Am 2007;38:363-72. abstract vi.


8. Chen KT, Jabri H, Lokanath YK, et al. The evolution of interlaminar endoscopic spine surgery. J Spine Surg 2020;6:502-12.



9. Dasenbrock HH, Juraschek SP, Schultz LR, et al. The efficacy of minimally invasive discectomy compared with open discectomy: a meta-analysis of prospective randomized controlled trials. J Neurosurg Spine 2012;16:452-62.



10. Akinduro OO, Kerezoudis P, Alvi MA, et al. Open versus minimally invasive surgery for extraforaminal lumbar disk herniation: a systematic review and meta-analysis. World Neurosurg 2017;108:924-38. e3.


11. Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine (Phila Pa 1976) 2002;27:722-31.

12. Mayer HM, Brock M. Percutaneous endoscopic discectomy: surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg 1993;78:216-25.


13. Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:931-9.

14. Liu Y, Kotheeranurak V, Quillo-Olvera J, et al. A 30-year worldwide research productivity of scientific publication in fullendoscopic decompression spine surgery: quantitative and qualitative analysis. Neurospine 2023;20:374-89.




15. Yang Z, Wang H, Li W, et al. Comparative effects and safety of full-endoscopic versus microscopic spinal decompression for lumbar spinal stenosis: a meta-analysis and statistical power analysis of 6 randomized controlled trials. Neurospine 2022;19:996-1005.




16. Xu DR, Luan LR, Ma XX, et al. Comparison of electromagnetic and optical navigation assisted Endo-TLIF in the treatment of lumbar spondylolisthesis. BMC Musculoskeletal Disorders 2022;23:522.




17. Kim HS, Wu PH, Kim JY, et al. Retrospective case control study: clinical and computer tomographic fusion and subsidence evaluation for single level uniportal endoscopic posterolateral approach transforaminal lumbar interbody fusion versus microscopic minimally invasive transforaminal interbody fusion. Global Spine Journal 2023;13:304-15.




18. Gadjradj PS, Rubinstein SM, Peul WC, et al. Full endoscopic versus open discectomy for sciatica: randomised controlled non-inferiority trial. BMJ 2022;376:e065846.



19. Hsu HT, Chang SJ, Yang SS, et al. Learning curve of full-endoscopic lumbar discectomy. Eur Spine J 2013;22:727-33.




20. Son S, Ahn Y, Lee SG, et al. Learning curve of percutaneous endoscopic interlaminar lumbar discectomy versus open lumbar microdiscectomy at the L5–S1 level. PLoS One 2020;15:e0236296.



21. Hasan S, Härtl R, Hofstetter CP. The benefit zone of full-endoscopic spine surgery. J Spine Surg 2019;5(Suppl 1):S41-56.



22. Zaina F, Tomkins-Lane C, Carragee E, et al. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst Rev 2016;2016:CD010264.



23. Katz JN, Zimmerman ZE, Mass H, et al. Diagnosis and management of lumbar spinal stenosis: a review. JAMA 2022;327:1688-99.


24. Schneider MJ, Ammendolia C, Murphy DR, et al. Comparative clinical effectiveness of nonsurgical treatment methods in patients with lumbar spinal stenosis: a randomized clinical trial. JAMA Netw Open 2019;2:e186828.



25. Benoist M, Boulu P, Hayem G. Epidural steroid injections in the management of low-back pain with radiculopathy: an update of their efficacy and safety. Eur Spine J 2012;21:204-13.




26. Osterman H, Seitsalo S, Karppinen J, et al. Effectiveness of microdiscectomy for lumbar disc herniation: a randomized controlled trial with 2 years of follow-up. Spine (Phila Pa 1976) 2006;31:2409-14.

27. Khandge AV, Kim JS. Modified interlaminar endoscopic lumbar discectomy for highly upmigrated disc herniation: a proctorship description of the technique via translaminar route. Neurospine 2020;17(Suppl 1):S66-73.




28. Choi G, Lee SH, Raiturker PP, et al. Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5–S1 using a rigid working channel endoscope. Neurosurgery 2006;58(1 Suppl):ONS59-68. discussion ONS59-68.



30. Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271-3.

31. Ruetten S, Komp M, Merk H, et al. Use of newly developed instruments and endoscopes: full-endoscopic resection of lumbar disc herniations via the interlaminar and lateral transforaminal approach. J Neurosurg Spine 2007;6:521-30.


32. Mansournia MA, Higgins JP, Sterne JA, et al. Biases in randomized trials: a conversation between trialists and epidemiologists. Epidemiology 2017;28:54-9.


33. Yang F, Ren L, Ye Q, et al. Endoscopic and microscopic interlaminar discectomy for the treatment of far-migrated lumbar disc herniation: a retrospective study with a 24-month follow-up. J Pain Res 2021;14:1593-600.




34. Yang CC, Chen CM, Lin MH, et al. Complications of fullendoscopic lumbar discectomy versus open lumbar microdiscectomy: a systematic review and meta-analysis. World Neurosurg 2022;168:333-48.


36. Simpson AK, Lightsey HM 4th, Xiong GX, et al. Spinal endoscopy: evidence, techniques, global trends, and future projections. Spine J 2022;22:64-74.


37. Bae J, Kim J, Lee SH, et al. Comparative analysis of transforaminal endoscopic thoracic discectomy and microscopic discectomy for symptomatic thoracic disc herniation. Neurospine 2022;19:555-62.




38. Kwon H, Park JY. The role and future of endoscopic spine surgery: a narrative review. Neurospine 2023;20:43-55.




39. Fujita M, Inui T, Oshima Y, et al. Comparison of the outcomes of microendoscopic discectomy versus full-endoscopic discectomy for the treatment of l4/5 lumbar disc herniation. Global Spine Journal 2022 Sep 22:21925682221127997doi: 10.1177/21925682221127997. [Epub].



40. Jitpakdee K, Liu YT, Kotheeranurak V, et al. Transforaminal versus interlaminar endoscopic lumbar discectomy for lumbar disc herniation: a systematic review and meta-analysis. Global Spine J 2023;13:575-87.




41. Shimada N, Igarashi T, Murai K, et al. Adhesions in the epidural space caused by frequent epidural blocks. JA Clin Rep 2017;3:57.




42. Sasauchi K, Sunada K, Nakamura T. Long-term evaluation of continuous epidural anesthesia in an improved canine model. Anesth Pain Med 2016;6:e35458.



43. Igarashi T, Hirabayashi Y, Shimizu R, et al. Inflammatory changes after extradural anaesthesia may affect the spread of local anaesthetic within the extradural space. Br J Anaesth 1996;77:347-51.


44. Li WS, Yan Q, Cong L. Comparison of endoscopic discectomy versus non-endoscopic discectomy for symptomatic lumbar disc herniation: a systematic review and meta-analysis. Global Spine J 2022;12:1012-26.



45. Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ 2012;344:d7762.


46. Higgins JPT, Savović J, Page MJ, et al. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, . In: Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023) Cochrane. 2023;Available from: www.training.cochrane.org/handbook.




























