A Narrative Review of Development of Full-Endoscopic Lumbar Spine Surgery
Article information
Abstract
In the first phase of development of lumbar endoscopic spine surgery, the focus was on removal of soft disc material through the working corridor of Kambin’s triangle using transforaminal endoscopic lumbar discectomy. With the introduction of the interlaminar approach and increased interest from both industry and surgeons, there has been an exponential development of endoscopic surgical equipment and a corresponding expansion of endoscopic techniques. Endoscopic treatment strategies are applied to conditions ranging from contained prolapsed intervertebral discs to noncontained migrated herniated discs, hard calcified discs, spinal stenosis in the central or lateral recess and the foraminal and extraforaminal region, and other combinations of degenerative conditions requiring decompression or fusion surgery. The further expansion of endoscopic surgical management involving complicated spinal cases and the final quartet of trauma, infections, tumors, and possibly deformities could be the future stage of endoscopic spine surgery development. This article covers the full range of current treatment strategies and presents possible future developments of endoscopic spine surgery for the management of lumbar spinal conditions.
INTRODUCTION
The aim of endoscopic spine surgery is to provide safe, direct, and targeted access to the compressive pathology with minimal soft tissue trauma while performing decompression and/or fusion. Three key components help achieve this objective: (1) an endoscope that provides a clear and magnified visualization of the region close to the pathology [1]; (2) a working channel within the endoscope with customized endoscopic instruments to deliver energy for coagulation, a laser to dissect soft tissue, and tools to resect as well as to retrieve loose fragments from the operative field [2,3]; and (3) a constant inflow and outflow irrigation system that allows clearance of debris and clarity of visualization [4].
Endoscopic surgeons often work in well-defined anatomical corridors that provide a safe working zone for equipment to pass through to achieve targeted decompression and/or fusion. In the early phase of endoscopic spine surgery, transforaminal endoscopic lumbar discectomy (TELD) achieved soft tissue decompression in the target working zone of Kambin’s triangle [5]. Advances in endoscopic equipment, such as endoscopic drills and customized forceps through the working channel of the endoscope, improved the optical system and provided the foundation for the development of other endoscopic spine surgical techniques [6,7]. The range of conditions that can be treated by endoscopic spine surgery has expanded. We describe common current indications for lumbar endoscopic surgery and explain the recent expansion of conditions being treated by endoscopic spine surgery that were relative contraindications in the past; then, we present perspectives regarding possible future developments in spinal endoscopy.
TRANSFORAMINAL APPROACH: TRANSFORAMINAL ENDOSCOPIC LUMBAR DISCECTOMY
1. Brief Historical Overview
The first technique described for endoscopic spine surgery is TELD (Fig. 1) [8]. Hijikata and Kambin separately introduced percutaneous nucleotomy [9] and Kambin further described the safe triangular zone for docking and working on the transforaminal region [5]. Various techniques exploring this safe working zone were described, which can be largely summarized as the inside-out, outside-in with foraminoplasty, and mobile outsidein techniques [10-12]. Additional techniques for exploring space around the region involved partial resection of the pedicle and facet complex [13] or resection directly through the pedicle [14,15] to allow wider exploration of the spinal canal adjacent to the disc in cases of migrated disc herniation [16,17].

Revision right L4/5 transforaminal endoscopic lumbar discectomy (TELD). A 28-year-old woman who had previous L4/5 left mini-open microscopic discectomy presented with recurrence of central L4/5 prolapsed disc with right extensor hallucis longus weakness of motor grade 3. The decision was made to perform right TELD L4/5 with the mobile outside-in technique, and postoperatively the patient’s extensor hallucis longus strength recovered to motor grade 5. Panel A shows a sagittal view of a large sequestrated downward migrated central disc at L4/5. Panel B shows the corresponding cut demonstrating removal of the sequestrated disc. Panel C shows an axial cut at L4/5; a large centrally located L4/5 prolapsed disc is seen causing compression in the central and lateral recess of neural elements. Yellow arrow in panel C showed previous laminotomy in the left L4 lamina. Panel D shows the corresponding axial cut demonstrating removal of the sequestrated disc. Panel E shows the entry point of the needle and its docking; the mobile outside-in method was used, with the paraspinal skin entry point along the center of the disc space using the manual back assessment method. In this method, the borderline is checked between the back muscles and the abdominal muscles. The skin entry points are marked just medial to this borderline at the mid-disc level in both anteroposterior and lateral x-rays. Panel F shows an intraoperative view of decompression after complete discectomy; the epidural space is well decompressed with a pulsating traversing nerve root under irrigation fluid pressure.
2. Common Current Indications of TELD
TELD is an option for treating contained or low-grade migration of prolapsed intervertebral discs in the L1–5 region. This technique could be used for disc herniation in the central, paracentral, and foraminal regions [18,19]. The patient typically presents with radicular pain for which conservative management has failed with concordant magnetic resonance imaging. The targeted lesion evolved over time, from indirect central nucleotomy [20] to discectomy to selective fragmentectomy [21].
According to early descriptions, the inside-out technique achieved good results in patients with noncontained intracanal discs [19,22]. Fluoroscopic-guided foraminoplasty was later introduced to allow a more horizontal angulation of the working channel to enable the surgeon to retrieve more central fragments [23]. Foraminoplasty increases the foramen diameter at 3 points closely related to the exiting nerve root: the lower endplate of the superior vertebra, the disk, and the upper endplate of the inferior vertebra [24]. This increase in foramen size reduces the risk of exiting nerve root injuries caused by working channel compression, which can occur in a narrow foramen. Foraminoplasty allows good foraminal decompression in patients with neurogenic claudication symptoms and has been shown to have good long-term results [25]. In cases with a high iliac crest reaching the midpedicle of L4, there is a higher likelihood that foraminoplasty will be required to achieve the targeted disc removal for L4/5 and L5/S1 [26]. Despite the popularity of TELD, more cases of L5/S1 discs with a high iliac crest are done with the interlaminar approach.
In addition to discectomy, the transforaminal approach has been utilized in other spinal canal pathologies with the expansion of its applications in spinal endoscopy.
3. Expansion of the Indications of Transforaminal Endoscopic Techniques
1) Highly migrated disc herniation
With modifications of the transforaminal approach, such as the mobile outside-in technique [12], the transpedicular approach [14], the foraminoplastic superior vertebral notch approach [27], and partial resection of the pedicle and facet complex [13], other expanded applications for more complex discectomy procedures have been described [12,14,28-31]. Controlled reaming of the superior vertebral notch or part of the pedicle did not seem to affect spinal stability and outcomes [24]. In fact, in mechanical study, the transforaminal approach produced wider decompression with less effect on stability than posterior foraminal decompression [32]. In high-grade upward-migration disc herniation, the disc fragment has migrated beyond the height of the disc, often approaching the inferior or medial aspect of the cephalad pedicle [31]. It is a challenge to gain access to the disc medial to the cephalad vertebra pedicle, as the exiting nerve root obstructs the vision and cannulation of the working cannula. Hence, direct reaming of the pedicle under fluoroscopy, or directly visualized endoscopic drilling, would be useful for accessing these highly migrated discs medial to the cephalad pedicle [17]. Transpedicular lumbar endoscopy has been performed with good clinical results for highly migrated disc herniations [15]. With the development of better equipment such as angulated drills, side-firing lasers, and flexible forceps, many cases of highly migrated disc herniation for which endoscopic discectomy was previously contraindicated could now be considered for TELD [33].
2) Calcified disc, high canal compromise, and cauda equina syndrome
Yu et al. [34] used an endoscopic osteotome to remove calcified herniations with TELD, achieving good results with no major complications, although seven of the 25 patients had postoperative dysesthesia. Previously, Lee et al. [35] described a high failure rate of endoscopy in high canal-compromised patients. However, more recently, TELD achieved good outcomes for large prolapsed discs with high canal compromise [36,37]. Nonetheless, careful consideration must be made in patients with neurological deficit and cauda equina syndrome, as failure secondary to disc retention can lead to delay in neurological recovery.
3) Recurrent disc herniation
Recurrent disc herniation after conventional open or endoscopic discectomy presents a clinical challenge (Fig. 1). The scarring of soft tissue could lead to difficulties in endoscopic manoeuvres and dissection. Dural adhesion could lead to higher risk of dura tearing, and additional bone resection might affect the stability of the spinal segment. Ruetten et al. [38] compared the clinical results of endoscopy and microscopic surgery for recurrent disc herniation, and showed that endoscopy was superior in terms of rehabilitation, complications, and minimizing soft tissue trauma. Ahn et al. [39] showed significant improvements in clinical outcomes, especially in patients younger than 40 years old, patients with a duration of symptoms of less than 3 months, and patients without lateral recess stenosis. Furthermore, Li et al. [40] showed that endoscopic lumbar discectomy achieved better outcomes than open discectomy in terms of operative time, blood loss, the complication rate, the MacNab criteria, and pain reduction.
4) Spinal stenosis
Disc herniations with concurrent spinal stenosis were a challenge for TELD due to the increased risk of incomplete symptom resolution. Despite this difficulty, some selected patients with unilateral asymmetrical lateral recess stenosis and concurrent disc herniation were treated with TELD. Through an extreme lateral transforaminal approach with foraminoplasty, it was possible to perform partial upper pediculotomy of the lower vertebra pedicle, removing the lateral ligamentum flavum covering lateral portion and even the dorsal portion of the traversing nerve root [41]. Ahn et al. [42] reported good clinical outcomes at 2 years using this technique. Chen et al. [43] showed similar positive clinical results of transforaminal lumbar lateral recess decompression in elderly patients (70–93 years old). Foraminoplasty targeted to the base rather than the tip of superior articular process was useful in decreasing neural dysfunction and reducing operative time in patients with lateral recess stenosis with concomitant disc herniation [44]. Li et al. [45] conducted a retrospective study with a direct comparison between interlaminar and transforaminal approach to decompress lateral recess stenosis and concluded that the transforaminal group had a longer operation time and more radiation exposure. Interestingly, there was no significant difference in clinical outcomes between the 2 groups.
5) Less common indications of transforaminal endoscopic techniques
Other less common applications of TELD have been described for the resection of facet cysts, discal cysts, and tumors. Discal cyst is associated with previous discectomy and/or spontaneous developments secondary to defects in the intervertebral disc with degeneration. TELD was described for the removal of this condition, which is included in the differential diagnosis of herniated intervertebral disc, since it has very similar clinical features [46,47]. Facet cyst is a common clinical entity that is traditionally decompressed via the posterior approach. However, selected patients with a facet cyst causing lateral recess stenosis can be treated with TELD with good clinical results, sparing excessive soft tissue injuries, reducing facet damage, and conserving spinal stability as compared to open posterior decompression [48-50]. Tumor debulking using the transforaminal approach has been described sparingly in the literature. The main application is for palliative relief of symptoms in patients who had a pathological tissue sample obtained previously with a primary tumor identified. Transforaminal endoscopic tumor decompression under local anesthesia is ideal for patients with a low life expectancy. This treatment strategy can prevent a prolonged hospital stay in a patient whose life span is already compromised by cancer [51]. Reports of transforaminal endoscopic debulking of both thoracic and lumbar metastatic tumors have presented positive results [51,52]. The literature on expanded applications of transforaminal approaches on facet cysts, discal cysts, and tumors mainly comprises small retrospective case series by endoscopic experts; hence, the effects of broad application of the transforaminal approach on these conditions need further evaluation.
6) Expansion of indications of interbody fusion: transforaminal interbody fusion through the Trans-Kambin route
As endoscopic techniques with the transforaminal approach evolved, their applications expanded to include interbody fusion. The advantages of this approach are that it preserves soft tissue and the facet, and that it can be performed under local anesthesia with monitored sedation, which is particularly helpful in patients who are unfit for general anesthesia [53]. This technique is a treatment option for patients who present with spinal instability and concurrent significant disc height collapse, causing foraminal stenosis [53,54]. However, as there are a limited safety window and working corridor in this approach, an expandable spacer is often required. In selected groups of patients, this technique yielded good outcomes [53,55]. There are significant concerns about transient neurologic complications and subsidence of the interbody cage, with a reported rate of 20%–30% [53,54,56]. Trans-Kambin transforaminal endoscopic fusion in extremely collapsed discs (> 50% decrease in disc height) using standalone cages had poor results in one study, with a significant rate of subsidence and revision [54]. However, another study showed although there was subsidence in most of the standalone cases, the clinical outcomes remained good [57]. Although the data on this technique appears promising, the lack of long-term fusion data coupled with a significant risk of postoperative dysesthesia and cage subsidence suggests that more investigations are required before wide adoption of this technique.
INTERLAMINAR APPROACH
1. Brief Historical Overview
The development of interlaminar endoscopy is by no means an accident. Instead, it is a by-product of conscientious efforts to continue to improve visualization, drawing upon the benefits of constant irrigation and the development of endoscopic equipment, such as endoscopic high-speed drills, rongeurs, forceps, and working cannulas. The interlaminar technique was applied by Ruetten et al. [58,59] in early series of interlaminar decompression. The initial applications were disc herniation, and the indications of this technique later expanded to stenosis in the central and lateral recess, as well as foraminal stenosis [60,61]. In particular, interlaminar endoscopic lumbar discectomy (IELD) for disc herniation at L5/S1 is gaining popularity as it can overcome the issues faced by the transforaminal approach, such as a high iliac crest, which often obstructs safe passage to the L5/S1 foramen, especially in patients with decreased foraminal height at L5/S1 [62]. The wide interlaminar window at L5/S1 has the benefit of decreasing the requirement for facet resection if needed for discectomy [63]. Variation exists in the nomenclature for the interlaminar approach to perform decompression in the literature; however, the most up-to-date and widely adopted names established by the AO minimally invasive task group for the endoscopic interlaminar approach involve a subclassification into (1) IELD, (2) interlaminar endoscopic lateral recess decompression, and (3) lumbar endoscopic unilateral laminotomy for bilateral decompression [64-66]. The endoscopic interlaminar approach has since become a popular surgical technique for discectomy and stenosis decompression.
2. Current and Previous Indications
1) Prolapsed intervertebral disc
The transforaminal approach to L5/S1 is limited by the high iliac crest, hypertrophy of the L5/S1 facet, and the naturally narrower L5/S1 foramen. IELD for disc herniation at L5/S1 is gaining popularity for both contained and non-contained disc herniations and high-migration disc cases [64,67]. Endoscopic lumbar discectomy has generally shown to improve health-related quality of life [68]. In a systematic review and meta-analysis of IELD in comparison with TELD, Chen et al. [69] showed that IELD and TELD are equally safe and effective for treating L5/S1 lumbar disc herniations, with IELD being superior in terms of radiation exposure and operative time. Similar positive findings in terms of clinical results have been found for L3/4 and L4/5 discectomy [70,71]. The narrow laminar window in L1/2 and L2/3 is more challenging for the interlaminar approach.
There are controversies regarding how to handle the ligamentum flavum. While ligamentum cutting and full ligamentum flavectomy provide unparalleled visualization of the dura and herniated disc, these procedures involve resecting a protective layer for the dura, which may induce more scarring. Proponents of ligamentum flavum splitting have suggested that maximum preservation occurs when the ligamentum flavum is split and the working channel is advanced to keep the slit open [64]. Although this issue is controversial, low-level evidence suggests that there is no significant difference in clinical outcomes and complications between ligamentum resection and splitting [72]. Further conservation and restoration of the annulus after discectomy is attempted by annular sealing, which applies the principle of thermal-induced necrosis leading to tissue shrinkage in an attempt to seal any gapping annulus to prevent the recurrence of disc herniation [73]. In the L5/S1 region, there is a preference for the interlaminar approach (IELD) for axillary discs and downward-migrated discs over the transforaminal approach (TELD) [63].
A head-to-head comparison of the cost-effectiveness of four surgical techniques for lumbar disc herniation—microdiscectomy, TELD, IELD, and unilateral biportal endoscopic discectomy—showed comparable costs of uniportal and biportal endoscopic surgery, with microscopic surgery having significantly higher costs [74].
2) Spinal stenosis
An attractive attribute of interlaminar endoscopy is the familiarity of training in interlaminar anatomy as part of spine practice in both orthopedic and neurosurgery residencies. Open and tubular microscopic approaches to the lumbar spine are well taught as part of neurosurgical and orthopedic spine surgeon training [75]. Although endoscopic spine surgery has a steep learning curve, it certainly helps that surgeons are operating in an area with familiar surgical anatomy. The steep learning curve is evident as complications tend to happen in earlier cases [76]. As endoscopic surgeons perform more cases, there is a trend for less soft tissue and bone resection, as well as improved perioperative pain and satisfaction scores [77,78]. As one gets more experience with the interlaminar endoscopic approach, the operative time and complication rates decrease significantly, maximising the benefits of minimally invasive surgery [79]. A large retrospective study in 2018 showed that endoscopic-assisted decompression was significantly better in terms of the surgical site infection rate, postoperative hospital stay, delirium rate, and total complication rate [80]. These results echo earlier prospective randomized controlled trial findings with better clinical outcomes at 2 years [60]. Clinical results from several studies have shown non-inferiority to traditional open spine surgery and minimally invasive microscopic tubular surgery in terms of outcomes, with fewer adverse events and shorter hospital stays [81,82]. A recent meta-analysis of central and lateral recess interlaminar endoscopic decompression showed significant improvements in visual analogue scale scores for back and leg pain and the Oswestry Disability Index, with statistical improvement fulfilling the criteria of a minimal clinically important difference [61].
3. Expansion of Indications for Interlaminar Endoscopic Techniques
1) Highly migrated disc herniation and high canal compromise
The effectiveness of discectomy versus sequestrectomy is a controversial topic. Caspar and Loew launched the field of lumbar spinal discectomy by removing the herniated disc with curettage of the intervertebral disc [83]. This idea of curettage of the disc had an evolutional shift to the concept of sequestrectomy, which involves removing the sequestrated, migrated, and herniated disc fragments and was popularized by Williams [84] Selective sequestrectomy has narrow applications, but it is inherently advantageous in terms of preservation of the intervertebral disc and prevents subsequent spinal segment instability. The recurrence of discectomy was nevertheless reported to be around 1%–20% [85]. Annular sealing and reductions in annular defects may decrease the risk of recurrence [73]. The development of angled scopes and flexible forceps has enabled sequestrated disc removal in areas of the spinal canal that were previously hard to access, but are now possible through the interlaminar approach [86]. Overall, IELD obtained good results for highly migrated disc herniation even for less experienced surgeons due to the familiar anatomy, similar to that encountered in open surgery [67]. High canal compromise is a challenging clinical problem that is a relative contraindication for IELD [35]. However, selected cases of high canal compromise can be candidates for IELD provided that the disc is soft and that a large portion of the disc is lateral to the thecal sac. The surgeon needs to be gentle with the dissection and handling of neural tissue in such cases (Figs. 2, 3).
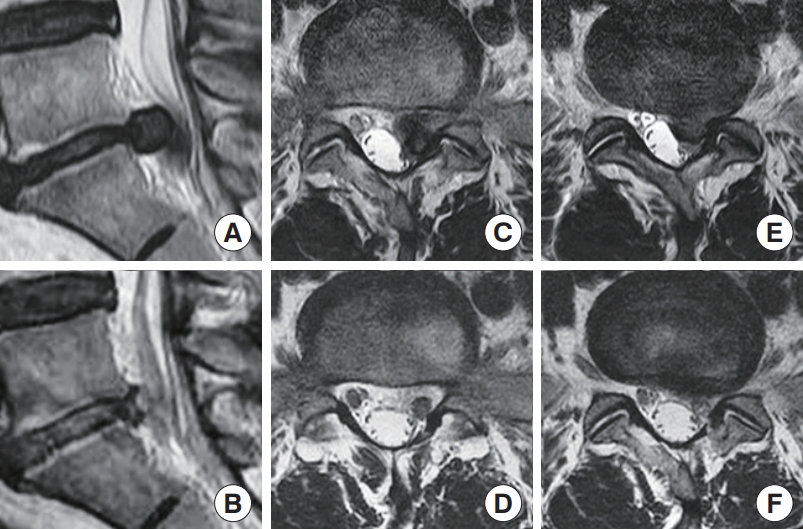
Left L4/5 prolapsed intervertebral disc with high canal compromise. A 30-year-old woman presented with sudden left extensor hallucis longus grade 3 weakness and radicular pain on the left L5 dermatome, and she underwent interlaminar endoscopic lumbar discectomy of left L4/5. Panel A shows a sagittal view of a large paracentral prolapsed intervertebral disc of L4/5 causing high canal compromise. Panel B shows the corresponding sagittal view with the decompressed L4/5 disc. Panels C–F are the corresponding preoperative and postoperative axial cuts of the left L4/5 prolapsed disc, showing effective discectomy of the large left L4/5 prolapsed disc.

Endoscopic interlaminar approach radiofrequency ablation and discectomy of the L5/S1 intervertebral disc space with axial buttock pain. Panel A shows dense adhesion and neovascularization around the disc space (grade 3 according to Kim and Wu’s classification of neovascularization and adhesion for probability of sinuvertebral and basivertebral neuropathy). Panel B shows the use of the working channel; the traversing nerve root was protected, out of harm’s way, and the adhered and neovascularized soft tissue was dissected, exposing the disc and lateral recess. Panel C shows radiofrequency ablation at the region around the ipsilateral superior S1 pedicle; Kim’s twitching occurred upon radiofrequency ablation, but subsided after the basivertebral nerve was ablated. Panel D shows radiofrequency ablation of the sinuvertebral nerve under the L5–S1 disc in a central location. Panel E shows exposure of the prolapsed disc with the working channel protecting the neural elements. Panel F shows discectomy performed with endoscopic forceps.
2) Recurrent disc herniation
Recurrent disc herniation is a challenging surgical scenario for both open and endoscopic spine surgery. A recent systematic review showed no significant difference in outcomes between revision discectomy and fusion [87]. A comparison of endoscopic versus open procedures showed that both transforaminal and interlaminar approaches resulted in shorter hospital stays, less blood loss, less operative time, and an earlier return to work in comparison to open surgery while maintaining similar visual analogue scale scores for back and leg pain as well as Oswestry Disability Index scores [77]. A further advantage of TELD as compared to IELD is that less scarring is encountered. Revision discectomy using TELD in patients with previous posterior open surgery is like primary discectomy, since usually no significant scarring is noted in the transforaminal route. In revision discectomy treated with IELD, one can encounter significant scar tissue; however, with the advantage of working channel protection and blunt dissection, exploration of scars around the nerve is not necessary in most cases, and one can safely explore scars around the facet joint and retract neural elements with the associated scar while still being able to explore and perform discectomy safely. Hence, the risk of incidental durotomy is reduced. Overall, endoscopic procedures preserve more soft tissue and have less scarring, as they are less traumatic than open surgery [40,88].
3) Concurrent decompression of lateral recess, foraminal, and extraforaminal lumbar spinal stenosis: interlaminar contralateral endoscopic lumbar foraminotomy
Foraminal and extraforaminal compression of the exiting nerve root is a difficult clinical challenge. The approach is further confounded by the fact that many cases of foraminal and extraforaminal compression are also associated with lateral recess stenosis, a condition that Wu et al. [89] termed as triple crush syndrome. The three areas of compression synergistically cause more symptoms in patients. Although transforaminal approaches have shown clinical success in both primary and revision procedures for foraminal stenosis [90], they have significant limitations in L5/S1 and to a certain extent in L4/5 cases with a high iliac crest. The steep angle required in extraforaminal compression, combined with the lower angulation required for foraminal decompression, makes the transforaminal approach less ideal in these scenarios [91]. Combining the paraspinal (extra-foraminal) and interlaminar approaches is a reasonable option, but this requires 2 operations from 2 separate approaches in the same session of regional or local anesthesia. Kim and Wu et al. described a technique of using a small working channel and a 30° endoscope to navigate within the interlaminar region safely and effectively with no-touch neural decompression, through working in the sublaminar region of the contralateral laminar decompression of the lateral recess, the foraminal region, and the extraforaminal region; essentially, in this procedure, the surgeon follows the exiting nerve root out the canal while safely decompressing any stenosis along the way (Fig. 4) [76,78,92].

Panel A shows interlaminar contralateral endoscopic lumbar foraminotomy of left L4/5 foraminal stenosis; the intraoperative image intensifier shows endoscopic forceps reaching beyond the foramen of the left L4/5 foramen. Panel B shows an intraoperative endoscopic view of the contralateral exiting nerve root (CENR), which was free and pulsating, with the superior articular process (SAP) being decompressed and the lateral foraminal disc being removed to facilitate foraminal decompression.
4) Expansion of interlaminar indications to interbody fusion: endoscopic posterolateral transforaminal lumbar interbody fusion
Unlike the uniportal transforaminal trans-Kambin facet-sparing approach in lumbar interbody fusion, which works within the Kambin triangle ventral to the facet joint, the uniportal posterolateral approach for transforaminal lumbar interbody fusion requires resection of the facet to gain access to the intervertebral disc space to perform interbody fusion (Fig. 5). Since the facet is resected, this procedure has the advantage of a larger corridor with a safe working region and less likelihood of exiting nerve root dysesthesia as compared to trans-Kambin transforaminal-approach interbody fusion. Kim et al. [93] described the uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion technique, which is an endoscopic modification of the surgical approach described by Harms [94] They safely resected the ipsilateral facet joint with an endoscopic drill under endoscopic vision, with the end plate prepared optimally under direct endoscopic vision. A large autografted titanium 3-dimensional-printed cage can be inserted under regional anesthesia while sedation is monitored safely. In the authors’ experience, having a larger working area allowed placement of a larger interbody cage and hence necessitated an expandable cage [95]. They demonstrated the technique in the treatment of a patient with grade 2 spondylolisthesis and instability [93]. There is little literature on posterolateral transforaminal endoscopic lumbar interbody fusion. More studies on this technique should be conducted to assess the safety and efficacy of this technique.

Left uniportal endoscopic transforaminal lumbar interbody fusion at L5/S1. Panel A shows a computed tomography (CT) scan of L5/S1 spondylolisthesis. Panel B shows an endoscopic view of endplate preparation, as direct visualization of the endplate is helpful to ensure optimal endplate preparation to prevent subsidence and/or pseudarthrosis. Panel C shows a special tubular guide used for protecting neural elements and the insertion of a bone graft and interbody cage. Panel D shows the insertion of a 3-dimensional-printed interbody cage packed with mixed autograft and allograft. Panel E shows an endoscopic view of a cage inserted with a free pulsating traversing nerve root. Panel F shows a postoperative CT scan with restoration of disc height and reduction of L5/S1 spondylolisthesis.
4. Paraspinal Endoscopic Approach
The paraspinal approach is popular for decompression of the exiting nerve root at the foramen and/or extraforaminal region. A steeper angle of approach means that there is less likelihood of an injury to the traversing nerve root and cauda equina, while preserving most of the facet integrity [96-98]. However, this approach has a steep learning curve. The main challenge of this approach is handling the dorsal root ganglion and its associated radicular artery. Injury to the radicular artery can pose a significant difficulty in the control of bleeding, leading to hematoma formation, which obscures the view of the endoscope and may require conversion to open surgery [99]. The current applications are foraminal and extraforaminal stenosis caused by disc herniation, foraminal osteophytes, and facet cysts [83,86-88].
5. Expansion of Indications for the Paraspinal Approach
The expanded applications of the paraspinal endoscopic approach now include far-out syndrome, where more lateral decompression is required to explore the nerve root exiting in the far lateral region. This approach is especially helpful in the L5/S1 region, where there is compression of the exiting nerve root between the transverse process of L5, the sacral ala, and/or the bony spur at the extraforaminal region [100,101].
CHALLENGES OF ENDOSCOPIC SPINE SURGERY
1. Intraoperative Endoscopic Complications With Endoscopy
1) Incidental durotomy
As surgeons expand the applications of endoscopic spine surgery in the lumbar region, an important consideration is the ability to handle endoscopic complications without conversion to open surgery. Incidental durotomy is a common complication in spine surgery that is likewise common in endoscopic spine surgery [102]. Patch-blocking dura repair has been commonly practiced in open spine surgery with equivalent results for small dura tears with no neural incarceration. This technique can also be applied in uniportal and biportal endoscopic surgery, using collagen fibrin patches such as Tachosil (Nycomed, Linz, Austria) [103]. Future developments of uniportal endoscopic equipment facilitating dura repair might eventually allow primary repair through a uniportal approach, decreasing the need for conversion to open surgery and hence giving confidence to endoscopic surgeons to advance their technique in more challenging revision cases.
2) Increased cerebrospinal fluid pressure and neurological dysfunction
Constant inflow of irrigation without proper outflow will cause fluid to accumulate in the confined spinal canal, which can lead to seizure, cerebral edema, and neurological dysfunction [104,105]. An in open spine surgery, careful placement of an epidural suction catheter after completion of an endoscopic spine procedure is essential to prevent the development of pseudohypoxic brain swelling [105]. This complication is less common in endoscopy performed under local or regional anesthesia, as the patient tends to feel neck pain as the cerebrospinal pressure builds up, which is a self-preserving mechanism to prevent further deterioration to the point of cerebral edema and seizure. Hence, it is important for endoscopic surgeons to be alert to patients’ reports of neck pain during the procedure, and perhaps to pause the procedure for a period of time to allow equilibration of the pressure. The surgeon should always seek to ensure a good inflow and outflow system, while maintaining irrigation pressure at an average of 25–30 mmHg [106].
3) Hematoma
Careful hemostasis before closure is a key aspect of preventing hematoma formation. The surgeon should pull out the working channel slowly, using the endoscope to directly visualize the soft tissue and to perform hemostasis along the way out. Although this is a minimally invasive procedure with relatively little soft tissue dissection, if bony drilling and decompression is performed, the surgeon can consider keeping a soft suction drain to drain irrigation fluids and blood for the first postoperative day.
SUMMARY OF CURRENT AND FUTURE EXPANSIONS OF INDICATIONS OF ENDOSCOPIC SPINE SURGERY
For any surgical technique development to be successful, six factors must interact with each other to generate positive synergy (Fig. 6). Currently, the achievements of endoscopic spine surgery are based on the fruitful work of the pioneers and early adopters of endoscopic surgery. Endoscopic training courses are being carried out on multiple continents and more endoscopic surgical fellowships are being offered by various institutions. Through the interactions of experts and academics interested in this topic, there has been a significant increase in the number of peer reviewed articles in the endoscopic spinal literature. This trend ensures an improved understanding of endoscopic anatomy and pathology, and provides a source for the evidence-based practice of endoscopic spine surgery. With the magnified field of the spinal canal, surgeons gain new insights into previously described conditions and make discoveries of new pathologies that might not have been understood before. Anesthesia techniques are concurrently being developed, with a focus on epidural and local anesthesia under sedation to complement the minimally invasive nature of these surgical procedures and to ensure a good perioperative experience for the patient. With the increasing popularity and adoption of endoscopic spine approaches as advanced equipmentdependent procedures, significant interest has emerged in industry for research and development to improve lens clarity, camera focus, lighting technologies, and the tissue-handling and coagulation properties of equipment, as well as slim, sturdy, and durable drills to ensure good ergonomics and safety for endoscopic procedures. Developments in navigation and robotics continue to be integrated with endoscopic spine procedures to provide a smoother learning curve with less radiation among budding endoscopic spine surgeons in the coming years. Such investments from industry may drive up the cost of the initial phase of endoscopic surgery. However, as more surgeons take up endoscopy, and the technique becomes more broadly accepted and applied, the costs may come down, which would increase the value-driven outcomes of endoscopic spine surgery. With optimism in endoscopic spine surgery, it is important to understand spine patients’ ideas, concerns, and expectations regarding endoscopic spine surgery, and one should be careful not to overpromise. Furthermore, surgeons should understand the limitations of endoscopic surgery and perform open surgery instead for patients who are not suitable for endoscopic spine surgery. As endoscopic techniques become more widely accepted, one must be wary of the Dunning-Kruger effect when surgeons first begin endoscopic surgery practice. Endoscopic spine surgery has a steep learning curve, which the surgeon will overcome with time. Currently, in some of endoscopists’ practice, we are able to perform up to 90% of procedures for uncomplicated lumbar degenerative conditions with endoscopic spine surgery. The current applications and the common and less common expanded applications are highlighted in Table 1. With further technical refinements and additional research and development into the 6 factors shaping the development of endoscopic surgery, there is potential for even wider clinical applications to tumors, infections, trauma and perhaps selected cases of deformities in the near future. With the adoption of spinal endoscopic practice, advances in techniques, and acceptance by public opinion, there is the potential to individualize spine care with varying degrees of invasiveness tailored to different spine conditions.
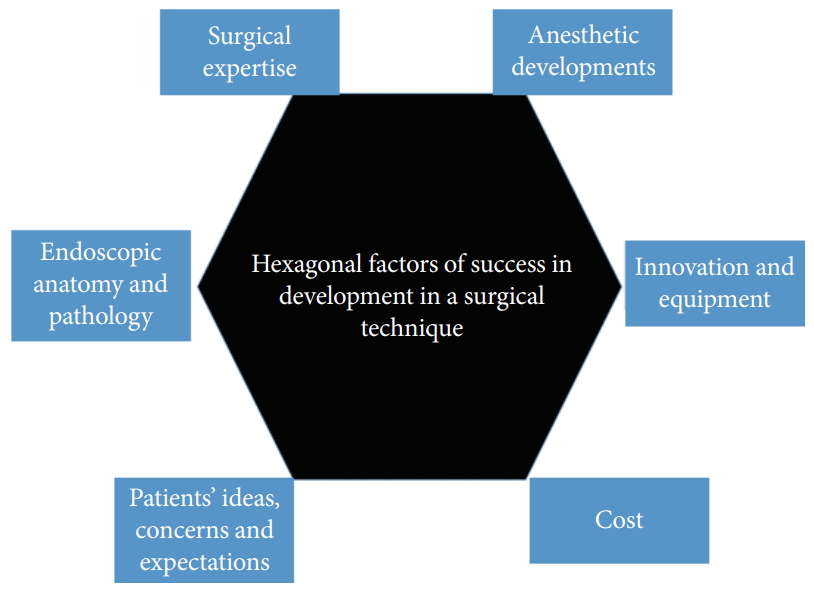
Hexagonal chart showing the 6 factors that work in sync to optimise the chance of success of a surgical technique, and in particular endoscopic surgical techniques.
Notes
The authors have nothing to disclose.