Evaluation and Surgical Planning for Craniovertebral Junction Deformity
Article information
Abstract
Craniovertebral junction (CVJ) deformity is a challenging pathology that can result in progressive deformity, myelopathy, severe neck pain, and functional disability, such as difficulty swallowing. Surgical management of CVJ deformity is complex for anatomical reasons; given the discreet relationships involved in the surrounding neurovascular structures and intricate biochemical issues, access to this region is relatively difficult. Evaluation of the reducibility, CVJ alignment, and direction of the mechanical compression may determine surgical strategy. If CVJ deformity is reducible, posterior in situ fixation may be a viable solution. If the deformity is rigid and the C1–2 facet is fixed, osteotomy may be necessary to make the C1–2 facet joint reducible. C1–2 facet release with vertical reduction technique could be useful, especially when the C1–2 facet joint is the primary pathology of CVJ kyphotic deformity or basilar invagination. The indications for transoral surgery are becoming as narrow as a treatment for CVJ deformity. In this article, we will discuss CVJ alignment and various strategies for the management of CVJ deformity and possible ways to prevent complications and improve surgical outcomes.
INTRODUCTION
Craniovertebral junction (CVJ) is the region around the skull base and the upper cervical spine (atlas and axis), and its alignment is unique regarding the segmental motion and biomechanical perspective [1-4]. The stability of CVJ is dependent on a robust ligamentous complex and the shape of the bony structures, which are also responsible for much of the axial rotation (C1–2 joint) and flexion-extension movements (C0–1 and C1–2 joint) [5-7].
Although deformity or malalignment around CVJ is a rare, CVJ deformity results in sagittal and coronal imbalances, which causes significant pain due to arthritis, instability, and C2 foraminal stenosis. Moreover, malalignment can be a potentially life-threatening due to swallowing difficulty and cervicomedullary compressive myelopathy.
Patients presenting intractable pain, functional disability, or myelopathy caused by the CVJ deformity usually require surgical treatment. Decompression of neural structures, sagittal and coronal spinal realignment, and stabilization are the primary goals of surgical intervention.
Surgical management of CVJ pathology could be complicated for various reasons; given the discreet relationships involved in the surrounding neurovascular structures and intricate biomechanical issues, access to this region is relatively tricky, and there are many risks involved [8-14].
Recently, there have been tremendous advances in CVJ surgery. Understanding normal alignment, segmental motion, compensatory reaction, and anatomical variation around CVJ is critical not only to decide optimal surgical options but also to prevent complications and improve surgical outcomes.
CVJ deformity has various etiologies, such as congenital anomaly, tumor, infection, rheumatoid arthritis (RA), and trauma [15]. One of the most common CVJ anomalies is basilar invagination (BI), either congenital or degenerative, as represented by the upward migration of the odontoid process into an already limited space of the foramen magnum, causing compressive myelopathy around the cervicomedullary junction [16,17]. Many bony anomalies are associated with BI, such as clivus and condyle hypoplasia, occipital assimilation, os odontoideum, bifid C1 arch, and Klippel-Feil syndrome [18,19].
RA is the leading cause of secondary BI, which is also called basilar impression, atlantoaxial impaction, vertical settling, and cranial settling [20]. Symmetrical rheumatoid destruction of the C0–1 and C1–2 joints allow for the cranium to settle on the cervical spine and for the dens to enter the foramen magnum. However, only one lateral mass involvement may result in a fixed rotational tilt of the head. Secondary BI often results in a reduction of atlantodental interval (ADI) due to the conical shape of dens and reduced motion, producing a false impression of anatomic improvement known as “pseudostabilization” [21].
CRANIOVERTEBRAL JUNCTION ALIGNMENT
The human cervical spine is located inferior to the foramen magnum. These characteristics ensure an energy-saving balance of the head and neck when at rest, allowing humans to effectively maintain horizontal vision without the significant effort of the neck extensor muscle [22]. However, quadrupeds with posteriorly positioned foramina magna require well-developed nuchal musculature and ligaments to bear the head’s weight to maintain horizontal gaze. When humans are at rest in standing position, the C0–1 articulation is held rather flexibly, whereas the C1–2 joints are held slightly in an extended position.
So, the C1 slope is backwardly slanted in the neutral position, and kyphotic angulation of the C0–1 segment allows some degree of freedom for neck extension as the space between the occiput and C1 posterior arch allows for upper cervical extension (Fig. 1A).
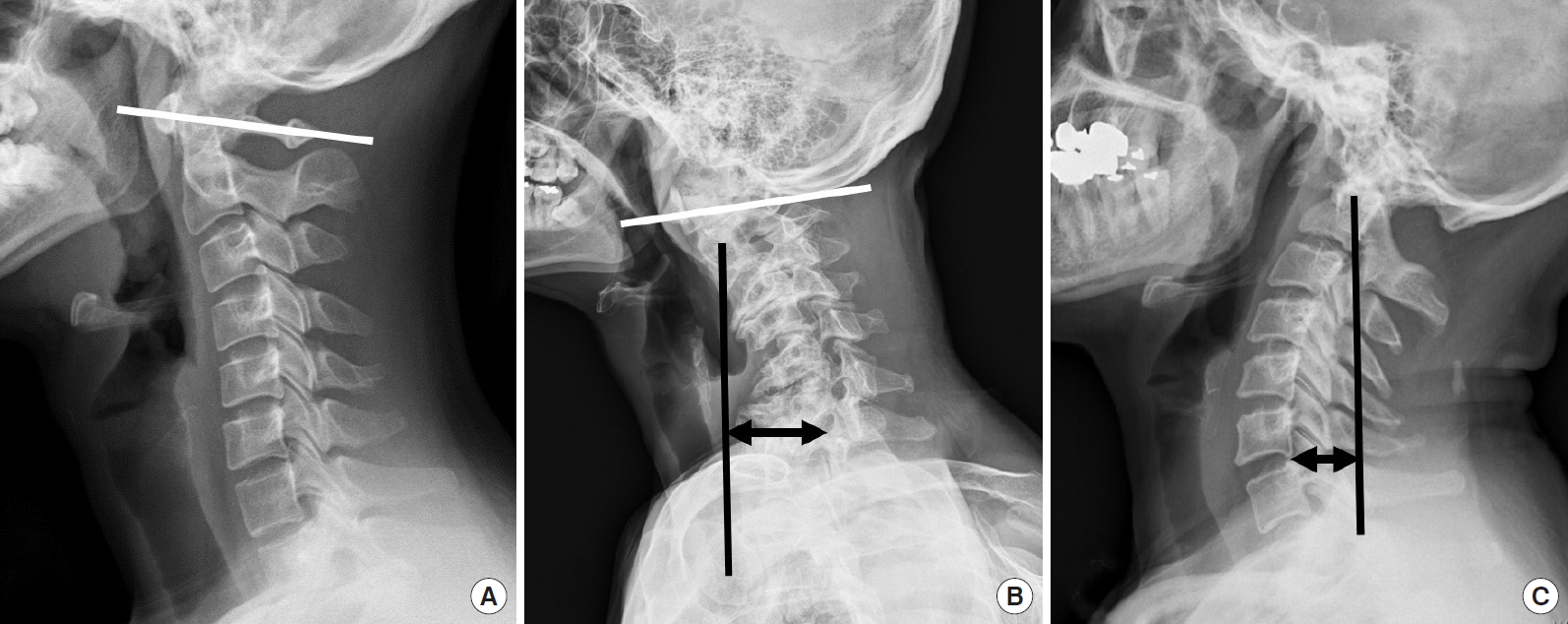
Lateral x-ray shows a normal alignment of the cervical spine. On most occasions, cervical lordosis occurs at the C1–2 segment. (A) C0–1 segment is kyphotic, and the C1 slope is posteriorly slanted. (B) C0–2 segment is a hyperlordotic, and the C1 slope is reversely slanted to maintain the patient’s horizontal gaze with sagittal malalignment and subaxial cervical kyphotic deformity patient. (C) Craniovertebral junction kyphotic deformity patient shows a hyperlordotic compensation in the subaxial cervical spine and negative sagittal imbalance to maintain gaze.
When patients have a kyphotic deformity in the lower cervical spine or thoracolumbar spine, the C0–1 segment can hold up the head to compensate for distal kyphosis, maintaining the sagittal balance and horizontal gaze. Therefore, a normal kyphotic angulation of the C0–1 segment could be referred to as a “tertiary curvature” to characterize human CVJ alignment, to differentiate between upper cervical spine alignment and primary/secondary curvature of the human spine as well as differentiate between the cervical spine curvature of humans and that of quadrupeds.
Although the asymptomatic population may have different types of cervical alignment, such as “straight” or “kyphotic” cervical spines ranging from 7% to 40%, it has been well accepted that cervical lordosis is a physiological posture in asymptomatic subjects, and a large percentage (about 75%–80%) of cervical lordosis is localized to the C1–2 segment. Only a small percentage (15%) of cervical lordosis exists in the lower cervical levels. On average, the C0-1 segment is kyphotic [23-28]. The loss of subaxial lordosis has been reported in CVJ fixation in which excessive hyperlordosis is created at the C0–2 segment (Fig. 1B) [29]. Moreover, craniometrics studies revealed that excessive CVJ kyphosis could cause the subaxial compensatory lordosis (Fig. 1C) [30-32]. The cervicothoracic junction has been shown to be the main determining factor for cervical lordosis. However, the CVJ angle has also been shown to be related to the subaxial cervical spine’s angle significantly.
The O–C2 angle was significantly greater in the patients with a straight or kyphotic subaxial cervical spine than those with lordotic lower cervical spine. When patients have a kyphotic deformity in the lower cervical spine, positive sagittal imbalance occurs in the cervical spine, and the hyperextended C0–2 segment holds up the head to compensate for distal kyphosis, sagittal imbalance, and maintaining horizontal gaze.
In the patients with CVJ kyphotic deformity, lateral radiographs show hyperlordotic compensation in the subaxial cervical spine and negative sagittal imbalance to maintain gaze (Fig. 1B, C).
These findings suggest that reciprocal interaction may likely affect not only global balance but also regional balance [33].
Another interesting finding is that neck flexion, and extension movement is initiated primarily by motions between the head and C1 [34]. As the head approaches full flexion and extension, contributions from the upper cervical segments decrease, whereas the lower cervical segments’ contribution increases.
Understanding the normal CVJ alignment and segmental motion is paramount to understand CVJ deformity better and to decide on a more appropriate surgical strategy according to the pathologies of CVJ.
PREOPERATIVE RADIOLOGIC ASSESSMENT
Irreducibility was defined as nonalignment of C1–2 after neck extension (determined on lateral x-ray) or after cervical traction. Lateral radiography with a dynamic study is necessary to evaluate ADI, degree of vertical subluxation, and reducibility. Furthermore, bony abnormalities, such as occipital assimilation, os odontoideum, bifid C1 arch, and C2–3 fusion, can be evaluated on lateral radiography.
Traction can be a useful tool in evaluating the reducibility and predicting intraoperative neurological worsening by surgical position. Preoperative cervical traction may correct the atlantoaxial dislocation (AAD) and vertical subluxation in some reducible deformity cases. This could especially be important for managing fragile patients because they can be treated with only a stabilization procedure postreduction, reducing the level of fixation. Gardner cervical traction was applied, depending on age and weight, starting with 2–5 kg for one or 2 days. The head of the bed was elevated to provide counter traction. Serial radiographs were assessed for reduction. These patients who demonstrated a reduction were classified as reducible AAD or BI.
Magnetic resonance imaging (MRI) was studied to evaluate cord compression, T2 signal change, Chiari malformation, and syringomyelia. Computed tomography (CT) scans can confirm bony abnormalities, the dens’ exact location, C1–2 joint destruction, and abnormal C1–2 facet angle. CT scans are also used to determine the extent of facet fusion and osteophytic bridging to assess the need for C1–2 osteotomy.
Computed tomography angiography (CTA) screening is a useful tool for getting 3-dimensional complex deformity information and identifying vertebral artery (VA) anomaly around CVJ [14,35]. The incidence of V3 segment anomalies has been reported to be as high as 10% [9,14]. In V3 segment anomalies, a more optimal entry point for C1 fixation should be selected to avoid significant morbidities associated with VA injury [36]. Although there could be a racial difference in the incidence of V3 segment anomaly, existing literature indicates that the V3 segment anomaly is more common in the group with the congenital bony anomaly. Quadrupeds, including humans, are more likely to have other CVJ bony abnormalities, such as atlantooccipital assimilation or dens anomalies, if atlantoaxial instability is present.
Therefore, we suggest that preoperative CTA might be informative for deciding on a surgical technique, especially in the high-risk group of the V3 segment anomaly, such as Asians and those with C1–2 instability with congenital bony anomaly [9].
TREATMENT OF CRANIOVERTEBRAL JUNCTION DEFORMITY
Fig. 2 provides decision-making pathways to treat CVJ deformity. The treatment goal of CVJ deformity is to restore normal alignment, relieve neural compression, and stabilization without neurological complications. Factors that influence the specific treatment of CVJ deformity are as follows: (1) the reducibility of deformity (i.e., restoring anatomic alignment, thereby relieving neural compression); (2) the direction of mechanical compression; (3) the presence of abnormal craniocervical angle and alignment; and (4) the presence of hindbrain herniation, syrinx, and vascular abnormalities.

The algorithm shows decision-making pathways for the treatment of the craniovertebral junction (CVJ) deformity. OC, occipitocervical.
Irreducible lesions can be divided into 2 types: The ventral compressive lesion and the dorsal compressive lesion. Doral compression cases need either a posterior or posterolateral decompression procedure, and if there is instability after decompression, posterior fixation is mandatory to gain stability. Irreducible CVJ deformity may require decompression at the site of compression or may require an osteotomy procedure to make the lesion reducible and relieve neural compression [33].
Transoral surgery is a procedure carried out through the oral cavity to gain access to the ventral compressive lesion of CVJ from the lower rim of clivus cranially to C23 disc space caudally. Transoral surgery could be necessary in case of irreducible CVJ deformity, where ventral compression to neutral structures is present [37]. Other indications for transoral surgery include CVJ tumor and infection, in which a simple posterior reduction [38] cannot decompress the anterior compression. Although the combined anteriorposterior approach may provide adequate neural decompression and fusion, it poses several disadvantages, such as the higher risk of surgical morbidities, prolonged postoperative intubation/nasogastric feeding, phonation difficulty, and potential infection [39-41]. The risk of infection is a big concern, mainly when the subarachnoid space had to be opened, which may cause catastrophic sequelae. Another issue is that transoral decompression can induce significant instability of CVJ, inevitably requiring a subsequent posterior stabilization procedure. These issues are why the initial enthusiasm vanished, and an indication of transoral decompression became less evident in the recent literature.
A C1–2 joint distraction technique, as introduced by Goel, has been gaining popularity recently as a possible treatment modality for selected cases of AAD and BI [42-45]. This technique seems to have several advantages over conventional transoral surgery and occipitocervical (OC) fixation. First, direct posterior reduction and fixation are possible for cervicomedullary decompression. Hence, a transoral surgery and its related complications could be avoided. Second, the fusion bed dimension could substantially be increased because C1–2 facet surface is rather large. Third, it can avoid occipital fixation and conserve C0–1 segmental motion. Fourth, C1–2 joint manipulation can make a fixed subluxation or deformity reducible, minimizing the need for head traction, before and during surgery.
1. Preoperative Planning
Preoperative cervical traction may correct AAD and vertical subluxation in some patients with reducible deformity. This could especially be important for managing fragile patients because they can be treated with only a stabilization procedure after reduction and decreasing the numbers of fixation levels.
The direction of the mechanical compression was the critical factor in deciding a surgical approach. With recent advancements in surgical techniques and instruments, the results of posterior C1–2 release and reduction have been satisfactory. However, difficult or insufficient reduction and loss of reduction have been observed after posterior reduction and fixation [37]. Thus, anterior release reduction and posterior fixation could still be available options for irreducible BI with AAD, especially in patients with severely deformed bony mass anteriorly compressing the cervicomedullary junction.
CVJ alignment is another crucial factor to decide surgical strategy. Clivus-canal angle and cervicomedullary angle is tended to be kyphotic in congenital CVJ anomaly, which is caused by the short clivus, platybasia, angulated odontoid process, or abnormal sagittal inclination of deformed C1–2 facet joints [30-32]. Craniocervical realignment through screws and rod systems may be an efficacious surgical technique for treating congenital craniocervical anomaly with abnormal angulation of the clivus. However, craniocervical realignment procedure, including distraction, compression, and skull extension, need additional decompression/fixation up to the cranium, and it should be cautiously performed, especially in cases with severe canal stenosis [46].
2. Surgical Techniques for CVJ Deformity
1) Fixed atlantoaxial rotatory fixation
Reducible AAD can be defined as C1–2 alignment on extension or cervical traction. If the dislocation cannot be reduced on extension position and preoperative traction, it is labeled as an irreducible or fixed AAD and rotatory subluxation (Fig. 3). Management becomes difficult with this type of fixed deformity. Since Goel introduced the C1–2 distraction method, the focus in CVJ pathology treatment has shifted to the dislocated C1–2 facets rather than the C2 dens, and the C1–2 joint manipulation has been the preferred method of treatment for irreducible AAD [21,43]. It is because the cause of irreducibility is in the C1–2 facets. In cases of irreducible AAD or rotatory fixation, C1–2 joint mutilation or osteophyte formation is so severe that dislocation cannot be reduced despite cervical traction. Therefore, C1–2 facet mobilization is necessary to make this fixed deformity reducible using facet osteotomies (Fig. 3C).
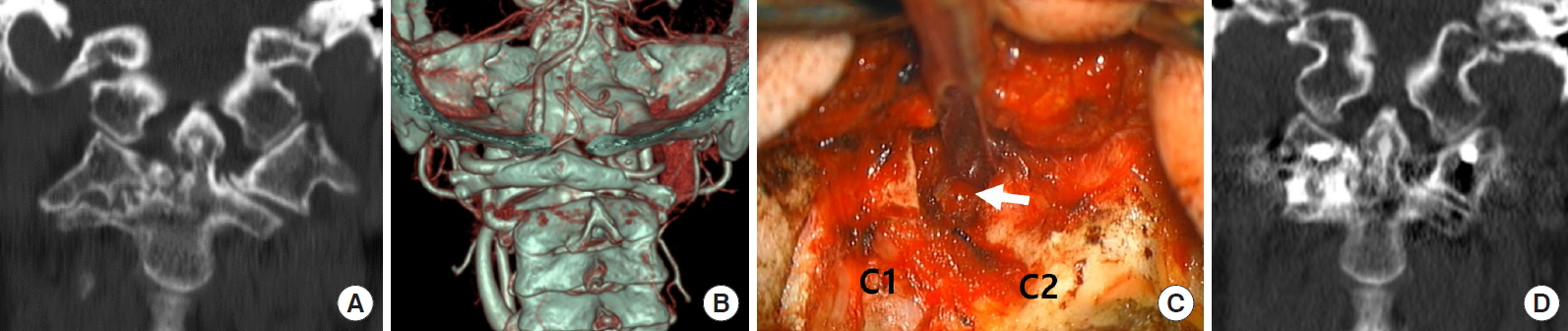
Illustrative case. Fixed C12 subluxation. (A) Coronal reconstructed computed tomography (CT) scan showing narrow C1–2 facet joint space and joint mutilation on the right side. (B) Three-dimensional reconstructed CT scan showing lateral tilt and C1–2 joint rotation. (C) Intraoperative photograph demonstrates small osteotomes inside the C1–2 joint space (arrow) on the right side for joint release. C2 root resection provides panoramic view of C1–2 facet joint. (D) Coronal reconstructed CT scan showing remodeling and reduction of the C1–2 facet tilt on the right side.
After releasing the C1–2 facet joint capsule and removing osteophytes, the mobility between C1 and C2 is usually identifiable intraoperatively. The cartilage endplate was curetted and drilled to increase mobility further and remodel the C1–2 facet joint plane. The rotated C1–2 facet joint could be mobilized and reduced in a neutral position after releasing the C1–2 facet joint (Supplementary video clip 1).
In the horizontal subluxation cases, the surgical assistant pressed down firmly on the C2 spinous process or pulled the C1 arch using cables to reduce horizontal subluxation. Adjusting the patients’ neck to allow for a slight extension should help the process of reduction. Bone block or cage filled with cancellous bone can be placed inside the decorticated C1–2 joint.
2) Rheumatoid BI
rheumatoid BI is caused by C1–2 joint destruction and subsequent upward migration of the odontoid process. These findings suggest that the primary pathology of rheumatoid BI is the C1–2 joint, not the C0–1 joint. Hence, spine surgeons should focus on C1–2 joint reduction to treat rheumatoid BI to spare the innocent C0–1 segmental motion. Rheumatoid destruction of the C1–2 joint could easily be confused with spontaneous fusion mass on CT scan due to narrowing the joint space and destruction of joint cartilage. However, the C1–2 joint of rheumatoid BI is unstable and easily reducible in vertical distraction.
The posterior edge of the foramen magnum, posterior arch of C1 and the C2–3 lamina were exposed through a midline skin incision. For patients with posterior compressive lesions around the foramen magnum, the posterior margin of the foramen magnum and C1 arch were removed. Intentional sacrifice of bilateral C2 ganglions was performed to obtain enough C1 screw entry point, joint manipulation, and bone fusion (Fig. 4). Subsequently, by applying the Harms technique, polyaxial screws were inserted to the bilateral C1 lateral masses and the C2 pedicle. The C1 and C2 screws were partially loosened. Distraction between the C1 and C2 screws was performed using a distractor to achieve vertical reduction; the nuts were subsequently locked. Vertical reduction results in the migration of the odontoid process inferiorly, reducing the neural compression around the ventral cervicomedullary junction. The C1–2 articular cartilages were removed widely using a curette and a high-speed burr. The facet cage or tricortical iliac bone autograft was inserted bilaterally as joint spacers to maintain the odontoid process vertically. Then compression between the C1 and C2 screw head increases the graft contact inside the C1–2 joint, creating adequate C1–2 lordosis. Subsequently, interlaminar fusion could be performed using autograft iliac bone blocks and cables.
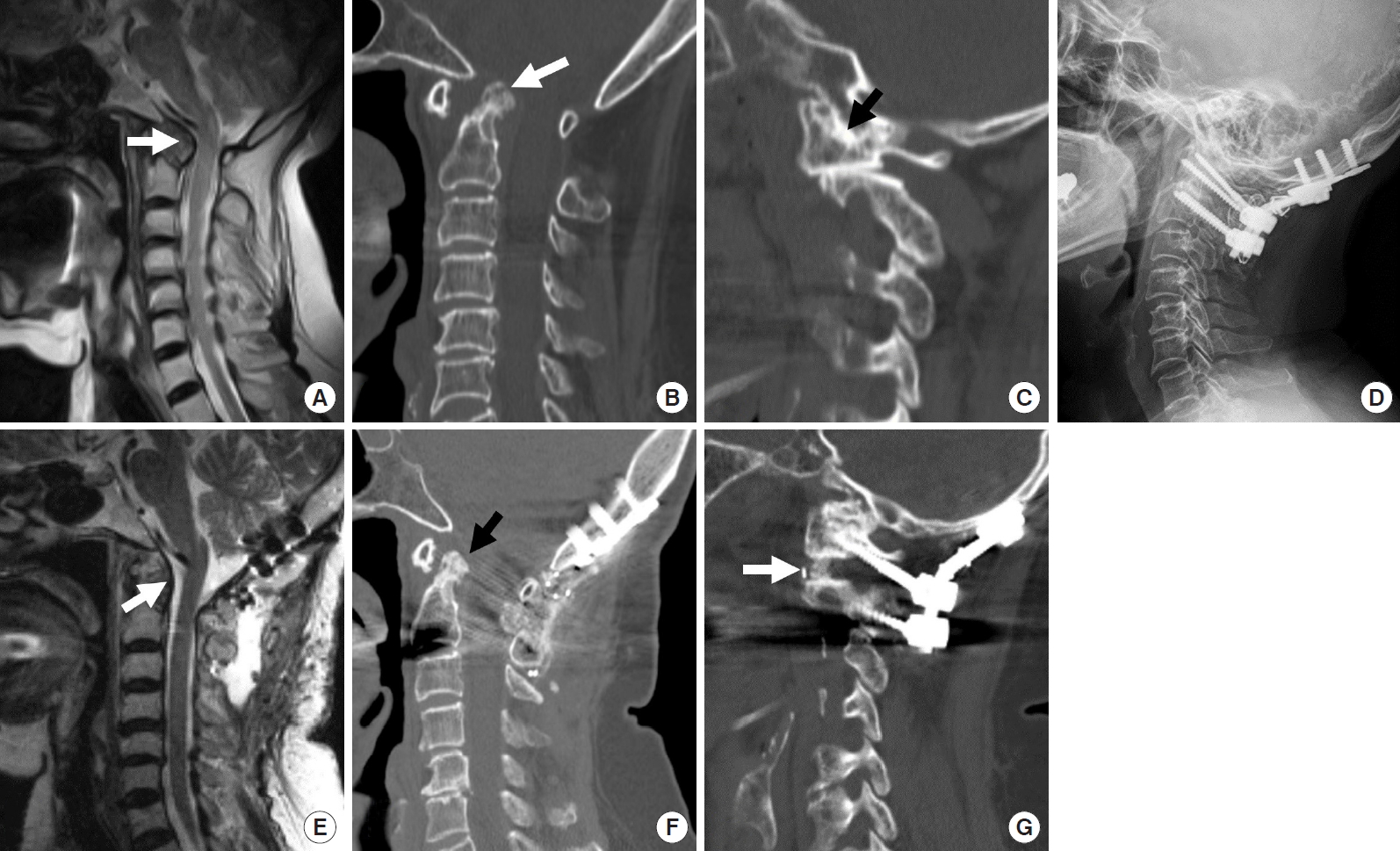
(A, B) Sagittal T2 magnetic resonance imaging (MRI) and sagittal reconstruction computed tomography (CT) image showing odontoid compression over the brain stem and upper cervical cord. Odontoid tip (white arrow) is located above the foramen magnum. (C) Parasagittal reconstruction CT scan showing C1–2 facet subluxation and severe joint mutilation of the C0–1 facet joint (arrow). (D) Postoperative x-ray showing the final construct of C0–2 instrumentation. (E, F) Postoperative sagittal T2 MRI and sagittal reconstruction CT image showing early reduction of retroodontoid rheumatoid pannus (white arrow) and odontoid tip (black arrow) is pulled down below the foramen magnum. (G) Parasagittal reconstruction CT scan shows vertical distraction and intra-articular fusion (white arrow) inside the C1–2 facet joints.
A substantial number of rheumatoid BI cases involve the spinal cord’s compression ventrally by the retroodontoid pannus, which needs transoral decompression. However, posterior atlantoaxial fixation with vertical reduction technique has been reported to provide an early reduction of retroodontoid rheumatoid pannus, as well as a reduction of BI and AAD [47-51]. This finding suggests that rheumatoid pannus could be a buckling of the ligamentous complex around CVJ caused by BI rather than granulation tissue caused by the inflammation.
3) Congenital kyphosis and BI
Congenital BI is usually irreducible. Irreducibility results from the combined bony malformation and the abnormal inclination of the C1–2 facet joints.
In C1–2 subluxation with oblique C1–2 facet joint orientation, the joint space is not visible even after C2 root resection. The drilling of the superior posterior corner of the C2 facet allows the C1–2 joint space visibility. Then the small osteotome could be inserted into the joint space to open it. Then drilling, reaming, and curettage was continued to release the fixed C1–2 joint space. Drilling and osteotome were focused on the inferior anterior corner of the C1 facet and the superior posterior aspect of the C2 facet to make the C1–2 joint flat. Osteotome was used as a fulcrum to open the C1–2 joint space and reduce the subluxation both for the vertical and horizontal translation. The reduction was then maintained using the C1–2 spacers (bone block or PEEK cage filled with bone), and C1–2 was fixed using the polyaxial screw system. In those who needed C1 laminectomy for the posterior decompression, C1 laminectomy bone could also be used as the C1–2 facet spacer.
If BI and CVJ kyphosis are not severe, C1–2 facet joint distraction and C1–2 joint remodeling may be sufficient to reduce and decompress the cervicomedullary compression (Fig. 5). However, in cases with a severe degree of CVJ kyphotic angulation and ventral cord compression, C1–2 joint vertical reduction and fixation may not be sufficient to obtain good CVJ alignment and decompression. Therefore, occipital fixation, distraction, compression, and head extension are mandatory to obtain a craniocervical realignment in CVJ kyphotic deformity (Fig. 6).
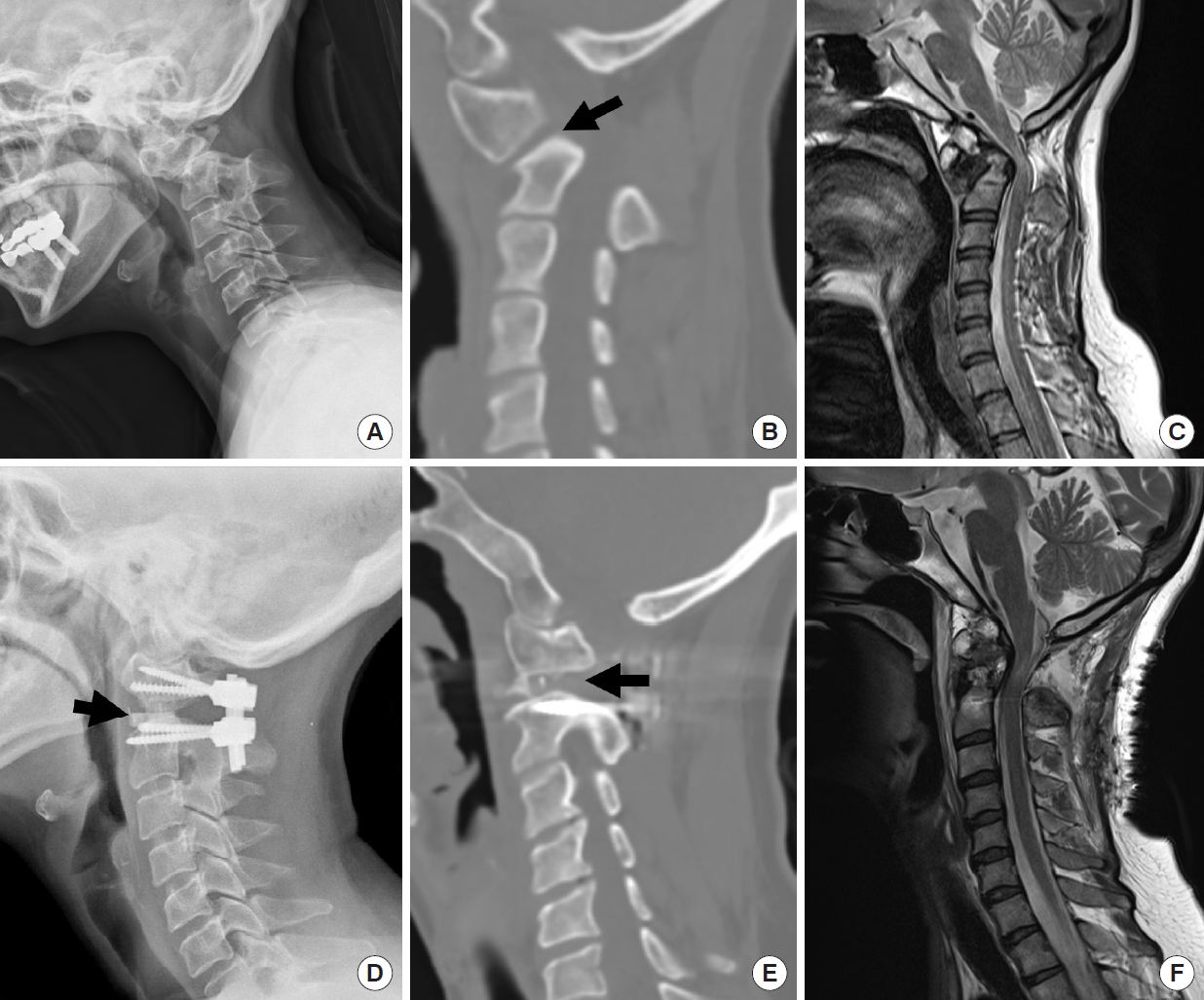
Illustrative case. Congenital craniovertebral junction kyphosis. (A) Preoperative x-ray shows the increased atlanto-dental interval. (B) Sagittal reconstructed computed tomography (CT) scan reveals the abnormal inclination of C1–2 facet joint (arrow). (C) Sagittal T2-weighed image magnetic resonance imaging (MRI) showing os odontoideum and cord compression by the C2 dens over the upper cervical cord. (D) Postoperative x-ray shows the kyphosis reduction and facet grafts inside of C1–2 joint (arrow). (E) Sagittal reconstructed CT scan showing remodeling of the C1–2 facet joint and facet graft inside (arrow). (F) Postoperative MRI showing the decompression of the spinal cord and C2 realignment.

Illustrative case. Congenital basilar invagination and craniovertebral junction (CVJ) kyphosis. (A) Preoperative x-ray shows the atlantoaxial dislocation and severe kyphotic CVJ deformity caused by the os odontoideum. (B) Sagittal reconstructed computed tomography (CT) scan reveals the abnormal inclination and radiographical stiffness of C1–2 facet joint (arrow). (C) Postoperative x-ray shows a reduction of C1–2 dislocation. CVJ kyphosis is also reduced and clivus-canal angle is increased. (D) Sagittal reconstructed CT scan reveals postoperative remodeling and flattening of the C1–2 facet joint and PEEK cage inside the joint (arrow).
After placing the screw for occiput, C1 lateral mass, and C2 pedicle, C2 screw heads were tightened to ensure that the screws, C2 body, and the odontoid process became an integrated unit. The C1 and occipital screw heads were not tightened yet. The C1 screw placement was not mandatory in cases with occipital assimilation and in cases where the space was not sufficient for C1 screw placement or rod application. The rod bent more than the patient’s CVJ alignment, and the head was extended using the Mayfield head holder to approximate the rod to the occipital screw head. The occipital screw and C1 screw head were only tightened loosely at this stage. Then, the longitudinal distraction between the screws, which pulled the odontoid process downward and anteriorly. Once the joint space was distracted, facet cages or bone blocks were placed bilaterally, and the occipital screws and C1 screws were tightened after head extension using the Mayfield skull pin, which reduced the AAD and CVJ kyphosis. For patients who need more horizontal reduction for AAD and forward movement of the odontoid process, the C2 pedicle screws were loosened, and vertical compression was performed between C1–2 screw heads after placement of facet spacers. Excessive vertical reduction and CVJ realignment may pose a higher risk of postoperative neurological impairment. Therefore, the C1-2 distraction and craniocervical realignment should be performed very carefully under intraoperative neuromonitoring (IONM) to avoid excessive distraction and subsequent stretching injury of the spinal cord or VA. Although we do not fully know the amount of distraction required for neurological recovery and prevention of neurological complications, our published data showed that the change rate of Modified Ranawat index after surgery was correlated with postoperative Hirabayashi recovery rate [52]. It is impossible to simplify clinical outcomes into a linear curve. However, the amount of radiological change has a substantial correlation with the clinical outcomes. After satisfactory reduction and confirmation were provided by fluoroscopy, the posterior bony surface was decorticated to prepare for the fusion bed. The bone grafts were placed over the decorticated fusion surface.
3. Complication and Prevention
Mechanical failure, various kinds of neurovascular complications, and postoperative swallowing difficulty can occur after the surgery in patients with CVJ deformity.
1) Mechanical failure (nonunion, instrument failure, and adjacent segment degeneration)
Despite recent advancements in the posterior cervical instrument, high biomechanical loading around this particular junctional area poses a risk for instrument failure or nonunion [10,12]. Fusion bed preparation and graft material selection are essential for increasing the fusion rate. We usually use iliac autograft as much as possible. Although the posterior surface of the occipital bone and C1–2 interlaminar space is a popular fusion bed, the C1–2 facet joint is the most critical area for fusion and malalignment correction for CVJ kyphosis patients, especially when C1 laminectomy is inevitable.
Moreover, the long-term fate of the adjacent C0–1 segment has rarely been reported in patients after receiving C1–2 vertical distraction procedure with respect to the segmental motion and adjacent segmental degeneration. Yoshida et al. [53] reported that C1–2 rigid fixation could provide prophylactic benefits against vertical subluxation and the subaxial subluxation in RA patients. Werle et al. [54] reported that C0–1 degeneration is very low after C1–2 fusion in RA patients. Therefore, the C0–1 segment sparing technique should be the preferred treatment option of CVJ fusion, especially for RA patients.
2) Subaxial alignment change following CVJ fixation
Several studies have investigated the relationship between postoperative C1–2 angle and C2–7 sagittal alignment change after C1–2 fixation [55-58], and most have reported a reciprocal relationship between changes in the upper cervical spine and subaxial cervical spine [57,58]. If the CVJ is fixed in the hyperlordotic posture, subaxial cervical kyphosis is inevitable, and upper cervical kyphosis causes lordotic compensation in the subaxial cervical spine. The most excellent care is necessary to determine the C1–2 fixation angle during surgery to reduce the risk of postoperative malalignment and subluxation of the subaxial cervical spine. Subaxial alignment change is not uncommon after CVJ fixation. Muscle detachment at the C2 spinous process was not a risk factor of kyphotic change. Our findings suggest small range of motion (ROM) of the C0–1 segment with or without occipital fixation and combined subaxial laminoplasty are risk factors of subaxial kyphotic change. Although the little study has sought to identify the risk factors of postoperative subaxial malalignment following CVJ fixation, we recently reported subaxial cervical spine alignment might change significantly during the first year after CVJ posterior fixation. The subaxial cervical alignment is significantly related to the upper cervical angle after CVJ fixation. Furthermore, the study suggested a small ROM of the C0–1 segment with or without occipital fixation and combined subaxial laminoplasty independently predict subaxial kyphotic change after upper cervical fixation. Interestingly, extensor muscle detachment at C2 itself was not identified as a risk factor of kyphotic change, but when combined with subaxial laminoplasty and wider dissection of deep extensor muscle, the risk of subaxial kyphotic change was significantly increased [59].
These results suggest the C0–1 segment is the dominant segment for compensating for abnormal angulation of the subaxial cervical spine after upper cervical fixation. These results imply it is important not to include occipital fixation during upper cervical fixation to avoid loss of subaxial cervical lordosis and preserve C0–1 segmental motion.
3) Occipital neuralgia
C1–2 joint distraction technique has a clinically significant concern regarding the C2 neuropathic pain after C2 root resection. Although C2 root resection is not mandatory for C1–2 facet opening, it is useful to make a broad exposure for the C12 facet surface, especially when C1–2 interlaminar space is narrow and venous plexus is prominent [44,60]. Thus, C2 root resection is one of the critical parts of the C1–2 joint distraction procedure. In the literature, most authors have reported that C2 neuropathic pain incidence is relatively low after C2 root resection [44,60]. However, C2 neuropathic pain could be troublesome for some patients. Therefore, surgeons should be aware of both the benefits and risks associated with C2 root resection. C2 neuropathic pain may cause significant postoperative occipital neuralgia, which could also occur due to the C2 root irritation by C1 screw, excessive manipulation, and traction on the C2 root during surgery. Several reports have advocated the deliberate C2 root resection or C1 screw without threads to minimize C2 root complications [61-63]. However, the relationship between C2 root resection and neuropathic pain in the literature remains controversial. Goel et al. [61] reported no significant concerns regarding C2 neuropathic pain among 160 patients who underwent the C2 root sacrifice. Dewan et al. [64] reported that C2 root transection is associated with increased occipital numbness. However, this finding does not affect patient-reported outcomes and quality of life, and the C2 root preservation can be associated with occipital neuralgia, which harms patient disability and quality of life. Elliott et al. [65] demonstrated that the C2 root transection decreases blood loss and operative time and significantly reduces postoperative neuralgia. However, Yeom et al. [66] reported that C2 root resection could cause a high incidence of postoperative occipital neuralgia. In our study, occipital neuralgia was significantly induced in the C2 root resection group than that of the C2 root-sparing group.
Furthermore, the C1 screw entry point is significantly related to postoperative C2 neuropathy. Although C2 root resection allows for better visualization for venous bleeding control, getting a broader view for screw insertion to prevent screw malposition and a wider bone fusion surface, C2 root resection is an independent risk factor of postoperative C2 root neuropathy. So, excessive C2 root manipulation and resection should be carefully determined because it can cause occipital neuralgia. However, C2 root resection could be inevitable for vertical reduction, especially in cases of rheumatoid BI. Given that C1–2 joint mutilation is so severe in rheumatoid BI, the height of the C2 foramen becomes too narrow for the manipulation of the C1–2 facet joint and insertion of screw without damaging the C2 nerve root.
4) Neurological worsening
Even though several reports of vertical reduction technique showed excellent clinical and radiological results without neurological complication [21,37,43,44], we experienced cases with transient neurological worsening after craniocervical realignment and encountered several cases with a significant change in IONM during the vertical reduction technique [67]. To date, we are not sure whether it is related to spinal cord stretch or spinal cord ischemia due to the VA stretch. However, we found that IONM change is usually recovered after reducing the amount of C1–2 joint distraction and the facet graft size. This finding shows the efficacy of IONM during craniocervical realignment or vertical reduction procedure for congenital craniocervical segmentation anomaly.
IONM change is more common in CVJ surgery than at any other level of the cervical spine. Preoperative T2 signal change on MRI and narrow canal diameter are 2 risk factors that are significantly related to IONM change in our CVJ surgery case series [46,67,68]. We suggest that IONM may help in preventing unexpected neurological deterioration during posterior craniocervical distraction and realignment.
5) Dysphagia
A “military tuck” posture (neutral head posture, the extension of the lower cervical spine, posterior translation of the occiput-C1 complex) usually reduces C1–2 subluxation, while optimizing the surgical exposure and allowing for a favorable screw trajectory [69]. It is a commonly used posture for posterior instrumentation in the upper cervical spine.
However, previous studies have shown that a decrease in the OC2 angle in the retraction position causes a reduction in the oropharyngeal airway space and postoperative dysphagia [70,71].
Dysphagia is not an uncommon complication after posterior cervical surgeries, as well as anterior cervical spine surgeries [72-74]. Moreover, dysphagia after posterior OC fusion has been recognized as a severe postoperative complication. Miyata et al. [70] reported that dysphagia after OC fusion is caused by glossoptosis, which is the downward displacement of the tongue root, resulting in the narrowing of the oropharynx and the impairment of the ability of the epiglottis to move sufficiently. They emphasized that a decrease in the OC2 angle may likely induce a reduction in the pharyngeal space and can be a predictor of postoperative dysphagia, which is not compensated by the middle or lower cervical spine. We reported that the upper cervical segments’ movement could be an essential compensation mechanism for swallowing, and the fixation of the upper cervical spine could be one of the risk factors for postoperative dysphagia.
Moreover, C0–1 angle change and movement during swallowing are significantly greater in the retraction posture than in the neutral posture, and the C0–1 segment is the dominant level of compensatory motion that occurs when subjects experience dysphagia in the retraction position [75]. Therefore, if we fix up to the cranium in the retraction position, the compensatory movement’s major segment cannot function properly to allow swallowing, leading to postoperative dysphagia [76]. Therefore, avoiding OC fixation in retraction posture and sparing C0–1 segment are critical technical tips for preventing postoperative dysphagia.
CONCLUSION
Each type of CVJ deformity has its characteristics and treatment challenges. Regardless of the underlying cause, this complicated disease entity has 2 types—reducible deformity and irreducible deformity—according to whether the dislocation is reduced after dynamic x-ray and traction. Simple posterior fixation and fusion are adequate for reducible CVJ deformity, and any type of reduction procedure is necessary for irreducible CVJ deformity. A reduction of irreducible deformity can be achieved using anterior transoral surgery or posterior C1–2 facet joint manipulation procedure, depending on the pathology’s primary focus. With recent advancements in surgical techniques and internal fixation devices, posterior only surgery can achieve ventral decompression and craniocervical realignment. The posterior reduction procedure principle is releasing the C1–2 facet joint, pulls down the odontoid process away from the cervicomedullary junction, and craniocervical realignment without neurological injury. C1–2 vertical reduction technique can be the optimal surgery for BI with or without abnormal CVJ kyphosis. Additional procedure for craniocervical realignment is necessary to treat congenital BI with severe CVJ kyphosis. Head extension maneuver through the occipital screws and rod system may be a safe and efficacious surgical technique for the craniocervical realignment.
Avoiding neurological complications could be the most crucial issue in this complicated junctional area, and preoperative radiological assessment is critical to detect the structural anomaly and decide an optimal surgical plan. IONM may help in preventing any unexpected neurological deterioration during posterior craniocervical distraction and realignment.
By understanding the nature of CVJ alignment, recent advancements in posterior cervical instrumentation, all available treatment options, and the decision-making process, surgeons can improve patients’ life quality.
Notes
The authors have nothing to disclose.
SUPPLEMENTARY MATERIALS
Supplementary video clip 1 can be found via https://doi.org/10.14245/ns.2040510.255.v1.
