 |
 |
- Search
| Neurospine > Volume 18(1); 2021 > Article |
|
|
See commentary "Commentary on “Robot-Guided Transforaminal Versus Robot-Guided Posterior Lumbar Interbody Fusion for Lumbar Degenerative Disease”" in Volume 18 on page 106.
Abstract
Objective
There have been no clinical studies comparing different robotic techniques. We compare minimally invasive, robot-guided transforaminal lumbar interbody fusion (RG-TLIF) and mini-open robot-guided posterior lumbar interbody fusion (RG-PLIF).
Methods
Using data from a prospective institutional registry, we identified 38 patients who underwent RG-PLIF. Propensity score matching using a nearest-neighbor algorithm was implemented to select RG-TLIF controls. Twelve-month patient-reported outcome measures are presented. A reduction of ≥ 30% from baseline was defined as the minimum clinically important difference (MCID).
Results
Among the 76 included patients, there was no difference between RG-TLIF and RG-PLIF in surgical time (132.3 ± 29.4 minutes vs. 156.5 ± 53.0 minutes, p = 0.162), length of stay (55.9 ± 20.0 hours vs. 57.2 ± 18.8 hours, p = 0.683), and radiation dose area product (310.6 ± 126.1 mGy × cm2 vs. 287.9 ± 90.3 mGy × cm2, p = 0.370). However, while there was no difference among the 2 groups in terms of raw postoperative patient-reported outcome measures scores (all p > 0.05), MCID in leg pain was greater for RG-PLIF (55.3% vs. 78.9%, p = 0.028), and MCID in Oswestry Disability Index was greater for RG-TLIF (92.1% vs. 68.4%, p = 0.009). There was no difference concerning back pain (81.6% vs. 68.4%, p = 0.185).
With the growing number of experienced minimally invasive surgery (MIS) surgeons, the influx of evidence in favor of MIS is rapidly increasing [1,2]. Due to the limited line-of-sight in MIS procedures, surgeons need to rely on imaging, navigation, and guidance technologies to operate in a safe and efficient manner [3,4]. Also, the use of instrumented spinal fusion has been increasing vastly in the past decade, and can lead to favorable outcomes for a range of degenerative, congenital, infectious, traumatic, and neoplastic pathologies [5,6]. Multiple hundred million dollars are spent on spinal fusion procedures every year in the United States alone, with an increasing trend [6].
To cope with the increasing demand created by the rise of MIS, a plethora of new and ever-improving navigational systems have been developed for pedicle screw insertion. These systems allow a consistent level of safety and accuracy, on par with results achieved by very experienced freehand surgeons, with a reasonably short learning curve [4,7-10]. Robotic assistance can guide the surgeon to a predefined point in the anatomy, at a specific trajectory, while providing stability of drilling [4]. Through advanced planning, robotic navigation can be very useful in instrumenting pedicles, especially in minimally invasive procedures and in anatomically challenging cases such as dysplastic, twisted, and scoliotic pedicles, or when previous implants limit trajectories [4,10].
There is some evidence that robotic guidance can decrease the incidence of both radiological [11,12] as well as clinically relevant [10] pedicle screw malposition. If these benefits alone, particularly in light of the high acquisition and maintenance costs, warrant the use of these computer assistance systems, is currently unclear [13,14]. More importantly, data on the effect of using robotic guidance on clinical outcomes other than screw malposition, such as patient-reported outcome measures (PROMs) are lacking [15]. Similarly, there are no published comparisons among different robotic surgical approaches and their respective clinical outcome profiles. Therefore, we aimed to compare minimally invasive, robot-guided transforaminal lumbar interbody fusion (RG-TLIF) and mini-open robot-guided posterior lumbar interbody fusion (RG-PLIF) in a cohort of patients with either discogenic chronic low back pain (CLBP) or lumbar stenosis with spondylolisthesis [16].
A prospective registry of all lumbar spinal procedures carried out at a specialized spine surgery clinic was queried for data between November 2013 and April 2018. We identified all patients who underwent RG-PLIF and met the inclusion criteria and subsequently matched patients who underwent RG-TLIF in a 1:1 ratio. All patients were operated on by a senior neurosurgeon (MLS) [4]. Patients with a body mass index > 33 kg/m2 or with an American Society Score of Anaesthesiologists (ASA) physical status classification> II or > 80 years old were not regularly considered for elective surgery due to local insurance policies. We only include patients with complete baseline and 12-month PROM records, and otherwise complete data. Patients were treated perioperatively according to an institutional enhanced recovery after surgery protocol [17]. In patients with lumbar spinal stenosis and concomitant low-grade spondylolisthesis, the decision to perform fusion in addition to decompression was based on a validated decision-making protocol [16].
The prospective registry has been approved by the local Institutional Review Board (Medical Research Ethics Committees United, Registration Number W16.065), and this study was conducted according to the 2013 Declaration of Helsinki. All patients in this study provided written informed consent. The STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) statement was applied [18].
All surgeries were carried out as described previously [4]. A blueprint of pedicle screw trajectories on the workstation was created on 1-mm slice thickness computed tomography (CT) scans, taking into account the individual variations in vertebral anatomy, entry point, screw length, diameter, and angulation. The patient was placed prone and the correct spinal level was identified fluoroscopically. For RG-TLIF, a paramedian incision was made and the facet joint exposed through a 2.5-cm tubular working channel. Facet joint and ligamentum flavum hypertrophy were resected. For RG-PLIF, a mini-open midline approach was used for bilateral decompression. In both methods, the intervertebral disc space was cleared and the endplates were prepared for fusion. A Crescent or Capstone cage (Medtronic plc., Dublin, Ireland) was filled with demineralized bone matrix (Nanostim or Grafton, Medtronic plc.) and inserted. The remaining disc space was filled with autograft bone. The SpineAssist Hover-T mounting frame was attached to a spinous process and the posterior aspects of the iliac crests and a 3-dimensional fiducial array were attached. Anteroposterior and 60° oblique fluoroscopic images were taken and automatically matched to the preoperative CT. Once the surgeon confirmed correct registration for each vertebra, the robotic guidance unit was dispatched to the preoperatively planned trajectories. An incision was made through the robot’s cannula, and a trocar advanced onto the desired entry point. The pedicle was drilled using a 3-mm diameter drill-bit limited to a depth of 30 mm by a positive stopper. A pedicle screw (Sextant, Medtronic plc.) was inserted percutaneously over the K wire. This process was repeated for all trajectories. Fluoroscopic control was used to confirm optimal implant placement. Reduction was achieved, if necessary, and 2 curved rods were inserted percutaneously.
Every patient included in the study completed a standardized questionnaire containing visual analogue scales (VAS) for leg and back pain, as well as a validated Dutch version of the Oswestry Disability Index (ODI) as a measure of functional disability. We defined clinical success as achievement of the minimal clinically important difference (MCID) at 12 months postoperatively of ≥ 30%, as defined by Ostelo et al. [19] The ODI was defined as the primary endpoint of this study. Furthermore, we collected surgical time in minutes, length of hospital stay in hours, and intraoperative radiation dose recorded by the fluoroscope as the dose area product (DAP) in mGy× cm2. Other variables were collected according to the set of information and predictors of outcomes after spinal fusion described by Khor et al. [20,21], including age, sex, smoking status, indications for surgery, ASA physical status classification≥ III, presence of asthma bronchial as a comorbidity, ethnicity, preoperative opioid therapy, and prior spine surgery.
Continuous data are given as mean ± standard deviation or median (interquartile range), and categorical data as numbers and percentages. RG-TLIF patients were matched in a 1:1 ratio to RG-PLIF patients using propensity score matching based on a nearest-neighbor algorithm [16,22]. Patients were matched for age, sex, and preoperative VAS back and leg pain and ODI. The exact version of the Wilcoxon rank-sum test, based on the “shift” algorithm described by Streitberg and Röhmel [23], as well as the Pearson chi-square test were applied to test intergroup differences. A p ≤ 0.05 was considered statistically significant. All analyses were carried out in R ver. 3.6.3 (The R Foundation for Statistical Computing, Vienna, Austria). The statistical code is attached for reproducibility (Supplementary material 1).
In the specified timeframe, 87 patients underwent RG-TLIF and 51 patients underwent RG-PLIF at our institution. The prospective registry contained 38 patients fulfilling the inclusion and exclusion criteria including data completeness who underwent RG-PLIF, and 62 who underwent RG-TLIF. After 1:1 propensity score matching, 38 RG-TLIF patients were selected for comparison to the RG-PLIF patients. Cohort characteristics are reported in Table 1. There were few differences between the 2 groups after matching, ensuring a moderately fair comparison of the 2 techniques. Most importantly, the RG-TLIF cohort included more patients with CLBP as a surgical indication, and thus featured higher preoperative back pain and lower preoperative leg pain scores than the RG-PLIF cohort.
There were no statistically significant differences in the static 12-month postoperative PROM values, including ODI values for subjective functional disability and pain scores (all p > 0.05) (Table 2). The same results were achieved when looking at dynamic PROM change scores (all p > 0.05) (Fig. 1). However, the RG-PLIF group achieved a greater improvement in VAS leg pain (78.9% vs. 55.3%, p = 0.028) when comparing achievement of the MCID. In contrast, the RG-TLIF group achieved a lower rate of MCID in terms of ODI (68.4% vs. 92.1%, p = 0.009). There was no difference in MCID achievement regarding back pain severity (p = 0.185).
There were no differences between RG-TLIF and RG-PLIF in terms of surgical time (132.3 ± 29.4 minutes vs. 156.5 ± 53.0 minutes, p = 0.162), length of hospital stay (55.9 ± 20.0 hours vs. 57.2 ± 18.8 hours, p = 0.683), and intraoperative radiation as measures by the DAP (310.6 ± 126.1 mGy× cm2 vs. 287.9 ± 90.3 mGy × cm2, p = 0.370). Complications were equally common among the 2 groups (both 5.2%), including only transient and persistent motor or sensory weaknesses (Table 1).
In an analysis of 76 patients from a prospective registry, we compared the RG-TLIF and RG-PLIF surgical approaches to identify any relevant differences in the outcome parameters and PROMs. The 2 surgical approaches compared in our study did not show a statistically significant difference either in the static 12-month postoperative PROM, nor in the ODI nor the dynamic PROM change scores. However, our study did show differences between the 2 groups in regards to the achievement of the MCID. The VAS leg pain seemed to be improved to a greater extent using the RG-PLIF approach, whereas the RG-TLIF approach achieved a higher rate of clinical success in terms of functional impairment. Concerning the MCID achievement of back pain severity, there was no difference between the 2 groups. The secondary endpoints surgical time, length of hospital stay, intraoperative radiation as measured by the DAP, and occurrence of complications showed no differences between the RGPLIF and RG-TLIF group. These results confirm that both techniques should be regarded as equally effective but suited for slightly different purposes. Thus, technique choice in robotic spine surgery should be tailored to the surgical goal (i.e., primarily fusion or primarily decompression of the neuroforamen) and patient.
A multitude of studies has shown a certain superiority of MIS for PLIF and TLIF approaches [1,2,24,25]. Perioperative outcomes like blood loss [1,2,24], length of stay [1,2,25], and operative time have shown to be improved in patients undergoing MIS compared to open procedures [1]. Additionally in an analysis conducted by Mummaneni et al. [2] the patients’ leg pain seemed to have significantly improved using a MIS approach compared to open surgery. But despite functional outcome scores being notably improved for the MIS cohort, a statistical significance was not shown for all of them [2]. Zhang et al. [26] report that complication rates as well as back and leg pain severity seem to be significantly lower for TLIF compared to PLIF [26]. In other studies, the complication rates and the patient-reported outcomes showed no superiority of the MIS approach when comparing with an open approach [1,24]. Some studies also show a shorter length of stay and a longer operative time compared with the open procedure [2,25]. Noticeably many outcome measures such as 90-day return to work, patient-reported outcomes, and length of stay have been equal among MIS and open surgery groups [2].
Robotic assistance for MIS potentially provides high precision during critical surgical procedures like PLIF and TLIF. The potential of robot-guided procedures to reduce revisions for screw malposition is supported by the current data found in the literature [4,10]. A short learning curve for robot-guided procedures, shorter operative time, lower complication rates, and shorter length of hospital stay are convincing benefits provided by robotic surgery [9,27]. Other systematic reviews state that while there are successful incorporations for robot-guided MIS and a convincing potential of the RG-MIS to improve the accuracy of pedicle screw insertion, the evidence is still insufficient to rank the robot-guided approach for pedicle screw placement superior to a non-robotic approach [12,27]. Furthermore it needs to be mentioned that there is still a limited number of high level of evidence studies evaluating robot-guided procedures, especially regarding the high associated acquisition and maintenance costs [9].
Further evaluations and evidence are needed to state whether robot-guided surgical approaches are superior to freehand approaches. The promising trials MIS ReFRESH and EUROSPIN are researching this very issue to fill these remaining voids of evidence. The MIS ReFRESH trial is currently investigating the differences between robot-guided and freehand techniques and the EUROSPIN trial aims to produce evidence on the potential comparative clinical benefits of robot-guided, navigated, and freehand surgical approaches [13,14].
In our study, there were no statistically significant differences between the 2 surgical approaches about the static 12-month postoperative PROM, the ODI, and the dynamic PROM change scores. Our results are supported by the literature review and meta-analysis performed by Zhang et al. [26]. They found that TLIF and PLIF were equal concerning radiographic fusion rate and clinical satisfaction [26]. The findings of Liu et al. [28] support this by showing an equal benefit in improving short-term functional outcomes and in decreasing VAS scores for patients with degenerative lumbar spondylolisthesis undergoing PLIF or TLIF [28]. Asil et al. [29], Fujimori et al. [30], and de Kunder et al. [31] did not find a significant difference between the 2 surgical methods regarding clinical improvement measured with the ODI [29-31]. In regard to our results, the RG-TLIF approach achieved a higher rate of MCID in terms of ODI. De Kunder et al. [31] in a meta-analysis find a slightly lower ODI scores for TLIF, hence supporting our findings.
Yet while in Asil and Yaldiz [29], patients undergoing the PLIF procedures reported a higher postoperative VAS value, and in Fujimori et al. [30], VAS of leg and lower back pain was significantly improved in their TLIF group, our postoperative PROM findings including VAS leg, and back pain did not show a significant difference between our 2 cohorts. However the MCID in turn showed a better improvement of the VAS leg pain for the RG-PLIF group, therefore standing in contrast with Asil and Yaldiz [29] and Fujimori et al. [30] Concerning complication rates Fujimori et al. [30] does not find a significant difference between the complication rates of PLIF and TLIF groups. However, in other studies, a higher rate of complications and longer time of surgery associated with the PLIF procedures are reported [26,28-30]. This stands in contrast to our results demonstrating equal complications and surgical time among the 2 groups.
Hence, it needs to be mentioned that while those studies compared TLIF and PLIF approach, they did not include the robot-guided TLIF and PLIF, as there has not yet been a comparison between the 2 robot-guided approaches. As mentioned above there is a multitude of benefits already evidenced for MIS and RG approaches [4,9,10,27]. The different approaches could therefore explain the slightly different outcomes in our study compared to literature found on comparisons between PLIF and TLIF. Hence the RG approach may improve the complication rate and surgery duration in the PLIF group, reported in non-robot-guided PLIF procedures [26,28,29,31].
In our study, an experienced neurosurgeon decided on the procedures based on his extensive knowledge and experience with the pathologies of discogenic CLBP and lumbar stenosis with spondylolisthesis and the TLIF and PLIF procedures. The neurosurgeon hypothesized that robotic PLIF may offer a slight advantage in decompressing the neuroforamen bilaterally compared to robotic TLIF in patients with bilateral symptoms. In this way, the neurosurgeon regarded both techniques as equally effective but suited for slightly different purposes, and suggests that technique choice in robotic spine surgery should be tailored to the surgical goal (i.e., primarily fusion or primarily decompression of the neuroforamen) and patient. This very selection process and the matter of this study is a single-center study, therefore, presents a possibility of bias. This might be demonstrated in our cohorts, by showing that the RG-TLIF group included more patients with CLBP as surgical indication. The indication for RG-PLIF included more patients with preoperative leg pain due to bilateral compression of the neuroforamen. Therefore, the RG-TLIF cohort displayed higher preoperative back pain and lower preoperative leg pain scores than the RG-PLIF cohort. Consequently, the improved leg pain in the RG-PLIF group can to the greatest extent be explained by the different indications for RG-PLIF and RG-TLIF.
In consideration of our cohort assignment not being randomized, we conducted a propensity score matching procedure matching for age-, sex-, and baseline PROM.
It is nonetheless important to consider the nonrandomized groups while studying the PROM change scores, which did not show a significant difference between the 2 cohorts. The matter of the 2 cohorts showing a difference in preoperative back and leg pain scores likewise needs to be considered. Looking at the MCID, the RG-PLIF cohort displayed a significantly superior improvement of VAS leg pain while there seemed to be no significant difference in the VAS back pain between the 2 groups. Nonetheless, the baseline differences among groups that persisted due to selection bias likely almost fully explain this difference in improvement.
Although all data were collected in a prospective registry and events captured systematically, and all patients with sufficient data and meeting the inclusion criteria were included, the presence of selection bias cannot be ruled out. In addition, all data stems from a single center and all procedures were carried out by one senior surgeon, possibly creating center/surgeon bias. Therefore, our findings may not generalize well to other centers with differing patient demographics. The analysis was not predefined and must thus be considered as a retrospective statistical analysis. Therefore, the sample size was also rather low. Most of our patients were relatively low-surgical-risk patients. This means that our data and conclusions should not readily be expanded to high-risk and elderly patients. Group allocation was not randomly assigned. Although we applied propensity score matching and although the aim of this study was never to present an unbiased comparison of the 2 techniques for the same patients, some baseline differences persisted, which likely influenced the outcomes and which can partially be explained by selection bias according to clinical criteria, as discussed above. Although we did carry out matching as our strategy to reduce intergroup bias, due to the relatively low sample size, a multivariable analysis was not deemed biostatistically sensible. Lastly, we did not have reliably radiographic follow-up data, in most cases because no follow-up imaging was carried out in clinically stable patients.
While robotic assistance in lumbar spinal fusion is supported by some evidence to reduce clinically relevant screw malposition, clinical outcome data is scarce, even more so when comparing different surgical approaches for robotic surgery. We evaluate robotic minimally invasive TLIF and robotic mini-open PLIF in a propensity score-matched comparison of clinical outcomes among patients undergoing surgery for discogenic CLBP or stenosis with spondylolisthesis. Our findings suggest that both RG-TLIF and RG-PLIF are viable and equally effective techniques in robotic spine surgery, achieving similarly excellent clinical results virtually without pedicle screw revisions. Further evaluation in prospective controlled trials with larger statistical power is encouraged.
Fig. 1.
Illustration of percentage change scores in the 3 main patient-reported outcome measures, stratified by TLIF/PLIF status. The dashed horizontal line represents a -30% change score, which defined the minimum clinically important difference (MCID) in this study according to Ostelo et al. [16] TLIF, transforaminal lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; VAS, visual analogue scale; ODI, Oswestry Disability Index.
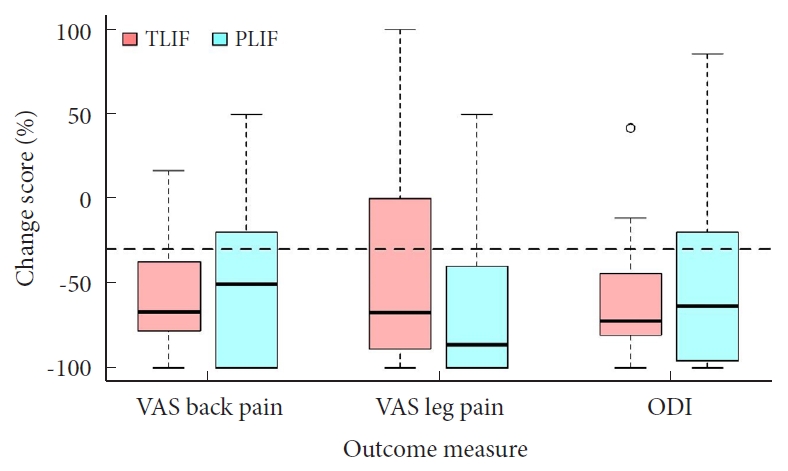
Table 1.
Patient summary statistics after propensity score matching
Values are presented as mean±standard deviation or number (%).
RG-TLIF, robot-guided transforaminal interbody fusion; RG-PLIF, robot-guided posterior lumbar interbody fusion; ASA PS, American Society of Anesthesiologists physical status; PROM, patient-reported outcome measure; VAS, visual analogue scale.
Table 2.
Comparison of outcome parameters and of PROMs between robotic transforaminal and robotic posterior lumbar interbody fusion
| Parameter | RG-TLIF (n = 38) | RG-PLIF (n = 38) | p-value |
|---|---|---|---|
| Surgical time (min) | 132.3 ± 29.4 | 156.5 ± 53.0 | 0.162 |
| Length of stay (hr) | 55.9 ± 20.0 | 57.2 ± 18.8 | 0.683 |
| Dose area product (mGy × cm2) | 310.6 ± 126.1 | 287.9 ± 90.3 | 0.370 |
| Postoperative PROMs | |||
| VAS back pain | 34.2 ± 25.3 | 30.2 ± 29.5 | 0.280 |
| VAS leg pain | 21.1 ± 24.9 | 20.0 ± 26.2 | 0.637 |
| Oswestry Disability Index | 16.5 ± 13.5 | 17.5 ± 16.9 | 0.822 |
| PROM percent change score (% change) | |||
| VAS back pain | -55.2 ± 31.4 | -46.88 ± 58.9 | 0.924 |
| VAS leg pain | -42.7 ± 62.4 | -65.7 ± 42.5 | 0.066 |
| Oswestry Disability Index | -64.4 ± 29.3 | -55.0 ± 43.5 | 0.451 |
| MCID of -30% | |||
| VAS back pain | 31 (81.6) | 26 (68.4) | 0.185 |
| VAS leg pain | 21 (55.3) | 30 (78.9) | 0.028* |
| Oswestry Disability Index | 35 (92.1) | 26 (68.4) | 0.009* |
REFERENCES
1. Goldstein CL, Phillips FM, Rampersaud YR. Comparative effectiveness and economic evaluations of open versus minimally invasive posterior or transforaminal lumbar interbody fusion: a systematic review. Spine (Phila Pa 1976) 2016 41 Suppl 8:S74-89.

2. Mummaneni PV, Bisson EF, Kerezoudis P, et al. Minimally invasive versus open fusion for grade I degenerative lumbar spondylolisthesis: analysis of the Quality Outcomes Database. Neurosurg Focus 2017 43:E11.

3. Härtl R, Lam KS, Wang J, et al. Worldwide survey on the use of navigation in spine surgery. World Neurosurg 2013 79:162-72.


4. Schröder ML, Staartjes VE. Revisions for screw malposition and clinical outcomes after robot-guided lumbar fusion for spondylolisthesis. Neurosurg Focus 2017 42:E12.


5. Bono CM, Lee CK. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine (Phila Pa 1976) 2004 29:455-63.

6. Kepler CK, Vaccaro AR, Hilibrand AS, et al. National trends in the use of fusion techniques to treat degenerative spondylolisthesis. Spine (Phila Pa 1976) 2014 39:1584-9.


7. Overley SC, Cho SK, Mehta AI, et al. Navigation and robotics in spinal surgery: where are we now? Neurosurgery 2017 80(3S):S86-99.


8. Ryang YM, Villard J, Obermüller T, et al. Learning curve of 3D fluoroscopy image-guided pedicle screw placement in the thoracolumbar spine. Spine J 2015 15:467-76.


9. Siccoli A, Klukowska AM, Schröder ML, et al. A systematic review and meta-analysis of perioperative parameters in robot-guided, navigated, and freehand thoracolumbar pedicle screw instrumentation. World Neurosurg 2019 127:576-87.e5.


10. Staartjes VE, Klukowska AM, Schröder ML. Pedicle screw revision in robot-guided, navigated, and freehand thoracolumbar instrumentation: a systematic review and meta-analysis. World Neurosurg 2018 116:433-43.e8.


11. Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine (Phila Pa 1976) 2007 32:E111-20.

12. Marcus HJ, Cundy TP, Nandi D, et al. Robot-assisted and fluoroscopy-guided pedicle screw placement: a systematic review. Eur Spine J 2014 23:291-7.


13. Schroerlucke SR, Wang MY, Cannestra AF, et al. Complication rate in robotic-guided vs fluoro-guided minimally invasive spinal fusion surgery: report from MIS refresh prospective comparative study. Spine J 2017 17(10 Suppl):S254-5.

14. Staartjes VE, Molliqaj G, van Kampen PM, et al. The European Robotic Spinal Instrumentation (EUROSPIN) study: protocol for a multicentre prospective observational study of pedicle screw revision surgery after robot-guided, navigated and freehand thoracolumbar spinal fusion. BMJ Open 2019 9:e030389.



15. Hyun SJ, Kim KJ, Jahng TA, et al. Minimally invasive robotic versus open fluoroscopic-guided spinal instrumented fusions: a randomized controlled trial. Spine (Phila Pa 1976) 2017 42:353-8.

16. Staartjes VE, Schröder ML. Effectiveness of a decision-making protocol for the surgical treatment of lumbar stenosis with grade 1 degenerative spondylolisthesis. World Neurosurg 2018 110:e355-61.


17. Staartjes VE, de Wispelaere MP, Schröder ML. Improving recovery after elective degenerative spine surgery: 5-year experience with an enhanced recovery after surgery (ERAS) protocol. Neurosurg Focus 2019 46:E7.

18. Von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007 335:806-8.



19. Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008 33:90-4.

20. Khor S, Lavallee D, Cizik AM, et al. Development and validation of a prediction model for pain and functional outcomes after lumbar spine surgery. JAMA Surg 2018 153:634-42.



21. Quddusi A, Eversdijk HAJ, Klukowska AM, et al. External validation of a prediction model for pain and functional outcome after elective lumbar spinal fusion. Eur Spine J 2020 29:374-83.


22. Ho D, Imai K, King G, et al. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Political Analysis 2007 15:199-236.

23. Streitberg B, Röhmel J. Exact distributions for permutation and rank tests: an introduction to some recently published algorithms. Stat Softw Newsl 1986 12:10-7.
24. Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015 1:2-18.


25. Zhao J, Zhang S, Li X, et al. Comparison of minimally invasive and open transforaminal lumbar interbody fusion for lumbar disc herniation: a retrospective cohort study. Med Sci Monit 2018 24:8693-8.



26. Zhang Q, Yuan Z, Zhou M, et al. A comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion: a literature review and meta-analysis. BMC Musculoskelet Disord 2014 15:367.



27. Stull JD, Mangan JJ, Vaccaro AR, et al. Robotic guidance in minimally invasive spine surgery: a review of recent literature and commentary on a developing technology. Curr Rev Musculoskelet Med 2019 12:245-51.



28. Liu J, Deng H, Long X, et al. A comparative study of perioperative complications between transforaminal versus posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Eur Spine J 2016 25:1575-80.


29. Asil K, Yaldiz C. Retrospective comparison of radiological and clinical outcomes of PLIF and TLIF techniques in patients who underwent lumbar spinal posterior stabilization. Medicine (Baltimore) 2016 95:e3235.































