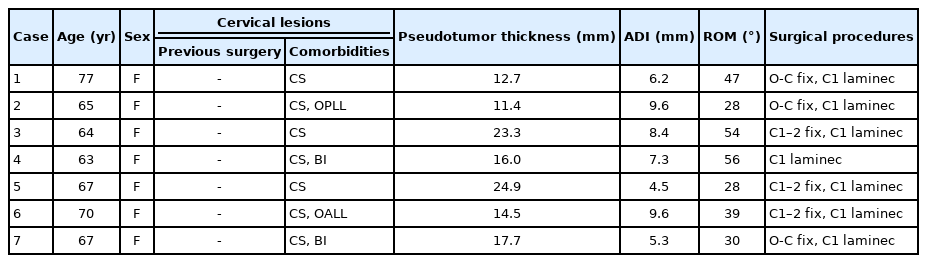
Nonrheumatoid Retro-Odontoid Pseudotumors: Characteristics, Surgical Outcomes, and Time-Dependent Regression After Posterior Fixation
Article information
Abstract
Objective
Although a retro-odontoid pseudotumor associated with rheumatoid arthritis is a well-known clinical entity, little is known about retro-odontoid pseudotumors not associated with rheumatoid arthritis due to their rarity.
Methods
Between 2006 and 2019, consecutive patients with nonrheumatoid pseudotumors were included and retrospectively compared with patients with rheumatoid pseudotumors.
Results
Nineteen patients had nonrheumatoid pseudotumors (mean age, 73 ± 6 years; male, 53%). All had cervical lesions including ossified anterior and posterior longitudinal ligaments with a history of cervical surgery in 5. The mean thickness of the pseudotumors at diagnosis was 8.1 mm (range, 4.2–17.2 mm). Pseudotumor thickness had a significant negative correlation with the atlantodental interval (p = 0.008) and the subaxial range of motion (p = 0.049). In comparison with 7 rheumatoid pseudotumor patients, nonrheumatoid pseudotumor patients were older (p = 0.042), had a higher proportion of males (p = 0.023), had a smaller atlantodental interval (p = 0.007), and had larger pseudotumors at diagnosis (p = 0.030). Of the 19 patients, 18 received posterior fixation with or without C1 laminectomy, while the other received C1 laminectomy alone. The percent pseudotumor thickness at follow-up to those at diagnosis was 91%, 77%, 68%, 46%, 58%, and 49% at 1, 3, 6, 12, 24, and 36 months after surgery, respectively.
Conclusion
This study revealed markedly clinical and radiological differences between nonrheumatoid and rheumatoid pseudotumors. The main etiology for nonrheumatoid pseudotumors was subaxial cervical degeneration and ossified lesions. There were good outcomes following posterior fixation and time-dependent pseudotumor regression within 12 months.
INTRODUCTION
A retro-odontoid pseudotumor is a nonneoplastic mass, which causes neck pain and/or myelopathy due to spinal cord compression at the craniovertebral junction. Rheumatoid arthritis is the most common predisposing factor for pseudotumors; however, special attention has been paid to nonrheumatoid pseudotumors since Sze et al. [1] reported 3 cases in 1986. Case reports of nonrheumatoid pseudotumors may be associated with cervical lesions such as atlantoaxial dislocation (AAD) [2,3], ossification of the anterior longitudinal ligament (OALL) [4], ossification of the posterior longitudinal ligament (OPLL) [5], diffuse idiopathic skeletal hyperosteosis [6,7], cervical spondylosis [8,9], os odontoideum [10,11], and atlanto-occipital assimilation [12]. These cervical lesions may increase mechanical stress on the craniovertebral junction for many years and induce degenerative pseudotumors [4]. Although reports on nonrheumatoid pseudotumors increased after the introduction of magnetic resonance imaging (MRI), little is known about the clinical and anatomical characteristics of nonrheumatoid pseudotumors due to their rarity.
The appropriate surgical treatment is still controversial, and direct pseudotumor resection [13,14], posterior fixation [15], posterior decompression [5,16], and combinations of these approaches [6] have been reported. Posterior fixation is emerging as a promising procedure, but previous reports have been limited to small case series [4,17,18]. Moreover, it is not clear how long it takes for pseudotumors to regress after surgery [19,20]. Review of literature is less than satisfactory.
The purpose of this study was to evaluate clinical and radiological characteristics, the etiology, surgical outcomes following posterior fixation including postoperative time-dependent changes in the thickness of nonrheumatoid pseudotumors. A literature review was conducted to integrate available reports on nonrheumatoid pseudotumors.
MATERIALS AND METHODS
1. Ethics
This study protocol was approved by the Institutional Review Board of Tokyo Metropolitan Neurological Hospital (#TS-R02-004). Since this was a retrospective and noninvasive study, written informed consent from patients was waived. Instead, a public notice that provided information on this study was provided.
2. Patients
Consecutive adult (> 20 years) patients with nonrheumatoid pseudotumors surgically treated between April 2006 and March 2019 were included. The control group was consecutive patients with rheumatoid pseudotumors treated during the same period. Rheumatoid arthritis was diagnosed by physicians and information was collected from medical records. Patients with a follow-up duration of less than one year after surgery were excluded. Clinical information including age, sex, previous medical history, cervical pain, neurological symptoms, radiological findings, surgical procedures, complications, follow-up duration, and postoperative symptoms was extracted. Neurological symptoms were evaluated using modified Rankin Scale (mRS) scores, grip strength, the grip-and-release test [21], and patella tendon reflex.
The thickness of each pseudotumor was evaluated on sagittal images in a neutral position. Fast imaging employing steady-state acquisition MRI, T2-weighted MRI, and computed tomography (CT) were used for evaluation. The thickness of each pseudotumor was determined as the maximal distance between the posterior border of the odontoid process and the posterior border of the pseudotumor.
The atlantodental interval and subaxial range of motion (ROM) were evaluated on sagittal CT images or lateral cervical x-rays. CT images were used to assess the calcification of pseudotumors as well as cervical instability. The presence of AAD was defined as the atlantodental interval in the neck flexion position being larger than 4 mm. The subaxial ROM was defined as the angle composed of the posterior lines of the C2 and C7 vertebrae in neck flexion and extension positions. Correlation between pseudotumor thickness and the atlantodental interval was assessed. Correlation between pseudotumor thickness and the subaxial ROM was also assessed. Data were collected from medical charts and telephone interviews in compliance with the Institutional Review Board at our institution.
3. Surgery and Outcomes
Under general anesthesia, patients were placed in a prone position. Posterior fixation was performed in all patients except for debilitated patients at high risk of perioperative complications. The decision to perform occipito-cervical (O-C) or C1–2 fixation was made individually based on a neurosurgical conference considering the atlantodental interval and cervical alignments. An O-arm-guided stereotactic navigation system was used for posterior fixation surgeries. The neck was placed in the neutral position to reposition C1 and decompress the spinal cord. C1 laminectomy was also performed on patients with AAD with severe spinal cord compression despite repositioning in the neutral neck position. Autologous bone grafts were obtained from the iliac crest bone or C1 lamina. Autologous grafts were secured between the posterior elements of O-C or C1–2 for bone fusion, posterior cervical decompression was performed concurrently for patients with severe cervical spondylosis. Foramen magnum decompression was performed for those with basilar impression and Chiari malformation. Patients were followed up at 1, 3, 6, 12, 24, and 36 months at an outpatient clinic. Variables for physical status included the presence of neck pain, grip strength, the grip-and-release test, and the mRS score. MRI was performed to evaluate spinal cord compression, and x-rays or CT images were used to assess postoperative AAD and bone fusion. Pseudotumor thickness was measured using MRI and CT images in the neutral neck position.
4. Pseudotumor Regression
The postoperative thickness of pseudotumors was measured at 1, 3, 6, 12, 24, and 36 months after surgery. The percent thickness of each pseudotumor at follow-up to that at diagnosis was calculated. Factors for insufficient (less than 50%) reduction in the thickness of pseudotumors were identified via univariate logistic regression analysis using the following variables: demography (age and sex), cervical lesions (previous surgery, cervical spondylosis, OALL, OPLL, and basilar impression), radiological findings (pseudotumor thickness at diagnosis, atlantodental interval, AAD, subaxial ROM, cyst formation, and calcification), surgical procedures (O-C fixation, C1–2 fixation, and C1 laminectomy), and follow-up duration. The time-dependent changes in pseudotumor thickness were evaluated and compared among follow-up periods.
5. Statistical Analysis
Statistical analysis was conducted using IBM SPSS statistics, ver. 23 (IBM Corp., Armonk New York, USA). Fisher exact probability test, the unpaired Student t-test, the Mann-Whitney U-test, a 1-way repeated-measures analysis of variance with the Bonferroni correction, and Pearson test were used to analyze the data. Univariate logistic regression analyses were performed to identify risk factors for pseudotumor regression of less than 50% after surgery. Values of p < 0.05 were considered significant.
RESULTS
1. Clinical and Radiological Characteristics
There were 19 patients with a nonrheumatoid pseudotumor and 7 with a rheumatoid pseudotumor (Tables 1, 2). Two patients were excluded due to the short follow-up period. Trauma, infection, and hemodialysis-associated pseudotumors were not observed during the study period. Patients with nonrheumatoid pseudotumors included 10 men (53%) and 9 women (47%) with a mean age of 73 ± 6 years (Table 1). All of them had congenital and/or acquired cervical lesions with a history of surgery in 5. Nine (47%) had cervical pain. The median mRS score was 3 (range, 1–5) and the median grip strength of 8 patients was 19 kg (range, 5–35 kg). The grip-and-release test of 6 patients showed a median of 14 times in 10 seconds (range, 6–30 times/10 seconds); and 9 of 17 patients (53%) showed hyperreflexia of the patella tendon. The median duration of preoperative symptoms was 1 year (range, 1 month to 6 years).
Preoperative thickness of nonrheumatoid pseudotumors was determined in all cases with a median thickness of 8.1 mm (range, 4.2–17.2 mm). Thirteen patients (76%) had AAD. The mean atlantodental interval was 4.8 ± 1.8 mm and the mean subaxial ROM was 35.9° ± 18.3°. Pseudotumor thickness had a significant negative correlation with the atlantodental interval (Pearson correlation coefficient= -0.622, p = 0.008) and the subaxial ROM (Pearson correlation coefficient= -0.499, p = 0.049) (Fig. 1). In comparison with rheumatoid pseudotumor patients (Table 2), nonrheumatoid pseudotumor patients were older (p = 0.042), had a higher proportion of males (p = 0.023), had a smaller atlantodental interval (p = 0.007), and had larger pseudotumors at diagnosis (p = 0030) (Table 3).

(A) Correlation between pseudotumor thickness and the atlantodental interval. (B) Correlation between pseudotumor thickness and the subaxial range of motion. Pseudotumor thickness had a significant negative correlation with the atlantodental interval (Pearson correlation coefficient = -0.622, p = 0.008) and the subaxial range of motion (ROM) (Pearson correlation coefficient = -0.499, p = 0.049).
2. Surgery and Outcomes
Eighteen patients underwent posterior fixation with or without C1 laminectomy, while the other underwent C1 laminectomy alone (Table 1). Three patients also underwent pseudotumor biopsy. One patient underwent cervical laminoplasty at C3 to C6 and 2 patients underwent foramen magnum decompression concurrently. Complications were vertebral artery injury due to malfunction of the O-arm navigation system (n = 1), venous plexus injury (n = 1), transient laryngopharyngeal edema (n = 1), and screw malposition which required reoperation (n = 1).
The median follow-up duration was 30 months (range, 15–90 months). Neurological symptoms improved within a median of 2 months (range, 1–8 months) after surgery. In 7 of 9 patients (78%), neck pain disappeared after surgery. The postoperative median mRS score was 2 (range, 1–4), the postoperative median grip strength was 20 kg (range, 8–42 kg) and the grip-and-release test showed a median of 19 times in 10 seconds (range, 14–28 times/10 seconds). After surgery, the mRS score and the grip-and-release test showed significant improvements (p = 0.005 and p = 0.025, respectively), while grip strength did not. The postoperative median of the atlantodental interval was 3.4 mm (range, 1.3–7.0 mm) and no patient showed any worsening of AAD.
3. Pseudotumor Regression
Successful pseudotumor regression was achieved following posterior fixation in the median follow-up of 30 months (range, 15–90 months) (Fig. 2). No pseudotumor regrowth was observed. Ten pseudotumors (53%) showed more than a 50% reduction in thickness. There were no risk factors related to less than a 50% reduction in the thickness of pseudotumors after surgery. The median percent pseudotumor thickness at follow-up to those at diagnosis was 91%, 77%, 68%, 46%, 58%, and 49% at 1, 3, 6, 12, 24, and 36 months after surgery, respectively. Significant pseudotumor regression was observed within 12 months following posterior fixation (p = 0.049) (Fig. 2). No significant pseudotumor regression was observed between 12 and 36 months after surgery (p = 1.0). Sixteen of the 18 patients (89%) who underwent posterior fixation achieved successful solid bone fusion. The other 2 patients had a clear zone around C1 screws on CT images; however, reoperation was not performed because pseudotumors regressed on MRI.
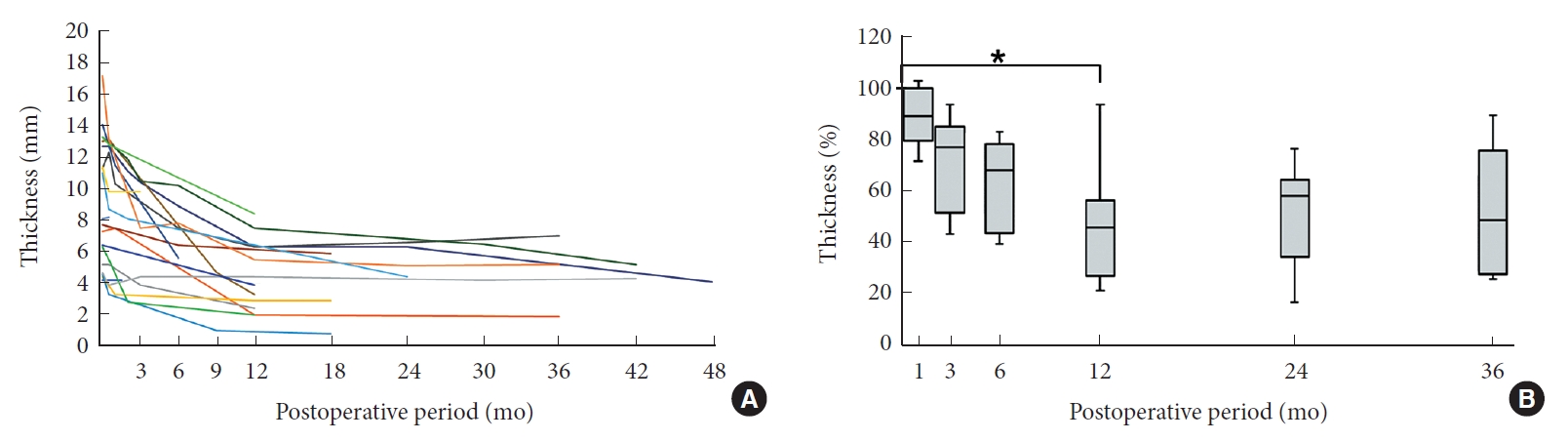
(A) The relationship between the thickness of nonrheumatoid pseudotumors and the postoperative follow-up period in each patient. (B) The relationship between the percent thickness of nonrheumatoid pseudotumors and postoperative follow-up periods at 1, 3, 6, 12, 24, and 36 months. *p < 0.05, statistical significance.
4. Illustrative Cases
1) Case 9
A 74-year-old woman presented with severe numbness of the upper and lower extremities. At another hospital, she underwent C1 laminectomy 6 years prior to admission to our hospital (Fig. 3); however, the thickness of the pseudotumor did not change and her symptoms gradually worsened. On admission, physical examinations showed bilateral weakness of the upper and lower extremities. Grip strengths were 8/10 kg on the right/left. The grip-and-release tests showed 11/13 times in 10 seconds on the right/left. She had hyperreflexia of the patella tendon. The sensations of touch, pain, and temperature were impaired on the right. She did not complain of neck pain. Her past medical history included crown dens syndrome, cervical calcification of the yellow ligament, chronic heart failure, diabetes mellitus, and angina pectoris. Radiological investigations revealed a retro-odontoid pseudotumor, which was 11.3 mm in thickness, compressing the spinal cord. Intramedullary hyperintensity on T2-weighted MRI indicated pyramidal tract disorder. The atlantodental interval was 5.1 mm and the subaxial ROM was 31°. After C1–2 fixation surgery, grip strengths and the grip-and-release tests improved (12/8 kg and 17/18 times/10 seconds, respectively), but sensory impairment worsened slightly. Gradual pseudotumor regression was observed after surgery, especially during the first 12 months. Pseudotumor thickness was 10.3 mm (91%) at 1 month, 7.5 mm (66%) at 6 months, and 6.3 mm (56%) at 12 months. The thickness did not change from 12 to 36 months after surgery.
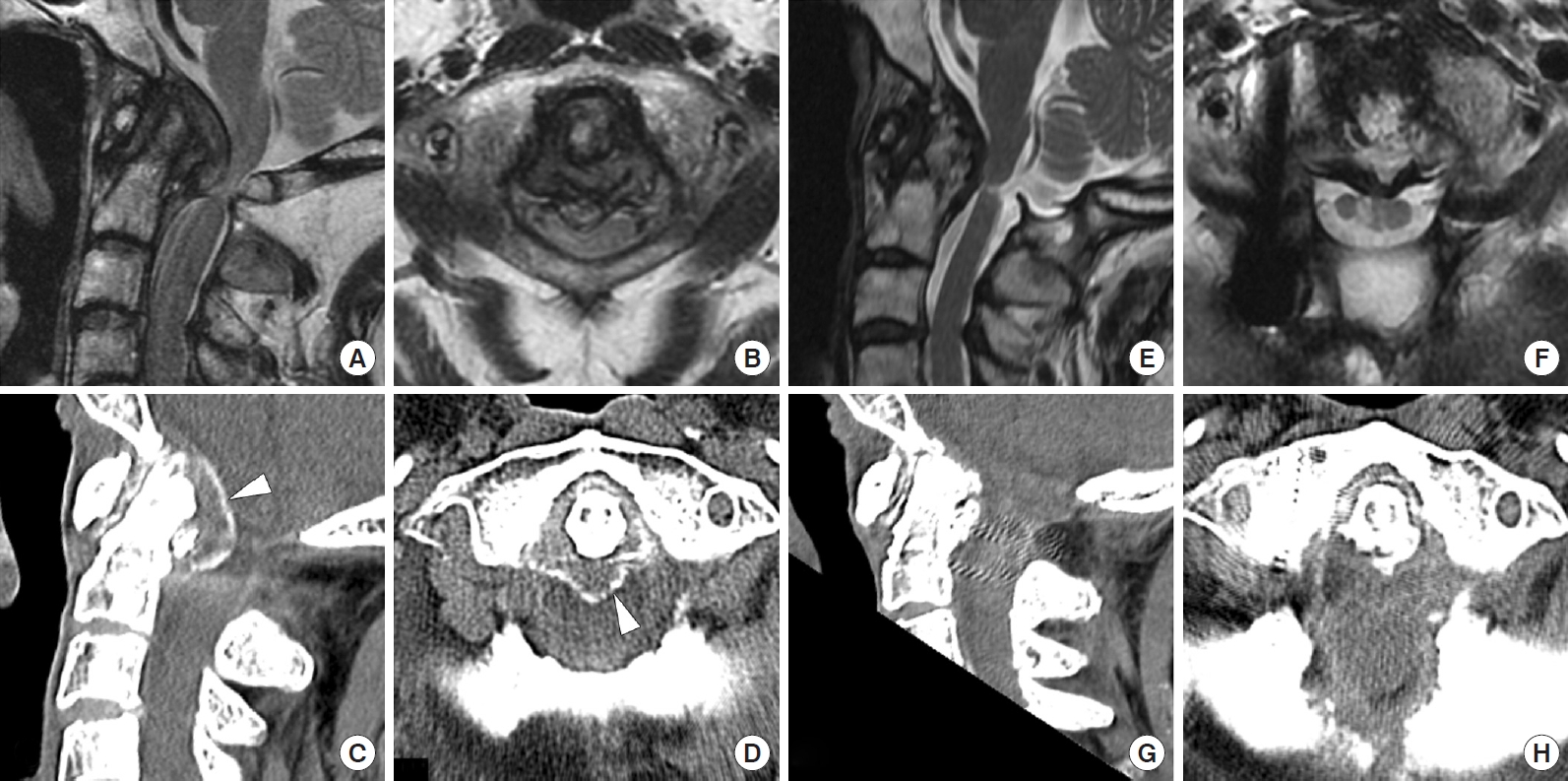
Case 9: A 74-year-old woman with a nonrheumatoid pseudotumor treated by 2 procedures: C1 laminectomy alone at another hospital and posterior fixation at the authors’ hospital. An initial sagittal (A) and axial (B) T2-weighted magnetic resonance imaging (MRI) image showing a retro-odontoid pseudotumor compressing the spinal cord diagnosed at the age of 68 years. Sagittal (C) and axial (D) images of computed tomography 6 years after C1 laminectomy alone. Note there was no pseudotumor regression and the spinal cord was compressed by the pseudotumor. Arrowheads indicate the calcified border of the pseudotumor. Sagittal and axial images of MRI (E, F) and CT (G, H) 1 year after C1–2 fixation surgery without pseudotumor removal. Note significant tumor regression and a pedicle screw on the right side.
2) Case 12
An 83-year-old man underwent C3–7 laminoplasty for myelopathy due to cervical OPLL at the age of 66 years. Preoperative MRI showed a straight cervical spine and slight retro-odontoid soft tissue thickness without mass (Fig. 4). The subaxial ROM was 27° at that time. In retrospect, follow-up MRI showed a pseudotumor at the age of 70 years, although his neurological symptoms did not worsen. He developed tetraparesis after a fall when he was 83. Physical examination showed bilateral weakness of the upper and lower extremities, especially on the right side. Grip strengths were 5/14 kg and the grip-and-release tests showed 6/15 times in 10 seconds. He had hyperreflexia of the patella tendon and bilateral sensory impairment. He did not complain of neck pain. His past medical history was complicated with fusion of the cervical spine, OALL, OPLL, lumbar stenosis, ossification of the yellow ligament, and thoracic compression fracture. MRI demonstrated enlargement of the pseudotumor, which was 13.3 mm in thickness. The atlantodental interval was 4.2 mm and the subaxial ROM was 0°. After C1–2 fixation surgery, grip strengths improved (12/17 kg). The grip-and-release tests also improved (14/18 times in 10 seconds on the right/left). Significant pseudotumor regression was observed after surgery, especially during the first 12 months. Pseudotumor thickness was 10.5 mm (79%) at 3 months, 7.5 mm (56%) at 12 months, and 5.2 mm (39%) at 42 months.
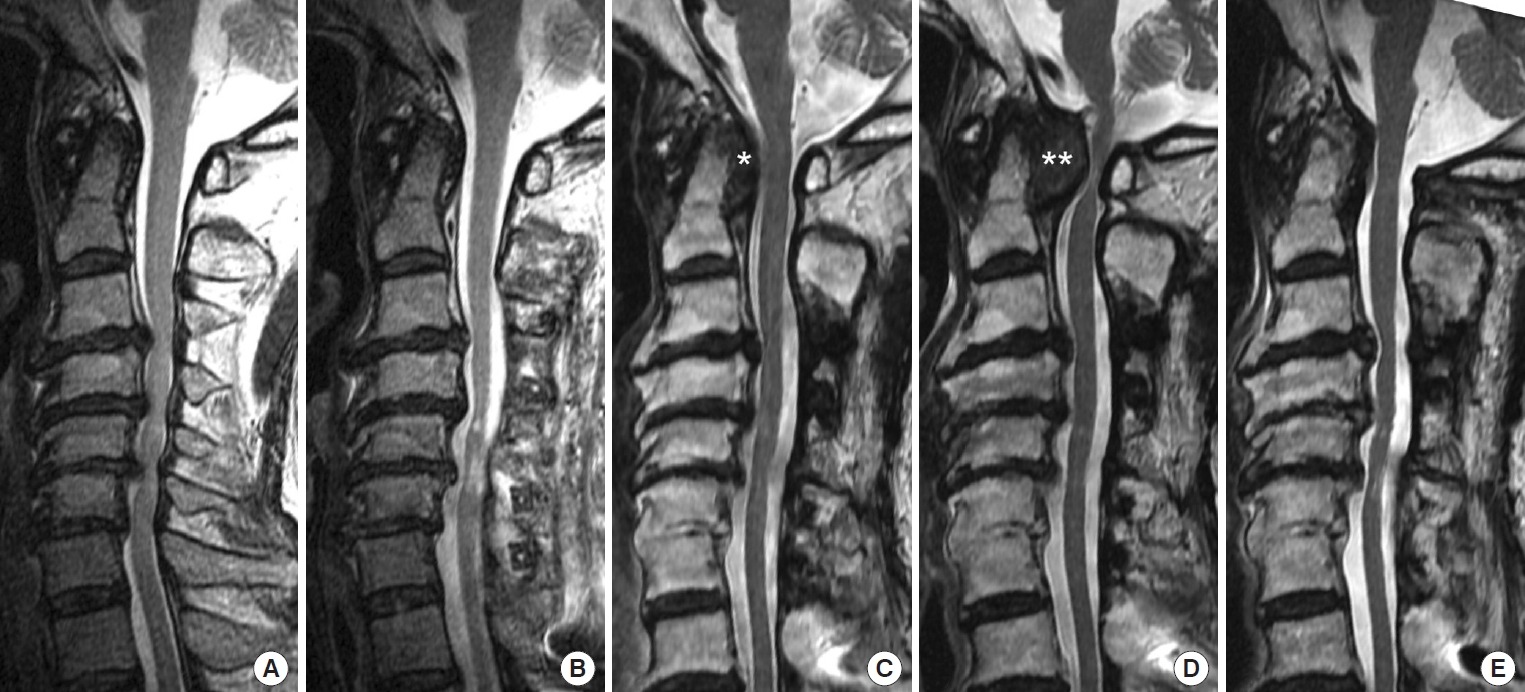
Case 12: An 83-year-old man with a newly developed pseudotumor after C3–7 laminoplasty for cervical ossification of the posterior longitudinal ligament (OPLL). (A) An initial sagittal T2- weighted magnetic resonance imaging (MRI) image showing severe spinal cord compression due to OPLL at the age of 66 years. No pseudotumor was observed at that time (The retroodontoid soft tissue thickness was 3 mm). (B) A sagittal T2-weighted MRI image following C3–7 laminoplasty showing decompression of the spinal cord. (C, D) Sagittal T2-weighted MRI images showing a newly developed pseudotumor at the ages of 70 (asterisk in C) and 83 years (double asterisks in D). (E) A sagittal T2-weighted MRI image 42 months after C1–2 fixation surgery showing significant pseudotumor regression.
DISCUSSION
To the best of our knowledge, this study has the largest number of patients with nonrheumatoid pseudotumors treated surgically. The results of our study revealed the following 3 important findings: firstly, there were marked differences in clinical and radiological characteristics between nonrheumatoid and rheumatoid pseudotumors; secondly, the main etiology for the nonrheumatoid pseudotumors was decreased subaxial ROM related to cervical degeneration and ossified lesions; and lastly, posterior fixation was effective for nonrheumatoid pseudotumors because significant time-dependent postoperative regression was observed after posterior fixation, especially during the first 12 months.
1. Clinical and Radiological Characteristics
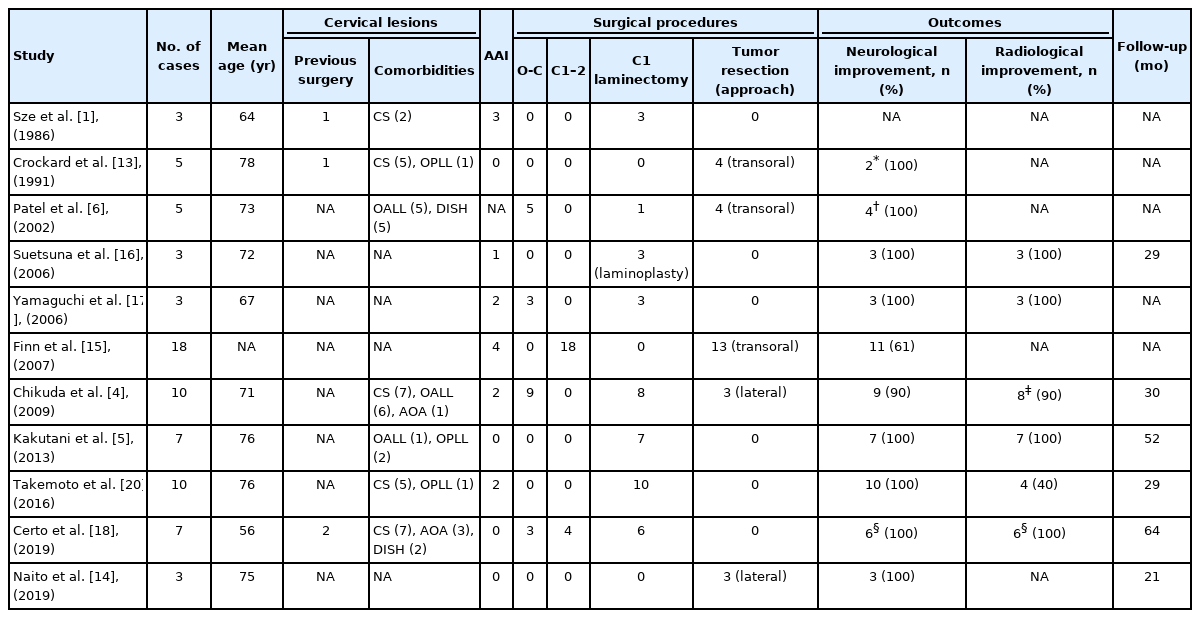
The nonrheumatoid pseudotumors in this study were diagnosed in patients around the 7th decade of life with a slight male predominance. They were diagnosed in patients who were significantly older than those with rheumatoid pseudotumors. We searched case series that included 3 or more cases of nonrheumatoid pseudotumors treated surgically. Our literature search identified 11 articles with a total of 74 cases of nonrheumatoid pseudotumors (Table 4) [1,4-6,13-18,20]. The mean age of the patients was 68 years and men comprised 66%. Our results were compatible with these reports.
In our study, OALL and/or OPLL was present in 12 of 19 patients (63%) and pseudotumor thickness had a negative correlation with the subaxial ROM in nonrheumatoid pseudotumors (Fig. 1). Our results were compatible with published reports, in which OALL and/or OPLL were common in cases of nonrheumatoid pseudotumors (Table 4). A number of etiologies for nonrheumatoid pseudotumors have been reported [22]. In our study population, restriction of the subaxial ROM was the main etiology of nonrheumatoid pseudotumors. Other etiologies included crowned dens syndrome (retro-odontoid calcium pyrophosphate deposition), cervical dystonia (cervical involuntary movement), and basilar impression or Chiari malformation (congenital lesions). Trauma, infection, and hemodialysis-associated pseudotumors were not observed. Interestingly, in case 12, a pseudotumor developed in his 80s, 17 years after C3–7 laminoplasty for cervical OPLL. This is the first case report, to our knowledge, that demonstrated a new developmental process of a pseudotumor, although 2 case reports described enlargement of an existing pseudotumor after laminoplasty [8,9]. One cause of postoperative development of the pseudotumor may be a reduction in the subaxial ROM from 27° to 0° due to cervical OALL, OPLL, and laminoplasty, which resulted in compensatory mechanical stress on the craniocervical junction [23].
The present study also showed that nonrheumatoid patients had significantly larger pseudotumors with a smaller atlantodental interval than rheumatoid patients. This may be due to the early detection of rheumatoid pseudotumors in routine follow-ups by physicians. Another possibility is that nonrheumatoid pseudotumors may develop in a different manner than rheumatoid pseudotumors; the pathogenesis of rheumatoid pseudotumors, which have been termed “pannus,” includes inflammatory, fibrous, or combined conditions, while that of nonrheumatoid pseudotumors includes noninflammatory conditions. However, the pathophysiological mechanisms underlying the development of nonrheumatoid pseudotumors have not yet been elucidated because there have been no comparative studies on the relationship between histopathological and MRI findings [22].
Furthermore, we found that pseudotumor thickness negatively correlated with the atlantodental interval in nonrheumatoid pseudotumors (Fig. 1). This may be due to the time gap between an increase in the atlantodental interval and the development of a pseudotumor: in case 12, an increase in the atlantodental interval was observed after laminoplasty (Fig. 4C), followed by enlargement of the pseudotumor. The atlantodental interval decreased when the pseudotumor reached its maximum thickness (Fig. 4D). Similar results were reported in the previous studies on rheumatoid pseudotumors, in which the retro-odontoid soft tissue thickness negatively correlated with the atlanto-dental interval [24,25]. In our study, AAD was not present in 4 of 19 patients (21%) with nonrheumatoid pseudotumors but it was present in all 7 patients in rheumatoid pseudotumors. These results were compatible with published reports, in which AAD was not present in some cases of nonrheumatoid pseudotumors (Table 4) [26].
2. Surgery and Outcomes
An appropriate surgical procedure for nonrheumatoid pseudotumors has not yet been established, although many procedures, such as anterior pseudotumor resection, posterior fixation, posterior decompression, or combinations of these, have been reported (Table 4). Anterior pseudotumor resection has been proposed as the best procedure, and it could achieve rapid decompression of the spinal cord by direct manipulation of the pseudotumor. However, this procedure carries a high risk of complications such as pulmonary complications, infection, cerebrospinal fluid leak, and spinal cord damage [6,13,27]. Recently, several reports on posterior fixation with or without decompression demonstrated good neurological outcomes and spontaneous pseudotumor regression [4,18]. In our study, with the largest number of patients to date, we found good outcomes and pseudotumor regression following posterior fixation. C1 laminectomy alone sometimes resulted in good neurological outcomes [5,16,20]; however, in some cases including our case 9, the pseudotumor did not regress [26]. Therefore, we propose that C1 laminectomy alone should be considered only for cases with a small pseudotumor.
3. Pseudotumor Regression
Ten nonheumatoid pseudotumors (53%) and 1 rheumatoid pseudotumor (14%) showed more than a 50% reduction in thickness. Regression was greater in nonrheumatoid pseudotumors, however, this difference was not statistically significant (p < 0.18). Our statistical analysis did not identify any factors related to less than a 50% reduction in the thickness of pseudotumors. This may be due to the small number of cases in our study. In the literature, contrast enhancement of pseudotumors has been reported as a factor for significant pseudotumor regression [5], although this was not evaluated in our study. More cases of pseudotumors should be accumulated to identify factors related to insufficient pseudotumor regression.
Time-dependent changes in thickness were not clear, although several studies reported pseudotumor regression. Chikuda et al. [4] reported that the thickness of pseudotumor at diagnosis was 9.4 ± 1.3 mm and this regressed to 3.4 ± 0.8 mm following posterior fixation. Takemoto et al. [20] found that C1 laminectomy succeeded in achieving slight pseudotumor regression from 9.5 ± 2.0 mm to 8.1 ± 2.9 mm, but they did not indicate when pseudotumor regression occurred. Barbagallo et al. [19] reported that “pannus reduction or disappearance required 4–14 months,” but they did not detail the specific thickness of pseudotumors. To the best of our knowledge, this is the first case series study to quantify time-dependent pseudotumor regression (Fig. 2). Significant pseudotumor regression was observed within 12 months following posterior fixation and no significant pseudotumor regression was observed subsequently. Our results indicate that follow-up MRI should be performed at around 12 months after posterior fixation. In cases in which neurological symptoms do not improve because of insufficient pseudotumor regression after posterior fixation, additional treatment such as anterior decompression should be considered.
4. Limitations
The limitations of the results obtained arise from the retrospective nature of the study. Even though this is the largest case series study, the relatively small number of patients in a single institution may limit the power of statistical significance. Despite these limitations, we believe that our findings contribute to making treatment choices and performing follow-up examinations for nonrheumatoid pseudotumors.
CONCLUSION
This study showed marked clinical and anatomical differences between nonrheumatoid and rheumatoid pseudotumors. Nonrheumatoid pseudotumors were diagnosed in patients who were significantly older (73 years) than those with rheumatoid pseudotumors, with a slight male predominance (53%). Nonrheumatoid patients had significantly larger pseudotumors (8.1 mm) with a smaller atlantodental interval (4.8 mm) compared with rheumatoid patients. The main etiology for the nonrheumatoid pseudotumors was decreased subaxial ROM related to cervical degeneration and OALL/OPLL. Posterior occipito-cervical or C1–2 fixation was effective for neck pain and myelopathy in nonrheumatoid pseudotumor patients. Significant pseudotumor regression occurred after posterior fixation, especially during the first 12 months. Follow-up MRI needs to be offered at around 12 months after surgery to evaluate pseudotumor regression.
Notes
The authors have nothing to disclose.