Emerging Technologies in the Treatment of Adult Spinal Deformity
Article information
Abstract
Outcomes for adult spinal deformity continue to improve as new technologies become integrated into clinical practice. Machine learning, robot-guided spinal surgery, and patient-specific rods are tools that are being used to improve preoperative planning and patient satisfaction. Machine learning can be used to predict complications, readmissions, and generate postoperative radiographs which can be shown to patients to guide discussions about surgery. Robot-guided spinal surgery is a rapidly growing field showing signs of greater accuracy in screw placement during surgery. Patient-specific rods offer improved outcomes through higher correction rates and decreased rates of rod breakage while decreasing operative time. The objective of this review is to evaluate trends in the literature about machine learning, robot-guided spinal surgery, and patient-specific rods in the treatment of adult spinal deformity.
INTRODUCTION
Adult spinal deformity (ASD) is characterized by loss of spinal alignment in the sagittal and coronal planes, including scoliosis, kyphosis, spondylolisthesis, and rotatory subluxation [1]. ASD may develop due to degenerative changes, deformities in childhood development, infection, trauma, or tumors that affect the vertebral column [1,2]. While rates of adult scoliosis are as high as 32% of the general population, spinal deformity may affect up to 68% of individuals 65 years or older [3,4]. Advances in medical care, increasing life expectancy, and a growing elderly population contribute to an increase in expenditures on spine-related care, accounting for over $86 billion annually [2,5].
Advances in surgical techniques and perioperative care have increased the prevalence of surgical treatment for ASD [2,4]. Challenges remain with respect to complication rates, patient selection, and outcomes prediction. Recent advances in outcome predictions have led to enhanced patient care through improved perioperative planning and risk counseling. Current models implemented computational tools that can analyze large sets of data to predict outcomes and complications [6]. Traditional statistical models have demonstrated some success in predicting postoperative length of stay and clinical outcomes. However, there is room for improvement [7,8].
Machine learning (ML) shows promise to improve clinical decision-making and patient outcomes. It is a collection of statistical techniques that uses large quantities of data to develop a model to determine nuanced patterns and predicting outcomes [9]. ML is poised to revolutionize the management of ASD, with its broad-ranging applications in preoperative planning, outcomes prediction, improving research quality, diagnostic tool development, and assistance with surgical performance [10].
Robotic-guided spine surgery (RGSS) is another area of rapid advancement in the treatment of ASD. In spinal fusion surgery, RGSS has led to increased intraoperative accuracy for pedicle screw placement while decreasing radiation exposure, complication rates, operative time, blood loss and recovery time for patients [11,12]. Historically, surgeons relied on fluoroscopy-assisted free-hand screw placement, but many studies have now shown the superior accuracy that RGSS provides [12]. The ability of robotics to guide surgeon screw placement can yield fewer mistakes and reduce inter-surgeon variability.
Another growing technology in ASD is the use of patient-specific rods (PSR). PSRs are rods made during preoperative surgical planning to provide a frame specific for each patient’s correction. This removes intraoperative rod bending, which has been shown through postoperative radiographs to often undercorrect [13]. By implementing PSRs, we can expect to see better corrections for patients, lower rates of rod breakage, and decreased operative times [14]. Additionally, this allows for more precise and reproducible results as opposed to manual bending which presumably cannot be recreated accurately. As the future of ASD surgery continues to advance technologies, we can expect more successful surgeries, more accurate predictions, and fewer complications for patients.
Thus, ML, PSR, and RGSS lead to improvements in preoperative planning and postoperative management of complications. The objective of this paper is to assess the current status of these tools in ASD, and discuss their future applications in spine surgery.
MACHINE LEARNING
1. Overview
ML is a collection of statistical techniques that allow algorithms to “learn” patterns contained in large quantities of data. The statistical algorithms span a wide range from simple to exceptionally complicated. More complicated algorithms can learn more nuanced patterns in data than might be possible using standard statistical methods [15]. Thus, the rise of ML has brought about more intelligent methods for data analysis. When applied correctly to high-quality data, complicated ML algorithms may obtain great fidelity to the phenomena they are designed to model [16]. Clinically, this means that a ML model might emulate surgeon thinking very well in complex tasks like diagnosis or outcomes prognostication. In addition to improved accuracy, ML techniques have simplified the application of computer modeling to complicated types of data such as images or text [15,17]. ML algorithms are often able to conduct both data preprocessing and analysis steps. Traditional statistical methods may associate surgeon-appreciated radiographic features with a diagnosis or with quality of life metrics. A ML algorithm may go a step further and examine the radiograph directly to find unique image features associated with the same diagnosis or quality of life metrics. With text, a ML model may read the medical record directly and obviate the need for a human-mediated coding step. In this way, ML models may perform both data preprocessing and analysis. Altogether, ML is an exciting emerging technology due to its sophistication, its improved performance relative to traditional statistical methods, and its ability to use complex datasets.
In recent years, ML has emerged as a prominent topic in spine research [9]. Armed with the unique features described above, ML algorithms bring high accuracy models for classification and regression tasks to the spine community. Within ASD, ML techniques are being applied clinically to assist preoperative planning, predict operative outcomes, and predict complications following spine procedures.
2. ML for Preoperative Planning
One area where ML use cases are emerging in spine surgery is in preoperative planning.
Radiographic measurement is an important step in preoperative planning for spine surgery, and several studies have investigated ML techniques for automating radiographic measurements for analysis of spinal deformity [18-22]. In one work, Galbusera et al. [23] developed an algorithm that automated measurement of important preoperative parameters such as T4–12 kyphosis, L1–5 lordosis, Cobb angles, and pelvic tilt (PT) from biplanar radiographs. Similar works by Schwartz et al. [18] and Cho et al. [22] have created algorithms to measure lumbar lordosis and pelvic parameters using lateral radiographs alone. These automated algorithms perform with similar accuracy to manual measurement by surgeons. Automated analysis of spine shape is useful because it saves time and energy in the preoperative planning process for an individual patient. In addition, automated measurement can remove the interrater variation seen when taking important measurements. Furthermore, automated analysis of spine shape may enable research on alignment goals to be conducted on an unprecedented scale. This may help build upon systems for guiding ASD correction, such as the Scoliosis Research Society (SRS)-Schwab classification [24]. In turn, this could translate into more personalized treatment plans for greater patient benefit.
In addition to automated measurement, ML techniques may assist in preoperative decision-making more directly. Lafage et al. [25] investigated a ML model to predict the selection of upper treated vertebrae by expert surgeons in ASD cases based on preoperative radiographic measurements and correction goals. Their model was able to identify upper treated vertebrae 87.5% of the time correctly. Although there may be room for growth before such an algorithm would be used clinically, the results demonstrate the capacity for ML methods to learn an important preoperative decision-making process. Algorithms such as this could garner deeper insight into surgeon decision making and help to generate improved objective decision methodologies in the future.
ML may be used in surgical planning to predict intraoperative events. Raman et al. [26] utilized a ML technique to predict the need for major intraoperative blood transfusion during fusion surgery. Studying risk factors for intraoperative events such as major transfusion may not be new. However, the application of ML models to these problems enables analysis of more complicated relationships between variables of interest. ML techniques may offer greater predictive power, and in turn, better resource allocation for events like major intraoperative blood transfusion.
3. ML for Outcomes Prediction
Patient-reported improvements in pain and function are an increasingly important metric of success in spine surgery [27]. Accurate prediction of surgical outcomes is useful to indicate patients for spine surgery properly, and several prediction tools have been previously developed to assist in this process [28]. More recently, newer ML techniques have begun to take the place of traditional statistical techniques to create these tools. In many instances, ML techniques such as neural networks offer improved predictive accuracy compared to more traditional techniques [16]. One notable study by Ames et al. [29] examined 8 different algorithms for predicting the achievement of minimal clinically important difference (MCID) in ASD surgery. The authors employed ML algorithms to predict postoperative changes in 8 patient-reported outcomes (PROs) measured at both 1 year and 2 years postoperative. The authors could produce models with mean absolute errors less than the MCID for each PRO instrument across each time horizon. These results indicate that the models are sufficient to predict if a procedure will achieve MCID for a given patient using a given outcome instrument across a given time interval. Clinical application of predictive tools such as those created by Ames et al. [29] may improve shared decision making and represent a step towards more customized care in ASD.
In addition to PROs, ML has been used to predict outcomes such as changes in spine morphology following ASD surgery. Lee et al. [30] created an algorithm to accurately predict thoracic kyphosis and PT following fusion from the lower thoracic spine to the sacrum. This algorithm could be utilized to minimize proximal junctional kyphosis following ASD surgery.
4. ML for Predicting Complications
Risk factors for readmissions and various postoperative complications have been well studied in spine literature. However, the application of ML techniques to identify risk factors and predict complications is new. For example, Kim et al. [16] compared the ability of an artificial neural network and logistic regression to predict cardiac complications, wound complications, venous thromboembolism, and mortality following elective surgery for ASD. They compared these 2 ML methods to the predictive ability American Society of Anesthesiologists (ASA) physical status classification. The authors found that the neural network outperformed logistic regression in predicting cardiac complication, wound complication, and mortality. In addition, both methods outperformed ASA physical status across all complication categories. Other studies have utilized similar ML models to predict adverse events. Martini et al. [31] utilized a ML algorithm to predict drivers of unplanned 30-day readmission. The authors applied their technique to a large, variable-rich dataset well-suited for analysis using ML techniques. As a result, the authors could elucidate several factors that contribute to readmission with greater nuance than may be seen using other statistical techniques. Taken together, these 2 studies demonstrate the capacity for ML models to improve the capacity to predict adverse events following spine surgery for ASD.
ROBOTIC-GUIDED SPINAL SURGERY
1. Role of Robotics in Spine Surgery Planning and Execution
Surgical correction of major spinal deformities associated with ASD (i.e., scoliosis, kyphosis, lordosis) necessitate precise placement of instrumentation, namely pedicle screw, plate, and rod fixation. In recent years, robotic-assisted surgery has been implemented with growing frequency in spinal procedures. The technology for RGSS first became approved for clinical use in 2004 with the introduction of the SpineAssist/Renaissance robot [12,32]. This initial system showed promising results with successful pedicle screw placements being achieved with high accuracy, igniting rapid growth in literature supporting the use of these systems in spine procedures [33,34]. And while the use of more advanced technology like unmanned-operative robotics still needs U.S. Food and Drug Administration approval, RGSS is actively being employed within the field. Currently, RGSS is utilized for screw-based spinal joint immobilization, pelvic instrumentation, dura mater tumor removal, and ASD procedures [12,35,36].
There is an extensive body of literature that evaluates the technical aspects of RGSS. While individual steps may differ based on the robot type being utilized, generally, preoperative computed tomography scans are uploaded to the robotic system to help construct a plan for screw size and course. Surgically, the RGSS system uses preplanned images to map the anatomical positioning of the patient where screw trajectories will be completed. Mapped positions are confirmed by perioperative fluoroscopy. In this role, robots act as a coautonomous system to help establish the optimal placement of stabilizing hardware, guiding the surgeon who ultimately places the screw [4,32,37]. In this work, we will impart focus on the current status of clinical results obtained using RGSS for ASD correction.
2. Current Status of RGSS Accuracy Compared to Traditional Means
The conventional standard of care for instrumentation placement, primarily pedicle screws, in spinal deformity correction is free-hand fluoroscopy-guided (FH) surgery. In a large-scale analysis of 6,816 pedicle screws placed using a FH approach, 51.2% of which were in deformity corrections, Parker et al. [38] found that only 115 screws (1.7%) breached the pedicle or vertebral body. In a scoliosis-specific study, Chang et al. [39] found a 93% accuracy out of 992 placed screws using this approach which corroborates with what Zhu et al. [40] found in an analysis of 625 screws placed in a pediatric scoliosis population. Nevertheless, a FH approach has inherent perioperative disadvantages that are propelling the frequency of RGSS utilization. In a systematic review comparing FH (672 screws) and RGSS (688 screws), it was shown that RGSS exposure and dosage were respectively 12.38 seconds (95% confidence interval [CI], -17.95 to -6.80; p<0.001) and 0.64 milli-Sieverts smaller on average (95% CI, -0.85 to -0.43; p<0.00001) [41]. Later, Fan et al. [42] expanded upon this finding showing that RGSS screw placement resulted in less radiation dosage compared to other guided techniques in ASD correction, including FH-CT-navigation. In a recent meta-analysis, it was found that RGSS can decrease both complication and revision rates when compared to FH [43]. Hu and Lieberman [44] showed that RGSS is associated with a flattened learning curve for experienced spine surgeons with rates of successful placement increasing from 82% to 93% after only 30 operations.
A principal source of perioperative and postoperative complications in ASD surgeries are mal-positioned pedicle screws. Thus, a major question within the field is what accuracy can RGSS can provide for pedicle screw placement compared to more traditional techniques? At present, there exist inconsistencies within the literature as to which approach is superior. Perdomo-Pantoja et al. [45] found that in a multiapproach analysis, FH (n=20,439 screws) (93.1%), fluoroscopy assisted (n=17,336 screws) (91.5%), and computed tomography navigation guided (n=10,848 screws) (95.5%) correction had better screw accuracy than RGSS (n=2,538 screws) (90.5%). However, multiple other studies do not corroborate these findings and in general trend towards RGSS outperforming FH. In a meta-analysis of 1,255 FH and 1682 RGSS placed screws, Fan et al. [46] demonstrated that RGSS significantly outperformed FH in screw accuracy (odds ratio [OR], 1.69; 95% CI, 1.38–2.07; p<0.01). In an analysis that compared FH to 2 separate RGSS systems, it was shown that the TINAVI robot system had significantly higher screw accuracy than FH (relative risk, 1.10; 95%, CI, 1.06–1.14; p<0.01), but the Renaissance robot system did not (OR, relative risk, 1.00; 95% CI, 0.96–1.05; p=0.95) [47]. However, in a separate meta-analysis, it was shown that both TINAVI (OR, 0.19; 95% CI, 0.09–0.38) and Renaissance (OR, 0.19; 95% CI, 0.07–0.56) systems promoted fewer cranial facet joint violations than FH [48]. Gao et al. [41] found that both proximal joint violation and pedicle screw accuracy were significantly improved in RGSS (688 screws) compared to FH (672 screws) procedures.
Given the current status of these meta-analyses on RGSS versus FH screw accuracy, it appears as though there are some inconsistencies within the literature, necessitating further research. Of the 5 meta-analyses above, 24 individual studies were analyzed in total, of which only 14 directly compared RGSS to FH. However, when these 14 reports are broken down by year, there is a noticeable improvement in RGSS results. Between 2012 and 2015, RGSS was found to either perform worse than or similarly to FH when directly compared in 5 separate studies analyzing pedicle screw accuracy [49-53]. Then, from 2016 to 2019, RGSS was found to perform similarly to FH in 5 analyses [16,54-57].
In that same period, 4 separate studies showed RGSS significantly outperforming FH with no studies reporting FH outperforming RGSS [58-61]. Undoubtedly, more research is needed to further ascertain the potential advantage RGSS has in this respect. Currently, there is a growing body of literature comparing these 2 approaches, and with billions of dollars expected to be invested in spinal robotics in the coming years, the clinical results for RGSS will likely continue to be strengthened [32].
The growing clinical success of RGSS has in part driven the publication of additional studies in just the past year that utilizes robotics in spinal deformity-specific cases. Using clinical results from 2018–2019 for the correction of ASD, Chen et al. [11] showed that RGSS (378 screws) improved blood loss (p<0.001) and pedicle screw accuracy (p<0.001) when compared to FH (786 screws) [11]. Additionally, they showed that both RGSS and FH had comparable operative times (p=0.31) and length of hospitalization (p=0.36). In 2020, Le et al. [62] reported that RGSS (46 screws) resulted in less superior facet joint violation compared to FH (109 screws) (p=0.04). Further in 2020, Edström et al. [63] showed that RGSS (2.2%) required less need for additional instrumentation (i.e., hooks) compared to FH patients (9.7%) (p<0.001). Lastly, Gonzalez et al. [64] accomplished 98.7% accuracy in the placement of 314 screws in adolescent idiopathic scoliosis (AIS) correction, one of the highest accuracies seen to date for RGSS. This surge in recent literature supporting RGSS in just the last year coincides with Hu and Lieberman [44] reporting a flattening of the curve to be expected for successful RGSS implementation. With surgeons likely growing more comfortable with robotic devices as they become a staple in the operating room, it should be expected that these high success rates will persist.
PATIENT-SPECIFIC RODS
1. Role of PSR in Adult Spine Deformity Surgery
Successful realignment of spinal deformities associated with ASD requires diligent preoperative planning and execution of the preoperative plan by a skilled surgeon. PSR has emerged as a novel technology that can reduce errors in executing a surgical plan due to imprecise intraoperative rod contouring. PSRs are relatively new in realignment surgery with the first set of rods implanted in 2013. Targets for realignment surgery in ASD follow the Schwab-Lafage recommendations and consider factors such as a sagittal vertical axis (SVA), PT, and a pelvic incidence lumbar lordosis mismatch (PI–LL) [65]. Despite established goals for realignment, neutral sagittal alignment was only achieved in 32% of patients following realignment surgery [66]. Failure in surgical correction of spinal angles in ASD patients can be attributed to either a failure in preoperative planning or a failure in execution of the preoperative plan [67]. The advent of rods specifically manufactured to fit the preoperative plan and the target alignment for an individual patient eliminates many of the sources of variability during a procedure.
2. How They Work
PSRs are designed from a preoperative surgical plan to fit the unique sagittal profile of each patient [68]. Surgeons use spine preoperative planning software to simulate realignment and develop a preoperative plan. These softwares allow surgeons to import a patient’s radiographs into the software, manipulate the image to achieve target PT angle and SVA, simulate planned osteotomies, and develop a finalized surgical plan with achievable target angles [67,69,70]. From the surgical plan developed in a planning software, rod curvature and length specific to the patient are determined and 2 identical PSRs are manufactured by MEDICREA (UNiD technology, MEDICREA, Lyon, France) to fit the precise specifications of the preoperative plan. Landmarks on the rods such as superior limit vertebra, S1 screw, and sagittal line are laser printed on the rods to aid in placement and all other surgical steps are similar to typical realignment surgery [67]. PSRs are delivered to the operating room ready to implant, reducing time of operation, eliminating notching in rods that can compromise integrity, and increasing precision and symmetry of the rod bending [71].
3. How Do PSRs Compare to Traditional Rods?
PSRs have led to excellent correction of ASD and spinopelvic alignment with postoperative radiographs demonstrating strong adherence to the preoperative plan [72,73]. In 2019, Solla et al. [68], in a study focused on PI–LL, found significant improvements with PSR implementation and found patients with PSR implants to be 2.6 times more likely to be optimally corrected in comparison to published data from conventional surgery. This study highlighted the added benefits of a prebent rod to include reduced operation time and decreased mechanical complications [74], conducted a study focusing on thoracic kyphosis correction and found a mean increase in kyphosis of 14° and found kyphosis at last follow-up to be close to or at target value [74]. A study examining 43 ASD cases using PSRs with no intraoperative adjustments to the prebent rods found improvements in SVA and PI–LL, confirming the clinical feasibility of their use [14]. Other studies have demonstrated strong and stable treatment effects at a 2-year follow-up in patients treated with PSRs, with particular improvements noted in SVA and PI–LL [75].
Two armed studies comparing PSRs to the current standard of care are lacking. A study comparing PSR use with preoperative planning to absence of planning in cervical decompression and fusion found patients with pre-contoured rods to have greater correction of T1 slope minus cervical lordosis [76]. SVA and cervical lordosis improvements were found to be similar between the PSR group and the conventional treatment group [76]. Additionally [77], found that PSRs demonstrated more acute radius of curvature and sagittal alignment than standard rods in spinal surgeries involving 4 or fewer levels [77]. Currently, a multicenter, controlled, double-blind, randomized trial entitled “The PROFILE Study” is underway which will provide an important comparison of this novel technology to the current standard of care [78].
While the advantages of PSR compared to traditional rods are not yet determined, several advantages to PSRs have already been established. By eliminating the need for intraoperative bending, PSRs reduce the time and difficulty of operation which decreases the likelihood of intraoperative infection [67,68,73,74]. PSRs demonstrate improved mechanical resistance than typical rods due to the absence of notching from intraoperative bending techniques [67,79]. Rod breakage was found in 2.2% of patients with PSR implants compared to 9.3% in patients with traditional rod implants [71,80]. Lastly, exact knowledge of rod curvature preoperatively will allow further postoperative analysis and improved surgical planning research [14,67].
THE FUTURE OF ASD
Advances in treatment for ASD will continue to improve preoperative planning and reduce complication rates for patients. Technological strides in ML, robotic-assisted spine surgery, and PSR are poised to reduce complication rates and improve patient satisfaction with surgical intervention for ASD.
1. ML: Increased Patient Satisfaction
Management of patient expectations can be challenging in spine surgery. Previous research has evaluated patient expectations about symptom relief, physical function, and mental well-being, and the results indicated that 66% of lumbar patients were only “somewhat fulfilled” 2 years postoperatively [81]. ML can improve patient satisfaction following surgery through its ability to generate postoperative radiographs using preoperative imaging and characteristics. Preliminary research within our institution has shown that ML can do this accurately. Thus, there is room for ML to be used on an individual patient level as well as its current role in analyzing large datasets.
Preliminary work by this lab has examined use of ML to predict postoperative radiograph appearance from preoperative radiographs (Fig. 1). A tool to accurately predict the postoperative appearance of patient radiographs could assist in shared decision-making and facilitate preoperative planning.
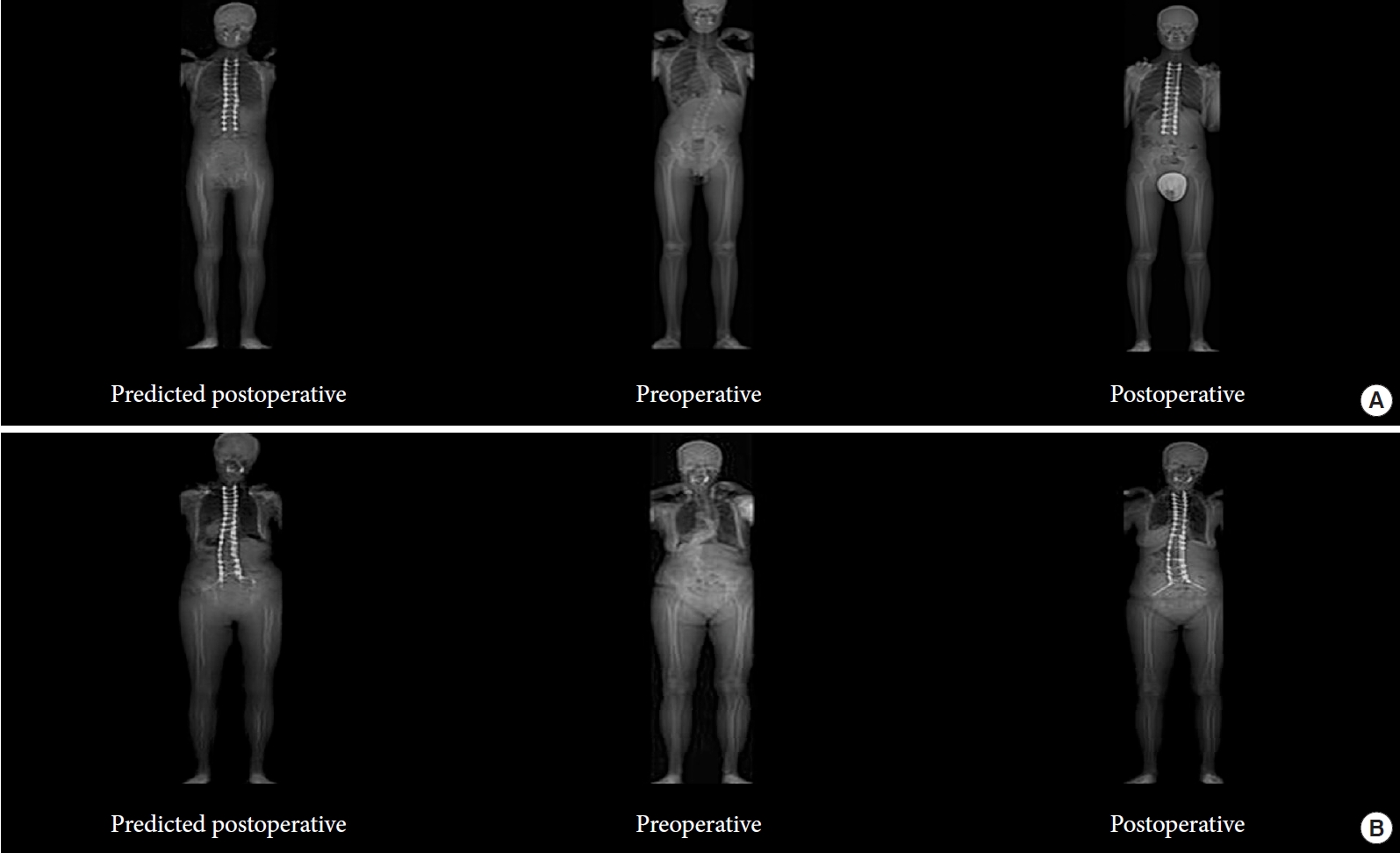
Two examples of utilizing machine learning (ML) in the prediction of postoperative radiographs. For panels A and B, the left is the predicted postoperative radiographs, middle is real preoperative radiographs, and right is the real postoperative radiographs for 2 separate patients. ML has the potential to predict outcomes for patients from a radiographic perspective accurately.
2. ML: Limitations
Despite the increasing integration of ML in ASD treatment, it still has some inherent limitations. ML algorithms conform very specifically to the data set used for training, and this frequently creates a lack of algorithm generalizability [17]. An algorithm created using a dataset of only male patients may not have accurate results if used to study trends in a dataset of female patients. The Black Box problem is worth acknowledging; it is sometimes difficult to understand the methodology, or “thinking,” behind a complex algorithm. This creates a barrier where both patients and providers lack trust in ML as they are unable to understand it. Additionally, there have not been any randomized control trials or prospective studies that evaluate the efficacy of ML in ASD care.
FUTURE OF ROBOTIC-ASSISTED SPINE SURGERY
1. Extension of RGSS Beyond Conventional ASD Correction
The utilization of RGSS in other aspects of spinal deformity include the correction of AIS and S2-alar-iliac screw (S2AI) placement. S2AI screws, in particlr, are utilized in ASD corrections extending to the sacrum, but are notoriously difficult to place. In an early study of S2AI placement for ASD correction at the sacrum, Schillingford et al. [82] saw that a fluoroscopy-guided FH approach only resulted in cortical breaches 5% of the time. In the first study of their kind, both Bederman et al. and Hyun et al. [55] saw the accurate placement of all 31 and 35 S2AI screws they analyzed, respectively, using RGSS [83]. Hyun et al. [55] noted no iliac or sacral breaches. However, Bederman et al. [83] saw that longer screws (≥ 80 mm) were associated with posterior pelvic violations in part because the software could not map beyond 60-mm projections; they later note that the software has since been modified. For AIS cases, Macke et al. [84] saw that 92.8% of screws were not misplaced by more than 2 mm in a report of 662 screws. This agrees with later reports that showed only 2.8% of screws being inaccurately placed in a sample of 844 screws as determined by electromyography stimulation [85].
2. PROs in RGSS
Given the novelty of RGSS, however, there is a paucity of literature on PROs associated with robotics in spinal procedures. In the limited literature, Li et al. [47] showed in their meta-analysis that visual analogue score (p=0.24) and Oswestry Disability Index (p=0.12) scores did not differ between FH and RGSS reported scores. Of note, these comparisons only included 3 studies that published PROs for RGSS procedures, only one of which had greater than 2-year follow-up time. Similarly, in a 2020 publication, Chen et al. [11] showed that SRS 22 pain scores were improved using both FH (n = 55) and RGSS (n = 31) at average 6-month follow-up in scoliosis correction; These scores did not significantly differ when compared between approaches. While the preliminary PRO results are promising, they are limited. To validate the clinical usefulness of RGSS in ASD correction, more studies will need to report on longer-term PROs. This data will come to light in the coming years with increasing consistency. However, given that RGSS is trending towards improvement in perioperative clinical spheres, it is a viable surgical technique in ASD correction.
FUTURE OF PATIENT-SPECIFIC RODS
The integration of PSR into everyday spine practices will heavily depend on it becoming a cost-effective alternative to the conventional options. At this time, PSR can cost 2–4x compared to traditional options, take about 2–4 weeks to prepare, and lead to greater implant waste as the manufacturer will provide multiple sizes to the surgeon [86]. Surgeons may not be able to delay care for 2–4 weeks in emergent cases, and patients may opt for conventional options if it means the surgery can be scheduled sooner. Additionally, the variability within each specific rod makes it challenging to conduct large-scale clinical trials to evaluate its safety and efficacy [86].
Preliminary research in small sample sizes has shown that PSR has positive outcomes, but further research and advances in accessibility are necessary to understand its role long-term in ASD. Overcoming the challenges related to cost and time for preparation will lead to significant improvements in preoperative planning and reductions in postoperative complications for spinal surgery patients.
CONCLUSION
Overall, given the success of ML, robotics, and PSR in ASD, it is likely that these 3 tools will become staples in spine surgery. We hypothesize that the body of literature surrounding these up-and-coming advancements will expeditiously grow to provide more clinical data, preoperative images, postoperative outcomes, and postoperative radiographs. With this predicted increase in available data, we suggest the future integration of ML with robotics and PSR to further improve patient care in ASD correction.
Notes
The authors have nothing to disclose.
