 |
 |
- Search
| Neurospine > Volume 18(3); 2021 > Article |
|
|
Abstract
Objective
This study aimed to identify the sagittal parameters associated with health-related quality of life and genetic variations that increase the risk of adult spinal deformity (ASD) onset in the older population.
Methods
We recruited 120 participants who had a sagittal vertical axis > 50 mm in a sagittal imbalance study. Sagittal radiographic parameters, cross-sectional area, and intramuscular fatty infiltration using the Goutallier classification in the paraspinal lumbar muscles were evaluated. Functional scales included the self-reported Oswestry Disability Index (ODI), 36-item Short Form Health Survey (SF-36), and visual analogue scales (VAS) for back and leg pain. We performed whole-exome sequencing and an exome-wide association study using the 100 control subjects and 63 individuals with severe phenotypes of sagittal imbalance.
Results
Pelvic incidence minus lumbar lordosis (PI–LL) mismatch was negatively associated with the SF-36 and positively correlated with ODI and VAS for back and leg pain. PI–LL was related to the quality and size of the paraspinal muscles, especially the multifidus muscle. We identified common individual variants that reached exome-wide significance using single-variant analysis. The most significant single-nucleotide polymorphism was rs78773460, situated in an exon of the SVIL gene (odds ratio, 9.61; p=1.15 × 10-9).
Conclusion
Older age, higher body mass index, and a more significant PI–LL mismatch were associated with unfavorable results on functional scales. We found a genetic variation in the SVIL gene, which has been associated with the integrity of the cytoskeleton and the development of skeletal muscles, in severe ASD phenotypes. Our results help to elucidate the pathogenesis of ASD.
Adult spinal deformity (ASD) consists of a heterogeneous spectrum of abnormalities of the lumbar or thoracolumbar spine in adult patients [1]. The causes of ASD range from de novo onset to progressive degeneration from a pre-existing deformity or accelerated development after previous spinal surgery [2]. Spinal deformity has a substantially debilitating effect on patients’ general health [3]. The prevalence of ASD is likely between 32% and 68% of the older population [2]. An understanding of sagittal plane alignment has become essential for improving the health care of older adults.
The radiological parameters most closely related to pain and disability are sagittal vertical axis (SVA), pelvic tilt (PT), and the balance of pelvic incidence (PI), and lumbar lordosis (LL) [4]. In particular, PI–LL mismatch (hereafter, PI–LL) has been reported as a critical radiological parameter for reducing postoperative pain and disability [4]. PI–LL is not correlated with preoperative symptoms because preoperative symptoms, including pain caused by nerve root compression, spinal instability, and spinopelvic alignment, are more complex than postoperative symptoms. Therefore, PI–LL is significantly correlated with postoperative lower back pain, but not with preoperative back pain [5].
With aging, the sagittal curvature of the normal spine tends to become stooped [6]. Multiple age-related factors are implicated in this development, including reduced bone mineral density (BMD), spinal degeneration, reduced mobility and balance, and fatty degeneration of the paraspinal muscle [2,6,7]. Individuals with ASD are usually characterized by back pain and an inability to stand erect. Significantly, low back pain while standing is more influenced by spinopelvic malalignment. Various radiological parameters have been reported to influence functional daily life activities.
In recent years, genetic involvement in the development of spinal deformities has garnered increasing recognition in clinical investigations of adolescent idiopathic scoliosis, and studies have provided new insights into the etiology and pathogenesis of spinal deformity [8,9]. Genetic factors include a wide spectrum of variations, such as single-nucleotide polymorphisms (SNPs), which may contribute significantly to the etiology of spinal diseases; furthermore, environmental factors may additionally complicate the impact of genetic factors. Genetic studies using next-generation sequencing have remarkable potential as means of elucidating the genetic background of a disease.
We evaluated the influence of postural changes, osteoporosis, and the quality and size of the paraspinal muscles on health-related quality of life (HRQoL). In addition, we investigated the influence of genetic variants on ASD by applying whole-exome sequencing obtained from the participants of an observational cohort. This study aimed to examine the relationship of radiological parameters associated with HRQoL and to identify genetic variations associated with ASD.
This study was approved by the Institutional Review Board of Chonnam National University Hospital (approval number: CNUH-2016-127). To perform an analysis of genetic associations, we recruited 228 Korean participants over 65 years old who had a SVA of > 50 mm on whole-spine standing x-rays in the Korean Elderly Sagittal Imbalance Cohort Study. A total of 228 adults with a nonneutral sagittal standing posture were recruited from July 2016 to December 2016. The inclusion criteria were as follows: (1) Korean men and women aged ≥ 65 years and (2) an SVA of > 50 mm as measured from a whole-spine standing lateral radiograph (Fig. 1). Participants who had previously undergone spinal surgery or had been diagnosed with present spinal disease, including acute compression fracture, tumor, trauma, and infectious diseases, were excluded. Out of 228 participants, we assessed radiographic and clinical data in 120 consecutive adults (52.6%) who demonstrated a stooping standing posture with a higher severity of ASD according to SVA imbalance. Severe sagittal imbalance was defined as an SVA ≥ 150 mm after adjusting for spinopelvic parameters. Wholeexome sequencing was performed in 63 participants (27.6%) with severe phenotypes of SVA (≥ 150 mm). Data regarding age, sex, body mass index (BMI), medical history, smoking status, alcohol consumption, nutritional status, education, occupation, and socioeconomic status were also obtained. This experiment was performed in accordance with the Declaration of Helsinki and with the approval of our institutional review board. All participants provided written informed consent.
Evaluations of the participants’ whole spine and general health were performed using sagittal radiographic parameters (SVA, thoracic kyphosis, PT, PI, LL, and PI–LL), blood laboratory examinations, BMD, cross-sectional area (CSA), and intramuscular fatty infiltration in the paraspinal lumbar muscles [7,10]. The Goutallier classification system was used to grade intramuscular fatty infiltration in the paraspinal lumbar muscles such as the multifidus muscle (MF), erector spinae (ES; including the longissimus muscle and iliocostalis muscle), and psoas muscle (PS). According to the Goutallier grade using axial T2-weighted images (T2WI), grade 0 was defined as no fatty infiltration, grade 1 as some fatty streaking of the MF, grade 2 as less fat than muscle, grade 3 as equal amounts of fat and muscle, and grade 4 as more fat than muscle. The CSA of the paraspinal lumbar muscles was evaluated on T2WI magnetic resonance images. All measurements were performed with a 3.0-T magnetic resonance imaging device (Skyra, Siemens, Germany). Functional scales were assessed through self-reported Oswestry Disability Index (ODI), 36-item Short Form Health Survey physical component summary (SF-36 PCS), and visual analogue scale (VAS) for back and leg pain.
Genomic DNA samples were purified from whole blood samples in 63 participants with severe sagittal imbalance (SVA ≥ 150 mm). The genomic DNA samples were used for exome capture with the SureSelect XT Human All Exon +UTR v5 exome kit (Agilent Technologies, Santa Clara, CA, USA). The Illumina HighSeq 2000 platform (Ilumina, San Diego, CA, USA) was used for sequencing with a mean coverage of 150x. We aligned the sequencing using the Burrows-Wheeler Aligner (bwa) algorithm and generated a binary alignment map file using the ‘bwa-mem’ package [11]. Following genome analysis tool kit best practices, the genomic variant call format file was generated by Haplotype Caller after recalibration, and the result was annotated using ANNOVAR (annotate variation) [12]. As control data, we used 100 samples from the whole-genome sequencing dataset obtained from the general Korean adult cohort of the Korean Genome and Epidemiology Study (KoGES) (Supplementary Table 1) [13].
Pearson correlation analysis was used to evaluate the relationships between ODI, VAS for back and leg pain, HRQoL, CSA, and sagittal radiographic parameters (SVA, PT, PI, LL, and PI–LL). Univariate and multivariate regression analyses were performed to examine the relationships between variables such as age, sex, BMI, radiographic parameters, CSA, and functional scales (SF-36 PCS). All statistical analyses were performed using MedCalc ver. 20 (MedCalc, Mariakerke, Belgium), and p-values of < 0.05 were considered to indicate statistical significance. We conducted linear mixed-model analyses in open-source PLINK/SEQ software (v0.10, released 14-July-2014) to test all associations. Plink/SEQ supports the -glm function for regression on every single variant and gene-based tests (low frequency and rare variants). Sex was used as a covariate in all statistical tests. The burden and SKAT-O (sequence kernel association test and the optimal unified test) were performed for low frequency and rare variants.
Sagittal imbalance was present in 120 persons (27 men and 93 women). The mean age was 70.57± 4.61 years (range, 65–80 years). Baseline patient demographics and information are represented in Table 1.
Pearson correlation analysis demonstrated that the SF-36 PCS, ODI, and VAS for back pain and leg pain were correlated with PI–LL. Furthermore, PI–LL was negatively associated with SF-36 PCS (r=-0.252, p=0.0056). ODI, VAS for back and leg pain, and outside working hours per week were positively correlated with PI–LL (r=0.276, p=0.0023; r=0.284, p=0.0017; and r=0.181, p=0.0478, p=0.0095, respectively) (Table 2).
Pearson correlation analysis showed that CSA and intramuscular fatty infiltration in paraspinal lumbar muscles correlated with recovery. In particular, the CSA of the MF was negatively associated with PI–LL (r=-0.520, p<0.0001), but not correlated with the CSA of the ES and PS. Spearman rank-order correlation analysis revealed that the Goutallier grade of the paraspinal muscles, such as ES (ρ=0.187, p=0.0411), MF (r=0.215, p=0.0186), and PS (r=0.302, p=0.0008), were positively associated with PI–LL.
Multivariate analysis revealed that age, BMI, and PI–LL were negatively associated with HRQoL (p=0.0120, p=0.0007, and p=0.0308, respectively) (Table 3). Based on our results, older age, higher BMI, and more significant PI–LL mismatch are significant predictors of poor HRQoL.
To identify disease-associated genetic variants, we annotated 23,844 SNPs in the 63 patients with severe ASD phenotypes as well as the 100 control subjects based on the following filtration criteria: (1) a depth of < 30×, (2) a Hardy-Weinberg p-value of < 10-3, (3) a genotype-quality score of < 20, (4) synonymous/nonfunctional variants, (5) a SIFT (Sorting Intolerant From Tolerant) score of 1, and (6) a difference in allele frequency of more than 100 times that of the Exome Aggregation Consortium.
As the clinical information of the control subjects was limited, we only used sex as a covariate for the single-variant regression test. We ranked SNPs based on the -logP; the top 4 genetic variants are presented in individuals with severe sagittal imbalance (Table 4). These 4 top SNPs were rs78773460, rs76740888, rs200926577, and rs140991770. The most significant SNP was rs78773460, situated in an exon of the SVIL gene (odds ratio, 9.61; p=1.15 × 10-9). Supervillin (SVIL) genetic variations have been associated with the integrity of the cytoskeleton and the development of skeletal muscles. Fig. 2 shows a Manhattan plot obtained from the single-variant association tests: its x-axis shows the positions of genetic variants across the chromosomes. In contrast, the y-axis shows the negative log of the p-values (higher values on the y-axis thus indicate higher significance levels). Fig. 3 shows the quantile and quantile (Q-Q) plot for the single variant test (the relevant fit of the observed to expected significance values after applying the covariates).
ASD is defined as a complex spectrum of spinal diseases, including adult scoliosis, degenerative scoliosis, sagittal and coronal imbalance, and iatrogenic deformity, with or without spinal stenosis, that present in adulthood [14]. We conducted a prospective observational cohort study to investigate the relationship of radiological parameters associated with HRQoL and to assess genetic factors in older adults with severe sagittal imbalance in Korea. Older age, higher BMI, and more significant PI–LL were significantly correlated with poor HRQoL. PI–LL was related to the quality and size of the paraspinal muscles, particularly the MF muscle. We found SNPs associated with the integrity of the cytoskeleton and the development of skeletal muscles in individuals with severe sagittal imbalance.
The prevalence of ASD among older people has been reported to be as high as 60% [15]. Although most cases of ASD are asymptomatic, others have pain, neural symptoms, functional limitation, or disability. Sagittal imbalance has a significant relationship with HRQoL deterioration and surgical outcomes in symptomatic adults with degenerative spinal disorders; therefore, correction of sagittal imbalance is essential for achieving good surgical results and HRQoL [4,16]. Among various sagittal alignment parameters, LL is the most changeable by positional adjustments or surgical operations, in contrast to PI, which is a fixed morphological parameter in each person [16]. With the gradual loss of LL that occurs with aging, there is a further compensatory increase in PI as the pelvis rotates to maintain global spinal alignment [17,18]. PI–LL was reported to be consistently associated with the quality of life of patients receiving operative treatment [10,19]. Schwab et al. stated that one of the target spinopelvic parameters for corrective surgery was that PI–LL should be within± 10° [4]. PI–LL is not correlated with preoperative symptoms because preoperative symptoms, including pain caused by nerve root compression, spinal instability, and spinopelvic alignment, are more complex than postoperative symptoms. Therefore, PI–LL is significantly correlated with postoperative lower back pain, but not with preoperative back pain [5]. However, this study revealed that PI–LL was negatively associated with HRQoL scores in older participants. ODI and VAS for back and leg pain were positively correlated with PI–LL. In individuals with sagittal imbalance, PI–LL had an impact on clinical symptoms, such as lower back and leg pain, back disability, and HRQoL.
BMI has been proposed as a potential risk factor for ASD. Obesity has traditionally been considered a protective factor against osteoporotic fractures [20]. In contrast, Gonnelli et al. [20] suggested that obesity might be a risk factor for fractures at various anatomical sites. Possible mechanisms include the production of inflammatory cytokines (interleukin-6 and tumor necrosis factor-alpha) in excessive abdominal fat and changes in 25-hydroxyvitamin D levels (a fat-soluble vitamin), which might lead to a reduction in bone strength. In the present study, higher BMI was correlated with poor HRQoL. This finding indicated that overweight individuals with sagittal imbalance had a poor HRQoL.
The most established metabolic factors related to ASD are osteoporosis and poor bone quality [2]. Vertebral fractures and lower BMD may confer reduced structural integrity within the spinal column, thereby decreasing the capacity of the spine to withstand the load and leading to more significant kyphosis. Vertebral compression fractures, for which osteoporosis is the major risk factor, contribute to sagittal plane deformity [2]. In the present study, a relationship between BMD and HRQoL was not found. Eight participants had experienced vertebral compression fractures; the lack of an observed association between vertebral compression fractures and sagittal imbalance most likely occurred because the number of vertebral fractures was too small to analyze. This discrepancy is likely related to the average characteristics of the older population recruited from the local community.
The paraspinal and psoas muscles have been considered crucial for stabilizing the spinal column, and fatty infiltration in the muscle decreases the proportion of contractile tissue capable of producing force [21]. Among the paraspinal muscles, including the MF, ES (including the longissimus muscle and iliocostalis muscle), and the PM, the lumbar MF muscle is essential for lumbar segmental stability, and defects in the paraspinal muscles are thought to be a cause of spinal deformity [22]. Muscular atrophy due to denervation, disuse, or other causes can manifest as decreased muscular size and increased infiltration by fat or connective tissue [21]. Parkkola et al. [23] reported that the amount of fat infiltration in the paraspinal muscles was related to muscle atrophy in chronic low back pain. In the current study, the quality of lumbar muscularity—as shown by the degree of fatty change of the paraspinal muscles—was positively associated with PI–LL. An increase in fat infiltration in the lumbar paraspinal muscles was correlated with severe sagittal imbalance in older individuals. The CSA of the paraspinal muscle compartment, especially the MF muscle, was significantly lower in individuals with ASD, consistent with a previous report [24]. Lee et al. [25,26] insisted that spinal sagittal imbalance may cause a discrepancy in muscle degeneration between the extensor and flexor muscles. In contrast, we thought that reducing the size and fat infiltration of the paraspinal muscle could lead to sagittal imbalance and chronic low back pain. Further research is needed to determine which of these factors causes degenerative spinal deformity.
Takemitsu et al. [27] suggested that lumbar degenerative kyphosis (LDK) was caused by degenerative changes such as disk narrowing, collapsed vertebral bodies due to osteoporosis, or atrophy of the lumbar extensor muscles without prior surgery. Recently, Lee et al. [28] suggested the name “primary degenerative sagittal imbalance” (PDSI), which includes degenerative sagittal imbalance of the whole spine of unknown origin and is associated with paraspinal muscle wasting. LDK may be regarded as a subgroup of PDSI related to agricultural occupations [28]. Bouxsein et al. [29] demonstrated that biomechanical stress on the spine increased vertebral wedging as BMD decreased, leading to more significant kyphosis. Hong et al. [30] reported that lifestyle factors common in Asia, such as squatting and sitting on the floor, were major causes of degenerative spinal kyphosis, back pain, and poor quality of life. They demonstrated that farmers had more sagittal imbalance and back pain in proportion to their working hours [30]. In the current study, PI–LL was positively correlated with outside working time (r=0.2357, p=0.0095) (Table 2). Participants who experienced increased outside working hours had more sagittal imbalance, but HRQoL and back VAS were not correlated with outside working time (p=0.0657 and p=0.3525, respectively).
Genetic variants associated with spinal disorders such as adolescent idiopathic scoliosis, ossification of the posterior longitudinal ligament, and intervertebral disc degeneration have been reported [8,9,31,32]. Until now, the genetic contribution of the development of ASD has been unknown, and it has been thought that ASD is influenced only by biomechanical or socio-environmental factors. To our knowledge, this is the first report describing genetic variations associated with ASD. We acquired exome-sequencing data to investigate potential genetic factors associated with severe sagittal imbalance phenotypes. In the present study, the supervillin protein, which is encoded by the SVIL gene, was found to be associated with ASD. Supervillin (SVIL) is a large eukaryotic protein from the villin/gelsolin superfamily of actin-binding proteins involved in many cellular processes [33]. SVIL is binds both myosin II and filamentous actin and interacts with several cytoskeletal proteins; it is most abundant in skeletal muscle, followed by the heart and other organs containing secretory or smooth muscle cells [34-36]. SVIL protein regulates all stages of cell motility, is involved in early cytokinesis, and plays a role in myofibrillar assembly [35]. Knockdown experiments involving SVIL reduced cell division and increase cell death in HeLa and U2OS cell lines [37]. This indicates that genetic variation in SVIL might be important for predicting the development of spinal deformity. Diseases associated with SVIL include myofibrillar myopathy, an autosomal recessive structural muscle disorder characterized by the onset of muscle pain, cramping, exercise fatigue, and then a slowly progressive course, leading to limited mobility in the first or second decades of life. Researchers recently demonstrated the importance of supervillin for the structural integrity of muscle fibers in humans. They showed that recessive loss-of-function mutations in SVIL caused a distinctive myopathy [38].
The current study did not prospectively evaluate genetic information from normal older adults without spinal deformity and those with severe ASD. We faced several obstacles, such as cost, recruitment of healthy volunteers, and research period limitations. We used 100 samples from a whole-genome sequencing dataset obtained from the general Korean adult cohort of the KoGES (Supplementary Table 1). As a result, one SNP (rs78773460) situated in an exon of the SVIL gene was detected in adults with severe ASD but not in the whole-genome sequencing dataset from KoGES. Due to the lack of a control group in this study and no previous whole-exome sequencing studies on the role of the SVIL gene in ASD, many questions remain about the genetics of ASD. The absence of normal candidate genes does not exclude a role for genetic variation at these loci in influencing severe ASD, but their contribution may be small compared with the genes identified. Nevertheless, this finding improved our understanding of ASD development and may assist in identifying a subgroup of severe ASD development.
Our results demonstrate the need for more extensive population-based longitudinal studies to illustrate the role of SVIL in the pathogenesis of ASD.
This study is subject to several limitations. First, it analyzed a small inhomogeneous older population recruited from the local community. Therefore, our results cannot be generalized as indicating factors associated with aggravated sagittal imbalance in other populations. Second, the genetic signature of ASD is not as clearly defined as is the case for Mendelian disorders. The application of a suitable model for the association was therefore limited. The nature of the disease favors the possibility of common diseases like heart disease, type II diabetes, and asthma, implying that genome-wide studies with much larger sample sizes that consider the effect size may be a more optimal method of detecting association signals. Third, as data from the control group were lacking, the case-control comparisons were not adequately controlled, and information from age/gender-matched control subjects with relevant covariates of clinical information was not incorporated into the regression analysis. The fact that the age group of the control population was largely biased to younger ages limited the usage of age as an acceptable covariate. The availability of information for normal controls may help to expand the interpretations of our results. However, a validation study applying targeted sequencing to a separate, older population of a relevant size that shows the effect of the risk allele on the susceptibility to the disease could be performed as an alternative approach.
PI–LL has been found to be useful for predicting individualized quality of life in inhomogeneous populations. Individuals with older age, higher BMI, and more significant PI–LL had poorer HRQoL. Reduced paraspinal muscle size and fat infiltration could lead to sagittal imbalance and chronic low back pain. The present study sought to find associations between SNPs and severe ASD phenotypes. Variants of the SVIL gene, which have been associated with the structural integrity of muscle fibers in humans, were among the SNPs identified. Further studies that recruit proper control subjects and conduct clinical validation are needed for our findings to be clinically applicable.
ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC15C1288) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2018R1A6A1A03023718). The authors wish to thank Prof. Sang Hyun Han for his contributions in drafting the manuscript and revising it for important intellectual content. The authors thank all of the subjects who participated in the study, the support staff, and the research coordinator.
SUPPLEMENTARY MATERIALS
Supplementary Table 1 can be found via https://doi.org/10.14245/ns.2142544.272.
Supplementary Table 1.
Demographics of control cohort of the Korean Genome and Epidemiology Study (n = 100)
Fig. 1.
Participants enrolled in the prospective cohort study. SVA, sagittal vertical axis; MRI, magnetic resonance imaging; BMD, bone mineral density; BMI, body mass index; SF-36, 36-item Short Form Health Survey; ODI, Oswestry Disability Index; VAS, visual analogue scale.

Fig. 2.
Manhattan plot for single-variant analysis. The y-axis shows -log10 (p-value) for common variants (minor allele frequency > 0.01), and the x-axis shows chromosomal positions for each variant. The threshold for statistical significance (p = 1 × 10-7) is shown by the pink horizontal line.

Fig. 3.
Quantile and quantile plot for the single-variant analysis, showing the observed versus expected ordered -log10 (p-value) for the single-variant analysis.
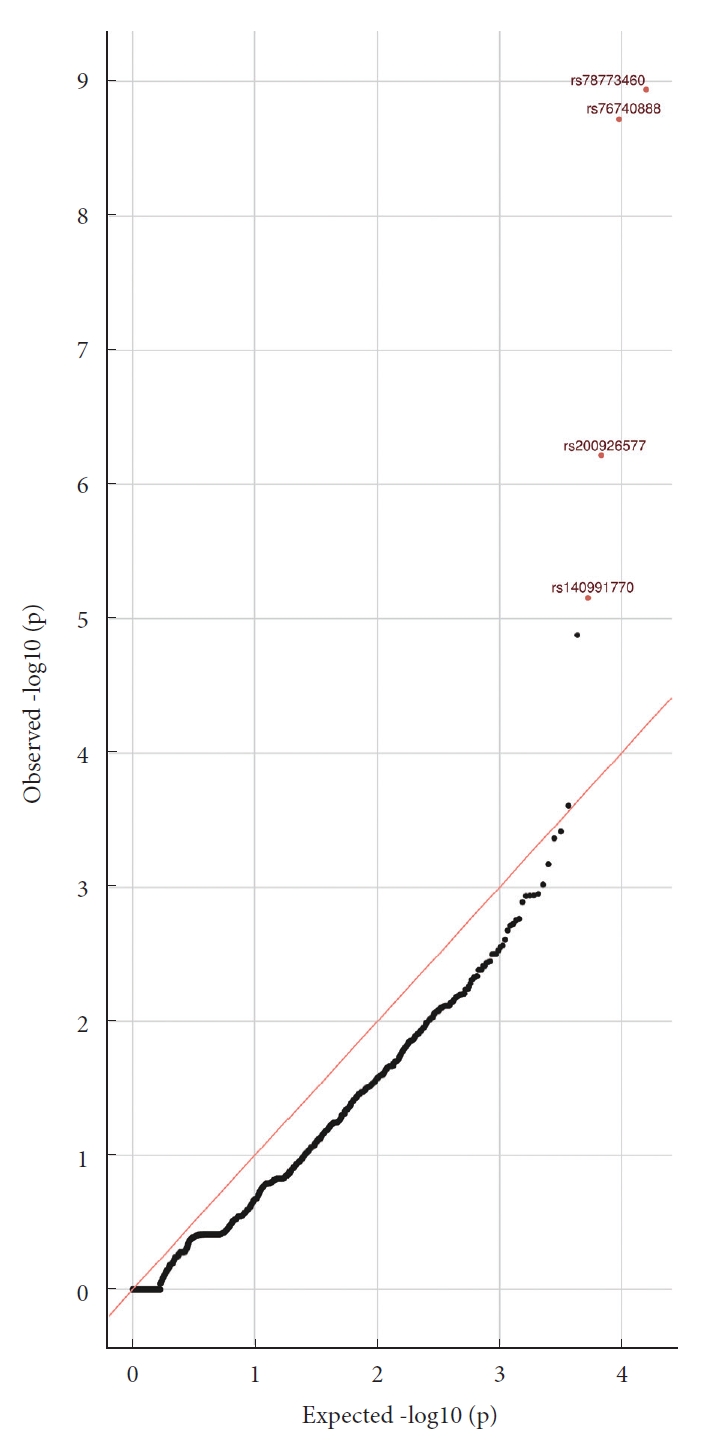
Table 1.
Sagittal imbalance patients’ demographics (n=120)
Table 2.
Pearson correlation coefficients for sagittal parameters, disability, and pain
| Variable | SVA | PI | TK | PI–LL | SF-36 PCS | ODI | VAS back | VAS leg | Working time |
|---|---|---|---|---|---|---|---|---|---|
| SVA | 1 | 0.002 (0.9848) | -0.178 (0.0516) | 0.277 (0.0022)* | 0.025 (0.7883) | -0.074 (0.4219) | -0.124 (0.1761) | -0.101 (0.9952) | 0.078 (0.3963) |
| PI | 1 | 0.009 (0.9206) | 0.490 (< 0.0001)* | -0.161 (0.0792) | 0.082 (0.3734) | 0.124 (0.1765) | 0.003 (0.9729) | 0.216 (0.0178)* | |
| TK | 1 | -0.510 (< 0.0001)* | 0.092 (0.3186) | -0.186 (0.0421)* | -0.124 (0.1787) | -0.055 (0.5474) | -0.045 (0.6263) | ||
| PI–LL | 1 | -0.252 (0.0056)* | 0.276 (0.0023)* | 0.284 (0.0017)* | 0.181 (0.0478)* | 0.236 (0.0095)* | |||
| SF-36 PCS | 1 | -0.770 (< 0.0001)* | -0.604 (< 0.0001)* | -0.610 (< 0.0001)* | -0.169 (0.0657) | ||||
| ODI | 1 | 0.630 (< 0.0001)* | 0.601 (< 0.0001)* | 0.101 (0.2729) | |||||
| VAS back | 1 | 0.643 (< 0.0001)* | 0.086 (0.3525) | ||||||
| VAS leg | 1 | -0.002 (0.9866) | |||||||
| Working time | 1 |
SVA, sagittal vertical axis; PI, pelvic incidence; TK, thoracic kyphosis; PI–LL, pelvic incidence minus lumbar lordosis; SF-36 PCS, 36-item Short Form Health Survey physical component summary; ODI, Oswestry Disability Index; VAS back, visual analogue scale for back pain; VAS leg, visual analogue scale for leg pain; Working time, outside working hours per week.
Table 3.
Univariate and multivariate regression analyses of health-related quality of life
| Variable |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Coef. (95% CI) | p-value |
Model: (R2 = 0.2299, p < 0.0001) |
||
| β-coefficient | p-value | |||
| Age | -0.2089 (-0.3741 to -0.03077) | 0.0221* | -0.9149 | 0.0120* |
| BMI | -0.2178 (-0.3822 to -0.04017) | 0.0168* | -2.0365 | 0.0007* |
| PI–LL | -0.2515 (-0.4122 to -0.0769) | 0.0056* | -0.2052 | 0.0308* |
| CSA-MF | 0.1385 (-0.04179 to 0.3100) | 0.1314 | 0.009630 | 0.3161 |
| Goutallier-MF | -0.2421 (-0.4039 to -0.06575) | 0.0077* | -2.0464 | 0.2695 |
| BMD | 0.1687 (-0.01089 to 0.3377) | 0.0655 | 2.8750 | 0.1133 |
Table 4.
Top 4 hits in the single-variant analysis of the severe sagittal imbalance phenotype
REFERENCES
1. Asai Y, Tsutsui S, Oka H, et al. Sagittal spino-pelvic alignment in adults: the Wakayama Spine Study. PLoS One 2017 12:e0178697.



2. Ailon T, Smith JS, Shaffrey CI, et al. Degenerative spinal deformity. Neurosurgery 2015 77 Suppl 4:S75-91.


3. Roussouly P, Gollogly S, Berthonnaud E, et al. Sagittal alignment of the spine and pelvis in the presence of L5-s1 isthmic lysis and low-grade spondylolisthesis. Spine (Phila Pa 1976) 2006 31:2484-90.


4. Schwab FJ, Blondel B, Bess S, et al. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: a prospective multicenter analysis. Spine (Phila Pa 1976) 2013 38:E803-12.

5. Aoki Y, Nakajima A, Takahashi H, et al. Influence of pelvic incidence-lumbar lordosis mismatch on surgical outcomes of short-segment transforaminal lumbar interbody fusion. BMC Musculoskelet Disord 2015 16:213.



6. Kado DM, Prenovost K, Crandall C. Narrative review: hyperkyphosis in older persons. Ann Intern Med 2007 147:330-8.


7. Park PJ, Lombardi JM, Lenke LG. The hybrid open muscle-sparing approach in adult spinal deformity patients undergoing lower thoracic fusion to the pelvis. Neurospine 2021 18:234-9.


8. Ogura Y, Kou I, Miura S, et al. A functional SNP in BNC2 is associated with adolescent idiopathic scoliosis. Am J Hum Genet 2015 97:337-42.



9. Takahashi Y, Kou I, Takahashi A, et al. A genome-wide association study identifies common variants near LBX1 associated with adolescent idiopathic scoliosis. Nat Genet 2011 43:1237-40.


10. Park PJ, Lin JD, Makhni MC, et al. Dual S2 Alar-iliac screw technique with a multirod construct across the lumbosacral junction: obtaining adequate stability at the lumbosacral junction in spinal deformity surgery. Neurospine 2020 17:466-70.



11. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009 25:1754-60.



12. Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010 38:e164.



13. Kim Y, Han BG, KoGES group. Cohort profile: the Korean Genome and Epidemiology Study (KoGES) Consortium. Int J Epidemiol 2017 46:1350.



14. Joshi RS, Haddad AF, Lau D, et al. Artificial intelligence for adult spinal deformity. Neurospine 2019 16:686-94.



15. Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine (Phila Pa 1976) 2005 30:1082-5.


16. Moon BJ, Ryu DS, Kim B, et al. Natural history and aggravating factors of sagittal imbalance in marked sagittal deformity compared with mild to moderate sagittal deformity: a prospective cohort study. Medicine (Baltimore) 2020 99:e19551.



17. Hanson DS, Bridwell KH, Rhee JM, et al. Correlation of pelvic incidence with low- and high-grade isthmic spondylolisthesis. Spine (Phila Pa 1976) 2002 27:2026-9.


18. Wui SH, Hyun SJ, Kang B, et al. Bicortical screw purchase at upper instrumented vertebra (UIV) can cause UIV fracture after adult spinal deformity surgery: a finite element analysis study. Neurospine 2020 17:377-83.


19. Yamada K, Abe Y, Yanagibashi Y, et al. Mid- and long-term clinical outcomes of corrective fusion surgery which did not achieve sufficient pelvic incidence minus lumbar lordosis value for adult spinal deformity. Scoliosis 2015 10:S17.



20. Gonnelli S, Caffarelli C, Nuti R. Obesity and fracture risk. Clin Cases Miner Bone Metab 2014 11:9-14.



21. Urrutia J, Besa P, Lobos D, et al. Lumbar paraspinal muscle fat infiltration is independently associated with sex, age, and inter-vertebral disc degeneration in symptomatic patients. Skeletal Radiol 2018 47:955-61.


22. Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol 2000 55:145-9.


23. Parkkola R, Rytokoski U, Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine (Phila Pa 1976) 1993 18:830-6.


24. Xia W, Fu H, Zhu Z, et al. Association between back muscle degeneration and spinal-pelvic parameters in patients with degenerative spinal kyphosis. BMC Musculoskelet Disord 2019 20:454.



25. Lee JC, Cha JG, Kim Y, et al. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: comparison with the normal controls. Spine (Phila Pa 1976) 2008 33:318-25.

26. Ryu DS, Shinn JK, Kim BW, et al. Prospective observational cohort study of health-related quality of life: marked adult sagittal deformity (ASD) in comparison with mild to moderate ASD. Spine (Phila Pa 1976) 2019 44:1723-30.

27. Takemitsu Y, Harada Y, Iwahara T, et al. Lumbar degenerative kyphosis. Clinical, radiological and epidemiological studies. Spine (Phila Pa 1976) 1988 13:1317-26.

28. Lee CH, Chung CK, Jang JS, et al. ‘Lumbar degenerative kyphosis’ is not byword for degenerative sagittal imbalance: time to replace a misconception. J Korean Neurosurg Soc 2017 60:125-9.



29. Bouxsein ML, Melton LJ 3rd, Riggs BL, et al. Age- and sex-specific differences in the factor of risk for vertebral fracture: a population-based study using QCT. J Bone Miner Res 2006 21:1475-82.


30. Hong JH, Han MS, Lee SK, et al. Is the agricultural work a risk factor for Koreans elderly spinal sagittal imbalance? J Korean Neurosurg Soc 2020 63:623-30.



31. Nakajima M, Takahashi A, Tsuji T, et al. A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat Genet 2014 46:1012-6.


32. Hanaei S, Abdollahzade S, Khoshnevisan A, et al. Genetic aspects of intervertebral disc degeneration. Rev Neurosci 2015 26:581-606.


33. Pestonjamasp KN, Pope RK, Wulfkuhle JD, et al. Supervillin (p205): a novel membrane-associated, F-actin-binding protein in the villin/gelsolin superfamily. J Cell Biol 1997 139:1255-69.



34. Chen Y, Takizawa N, Crowley JL, et al. F-actin and myosin II binding domains in supervillin. J Biol Chem 2003 278:46094-106.


35. Smith TC, Fang Z, Luna EJ. Novel interactors and a role for supervillin in early cytokinesis. Cytoskeleton (Hoboken) 2010 67:346-64.



36. Pope RK, Pestonjamasp KN, Smith KP, et al. Cloning, characterization, and chromosomal localization of human superillin (SVIL). Genomics 1998 52:342-51.



- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























