 |
 |
- Search
| Neurospine > Volume 18(3); 2021 > Article |
|
|
Abstract
Objective
Anterior cervical discectomy and fusion (ACDF) is a common surgical method used to treat patients with Hirayama disease. And sagittal balance indexes have been revealed to be predictors of clinical outcomes in patients with cervical diseases, but their relationships with ACDF-treated Hirayama disease outcomes remain unknown. The purpose of this study is to evaluate the relationship of preoperative cervical sagittal balance indexes and clinical outcomes in ACDF-treated Hirayama disease patients.
Methods
Eighty patients with Hirayama disease treated by ACDF were reviewed retrospectively. Six cervical sagittal balance parameters were collected including Cobb angle, T1 slope, C1–7 sagittal vertical axis (SVA), C2–7 SVA, center of gravity of the head (CGH)-C7 SVA, range of motion. The recovery outcomes of the patients were divided into 2 groups by Odom score and the differences in recovery between the 2 groups were confirmed by electromyography. The correlation between imaging parameters and postoperative outcome was evaluated with logistic regression. The receiver operating characteristic (ROC) curve and area under the ROC curve (AUC) were used to evaluate the significant result of logistic regression and the optimal diagnostic value.
Hirayama disease is a progressive disease caused by compression of anterior horn of cervical spinal cord under cervical flexion. It is mainly seen in young men of Asian descent. The onset age is generally less than 20 years old [1-3]. The patients were mainly manifested as asymmetric muscle weakness and atrophy at the distal extremity, leaving them with different degrees of hand and forearm dysfunction [4]. For some patients with early onset, short course of disease, and mild spinal cord atrophy, nonsurgical treatment like neck brace can be used. While surgical treatment is recommended for patients with ineffective conservative treatment and rapid progress of symptoms [5]. And the operation effect is good, most patients’ condition no longer progress [6].
Studies have shown that sagittal balance of cervical spine exerts a significant impact on the prognosis of patients undergoing spinal surgery [7,8]. And compared with normal people, patients with Hirayama disease have differences in sagittal balance parameters of cervical spine, and surgery can reduce this difference [9]. Many factors were confirmed associated with progression and poor prognosis of Hirayama disease [10], however, the relationship between the postoperative effect and the sagittal balance of cervical spine has not been studied. Sagittal imbalance of spine has been proven to be correlated with many other diseases such as cervical spondylosis, ossification of the posterior longitudinal ligament [8]. The purpose of this study is to analyze the correlation between the sagittal balance parameters of cervical spine and the postoperative outcomes of Hirayama disease.
The data of patients with Hirayama disease who were diagnosed and treated surgically in authors’ institution from August 2010 to February 2019 were retrospectively analyzed.
1) Inclusion criteria: (1) the diagnosis was clear, with typical amyotrophy and weakness of the distal upper limbs; (2) flexion position magnetic resonance imaging showed that the epidural space on the dorsal side of the spinal cord widened, vascular emptiness could be seen in it, and the corresponding segment of the cervical spinal cord was obviously compressed; (3) electromyography (EMG) showed that the number of multipoint motor units of thenar muscle and/or hypothenar muscle was less than the normal value; (4) conservative treatment such as neck brace was ineffective for 3 months; (5) the disease progressed rapidly and significantly affected the life of the patients.
2) Exclusion criteria: (1) unclear diagnosis; (2) improvement of symptoms within 3 months after neck brace treatment; (3) local infection in neck operation area or infection in other parts of the body; (4) titanium metal allergy. A total of 80 cases were enrolled, and the surgical procedures were performed by doctors in the same specialty group (Fig. 1).
The patient was placed in a flat position after general anesthesia, the right lateral incision of the anterior neck was taken, which was approximately 3.5 cm in length, and the anterior vertebra was exposed along the inner edge of the sternocleidomastoid muscle, and the operation segment was determined after fine-needle positioning. The anterior cervical distractor properly opened the intervertebral space and removed the intervertebral disc of the target segment to the posterior edge of the vertebral body to expose the posterior longitudinal ligament. The posterior longitudinal ligament was preserved, the endplate cartilage was removed with curette, and the interbody fusion device with appropriate height and artificial bone was placed into the intervertebral space. Titanium plate fixation was placed in front of the corresponding vertebral body after decompression of the intervertebral space at all target segments. Close the incision layer by layer after placing the drainage. The drainage tube was removed 48 hours after operation. The neck brace was fixed and immobilized for 4–6 weeks.
In order to minimize the error, the measurement was carried out by 2 doctors independently, and the final results were averaged. The preoperative and postoperative sagittal balance index of cervical spine was measured on the lateral x-ray, and the required measurement indexes were defined as follows (Fig. 2). Make a horizontal line under the inferior endplate of C2 and C7, and then make 2 vertical lines respectively. The angle between the 2 vertical lines is called Cobb angle. T1 slope is the angle between the superior endplate of T1 and the horizontal line. Make a vertical line at the anterior edge of C1 body, and the distance from the posterior upper corner of C7 to this vertical line is called C1–7 sagittal vertical axis (SVA). Make a vertical line in the center of C2 body, and the distance from the posterior upper corner of C7 to this vertical line is called C2–7 SVA. Make a vertical line at the front edge of the external auditory meatus, and the distance from the posterior upper corner of C7 to this vertical line is called center of gravity of the head (CGH)-C7 SVA. The difference of Cobb angle between hyperextension and hyperflexion was range of motion (ROM).
All surgical patients were followed up by 2 professionally trained clinicians in the outpatient clinic. X-ray of lateral cervical vertebrae after surgery was included for imaging evaluation. The clinical assessment was based on the Odom scale [11] and the Selected Brief-Michigan Hand Questionnaire (SB-MHQ) [4]. SB-MHQ was described as Table 1. Divided into A, B, C, and D 4 levels, a Odom score of A or B was defined as the ideal improvement of postoperative symptoms, and the rest was defined as the poor improvement (Table 2).
All neurophysiological measurements were performed by a Nihon Kohden MEB-940 EMG unit (Tokyo, Japan). The oscilloscope was scanned at a rate of 5 msec/cm with a magnification of 200–500 volts/cm, and the room temperature was controlled at 25°C. The skin temperature of the forearm was kept between 32°C and 34°C. To exclude the effect of intermeasurer variation, the same experienced neurophysiologist performed all tests. The median nerve was stimulated at the wrist and elbow. The ulnar nerve was stimulated at the wrist, above and below the elbow. During the motor nerve examination, the maximal compound muscle action potential (CMAP) values of the abductor pollicis brevis (APB) and abductor digiti minimi were recorded during stimulation of the median and ulnar nerves, respectively [12]. We also recorded the fibrillation and positive sharp wave of APB and abductor digiti minimi pre- and postoperative, presented as the number of plus signs.
Stata 16.0 (StataCorp LLC, College Station, TX, USA) was used for all the statistical calculation. We divided the patients into 2 groups according to the Odom score, the ideal improvement group and the poor improvement group. Then the basic situation and sagittal balance parameters of cervical spine preand postoperation were compared between the 2 groups. T-test was used for continuous variables and chi-square test was used for discrete variables. Logistic regression was used to analyze the correlation between the 5 parameters of sagittal balance of cervical spine and the Odom score of postoperative curative effect. Whether the improvement of postoperative symptoms is ideal or not is regarded as the dependent variable, and the sagittal balance parameter of the cervical vertebra is the independent variable. The value of test level α is 0.05 on both sides. For the positive results obtained from the analysis, we plotted the correlation curve with the Odom score as the horizontal coordinate. The positive results obtained by logistic regression analysis and their judgment threshold were tested and quantitatively analyzed by the receiver operating characteristic (ROC) curve and the area under the curve (AUC).
Six of 80 were excluded because preoperative EMG data could not be obtained, 5 withdrew from follow-up halfway for personal reasons, and finally, only 69 (86.25%; 65 males, 4 females) had complete follow-up data. The follow-up time range 2.16 to 10.38 years (5.17±1.76 years). The preoperative Cobb angle range -28.90° to 27.00° (3.95°±10.93°), T1 Slope range 4.50 to 43.30 mm (21.73±7.63 mm), C1–7 SVA range 0.00 to 56.80 mm (31.33±12.60 mm), C2–7 SVA range -7.69 to 44.02 mm (18.91±10.17 mm), CGH-C7 SVA was -17.73 to 45.57 mm (15.92±14.87 mm), and ROM was 24.20° to 109.60° (69.37°±17.34°). Comparing the sagittal balance parameters of cervical spine before and after operation, we could find that there are obvious differences between some parameters, such as Cobb angle, T1 slope, and CGH-C7 SVA (Table 3). The differences between radiological parameters indicate that the operation has successfully interfered with the balance of the cervical sagittal plane, which is consistent with our previous studies [9].
Imaging evaluation: lateral x-ray of cervical vertebra showed that the position of internal fixation was satisfactory in all cases, and there was no loosening or fracture of internal fixation. As for clinical evaluation of patient status before and after surgery, the hand functional scores in Table 4 suggested a remarkable progress in patient recovery. During the postoperative follow-up for Odom scoring, 69 patients were scored as follows: A, 7 cases; B, 25 cases; C, 37 cases; and no cases were evaluated as D. Besides, we reconfirmed the existence of differences between the 2 groups using EMG (Table 5). The results suggested no significant difference in the parameters between the 2 groups preoperatively. And there were significant differences in CMAP of the median and ulnar nerves, and the number of the fibrillation and positive sharp wave of APB postoperatively (Table 5, Fig. 3).
From Table 6, the age of patients at the time of operation, the number of operative segments, and the follow-up time had no relationship with the surgical effect. For the 6 preoperative imaging parameters, only there were differences between Cobb angle and CGH-C7 SVA, and the patients with better surgical outcomes had smaller CGH-C7 SVA and larger Cobb angle (Fig. 4). Since only anteroposterior and lateral x-ray of cervical vertebra was routinely performed in postoperative reexamination, there were only 5 postoperative parameters excluding ROM, and only C1–7 SVA had a significant difference.
Meanwhile, we also analyzed the correlation between preoperative imaging parameters and EMG, which showed that the degree of EMG recovery was positively correlated with preoperative Cobb angle and negatively correlated with preoperative CGH-C7 SVA (Fig. 5).
Logistic regression analysis showed that among the 6 preoperative parameters of sagittal balance of cervical vertebrae, the Cobb angle (p=0.037) and the CGH-C7 SVA (p=0.007) were correlated with the evaluation of curative effect during postoperative follow-up. As for the 5 postoperative indicators, the results showed that they were not the factors affecting the surgical effect (Table 7).
ROC curve analysis showed that the AUC (95% confidence interval) of Cobb angle and CGH-C7 SVA were 0.559 (0.421–0.697) and 0.702 (0.580–0.824), respectively. The reference value of CGH-C7 SVA is higher than that of Cobb angle. When the sum of sensitivity and specificity reached the maximum, the best predictive thresholds for judging Cobb angle and CGH-C7 SVA were 1.50° and 5.40 mm, respectively (Fig. 6).
The pathogenesis of Hirayama disease is still unclear, and researchers have advanced numerous hypotheses, mainly including the doctrine of spinal cord motility, the doctrine of growth and development, the doctrine of motor neuron disease, and the doctrine of immune mechanisms [13-16]. In addition, some scholars believe that Hirayama disease is due to abnormal dural traction and restriction, which not only injure the spinal cord in the upright position but also aggravate the injury when flexing the neck [17]. In view of the fact that cervical flexion may be the pathogenic factor of Hirayama disease [18,19], the application of neck brace fixation in the early stage of the disease can prevent the progression of the disease [20]. For patients with a long course of disease and severe atrophy of the muscles of the hand and forearm, it is difficult for patients to wear a neck brace for a long time, so it is not suitable for this treatment [21]. Surgical treatment can limit the further development of the disease. Anterior cervical discectomy and fusion (ACDF) limits cervical hyperflexion activity, fuses unstable segments, and restores the normal physiological arc of the cervical spine, thus limiting the progression of neurological symptoms [22-24]. Paredes et al. [25] suggested that ACDF is able to directly decompress and to correct cervical kyphosis better compared with posterior surgery and is recommended as the preferred procedure for the treatment of Hirayama disease. However, it still needs to be confirmed by further studies with long-term follow-up.
Unlike cervical spondylosis myelopathy, there are few obvious compression-causing objects, such as herniated nucleus pulposus, in patients with Hirayama disease. The vast majority of patients present with anterior displacement of the spinal cord in the flexed cervical position. This displacement results in dynamic anterior compression of the spinal cord. Rebuilding the curvature of the cervical spine becomes more crucial for patients with Hirayama disease [26].
In recent years, as research into cervical spondylosis has advanced and surgical approaches have advanced, spine surgeons have placed greater emphasis on cervical sagittal balance. Previous studies have shown that parameters of the cervical sagittal plane can predict the outcome of surgery. For example, it is used to predict the postoperative effect of patients with ossification of the posterior longitudinal ligament [8]. Patients with Hirayama disease may possess lower uncinate process, smaller inclination angle of inferior endplate of the upper vertebra, and greater discfacet angles [27,28]. These abnormal structures may be the cause of cervical sagittal imbalance in patients with Hirayama disease [9], the consequential cervical instability may play a significantly important role in the pathogenesis and progress of Hirayama disease. It has been reported that the improvement of neurological function after ACDF in patients with sagittal imbalance is limited [29,30]. However, the relationship between the effect of ACDF and preoperative cervical sagittal balance in patients with Hirayama disease has not been studied.
In our present study, we first confirmed that the included patients had differences in their sagittal parameters and clinical symptoms (SB-MHQ scoring) after ACDF surgery. The patients were divided into 2 groups by a simple and easy-to-use Odom score. Since the Odom score is more subjective, we reconfirmed the statistical difference in the recovery of EMG between the 2 groups, which reaffirmed the simplicity and ease of the Odom score. In the better recovered group, we noticed higher CMAP and less amount of fibrillation and positive sharp wave. Then we further evaluated the differences in sagittal parameters and tried to find the reason for the difference in recovery between the 2 groups.
According to the result of multivariate regression analysis, only Cobb angle and CGH-C7 SVA are the key factors that affect the effect of operation. Patients with a smaller Cobb angle and a larger CGH-C7 SVA seemed to have a poor recovery. The AUC of the 2 is 0.559 and 0.702 respectively, which indicates that they have a certain reference value. A cutoff of 1.50° for the Cobb angle was associated with a sensitivity of 85.19% and specificity of 38.10% in predicting operative effect, and a cutoff of 5.40 mm for the CGH-C7 SVA was associated with a sensitivity of 100% and specificity of 35.71% in predicting operative effect. The balance of the gravity center of the head is a better index, which can more truly reflect the load of the cervical spine, so it can be used to predict the surgical effect of cervical degenerative diseases [31]. Our study shows that sagittal balance of the gravity center of head can affect the final outcome and cervical alignment in patients with Hirayama disease after ACDF, which is consistent with previous reports that larger CGH-C7 SVA may lead to postoperative adjacent segment disease or increase the risk of other complications [32]. Since the introduction of the Cobb angle in 1948, it has been an important tool for spine surgeons to examine the alignment of the cervical spine [33]. In a report by Lau et al. [34] C2–7 Cobb angle was (9.1±11.4) in 145 patients with cervical spondylotic myelopathy. In another research by Wang et al. [26] C2–7 Cobb angle was (5.27±10.68) in 50 patients with Hirayama disease. From these reports, we may conclude to some extent that the degree of cervical lordosis in patients with Hirayama disease may be less. A smaller degree of lordosis results in more tension on the spinal cord in the flexed position, which may be the reason why we found a larger Cobb angle in the better recovered group.
This study also has some limitations. First of all, this study is retrospective, and there may be selection bias. Secondly, the number of people included in the study is not enough, and the sample size needs to be increased in the future. Finally, Odom score is more subjective, and we need to introduce some more objective scoring standards in the future.
In summary, according to the average follow-up of more than 5 years in patients with Hirayama disease treated with ACDF, surgical treatment can control or improve the clinical symptoms of the patients. Patients with lager preoperative Cobb angle and smaller CGH-C7 SVA have a high probability of developing sagittal imbalances, and these 2 parameters can be used as predictors of outcomes in ACDF-treated Hirayama disease patients.
Fig. 1.
A 20-year-old male patient underwent anterior cervical discectomy and fusion because of ineffective conservative treatment in the diagnosis of Hirayama disease. (A) Muscular atrophy of distal upper extremity. (B-D) Preoperative lateral, hyperflexion, and hyperextension x-ray. (E-G) Preoperative neutral, flexed T2-weighted magnetic resonance imaging on sagittal plane and flexed horizontal plane. (H, I) Frontal and lateral views in x-rays 5 years after operation.

Fig. 2.
Sagittal balance parameters of cervical spine. SVA, sagittal vertical axis; CGH, center of gravity of the head.

Fig. 3.
The relationship between postoperative electromyography and Odom score. (A) Odom score and CMAP in median nerve. (B) Odom score and CMAP in ulnar nerve. (C) Odom score and amount of sharp wave. CMAP, maximal compound muscle action potential; APB, abductor pollicis brevis. Odom score: A, All preoperative symptoms were relieved and daily life was not limited; B, Leave mild preoperative symptoms, daily life was not significantly affected; C, Some of the preoperative symptoms were relieved, but life was
obviously affected.
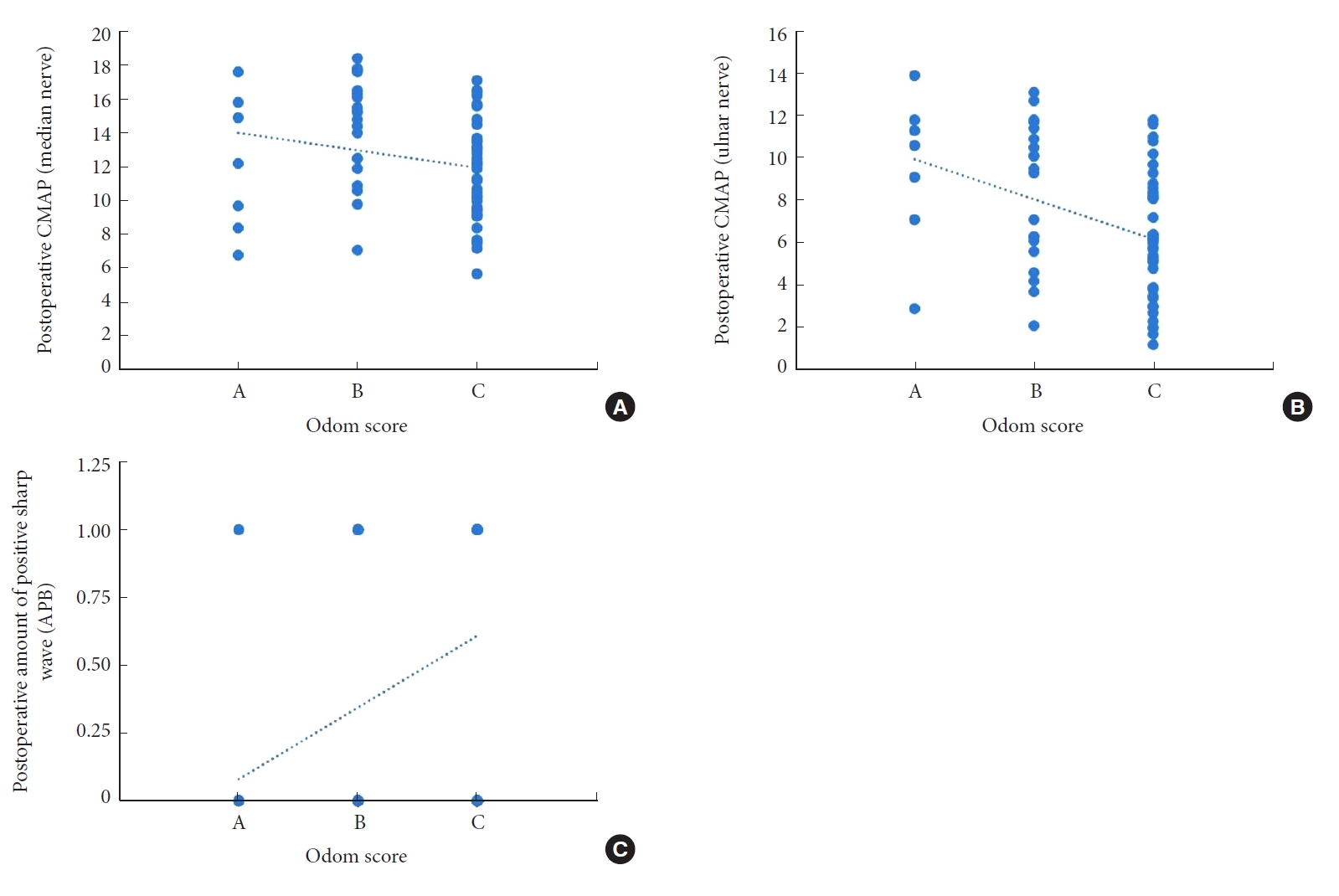
Fig. 4.
The relationship between preoperative imaging parameters and Odom score. (A) Odom score and CGH-C7 SVA. (B) Odom score and Cobb angle. CGH, center of gravity of the head; SVA, sagittal vertical axis. Odom score: A, All preoperative symptoms were relieved and daily life was not limited; B, Leave mild preoperative symptoms, daily life was not significantly affected; C, Some of the preoperative symptoms were relieved, but life was obviously affected.

Fig. 5.
The relationship between preoperative imaging parameters and EMG. (A) EMG and Cobb angle. (B) EMG and CGH-C7 SVA. EMG, electromyography; CGH, center of gravity of the head; SVA, sagittal vertical axis. APB, abductor pollicis brevis.
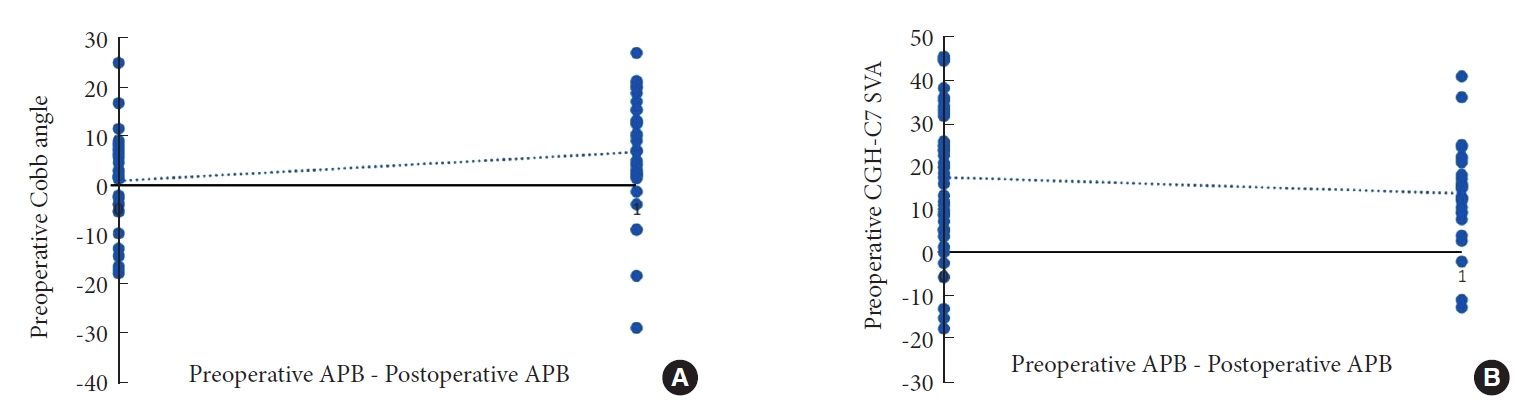
Fig. 6.
The receiver operating characteristic (ROC) curve of preoperative imaging parameters. (A) The ROC curve of CGH-C7 SVA. (B) The ROC curve of Cobb angle. CGH, center of gravity of the head; SVA, sagittal vertical axis.

Table 1.
Selected Brief-Michigan Hand Questionnaire
Table 2.
Odom scoring criteria
Table 3.
Comparison of imaging parameters before and after operation
| Parameter | Preoperative | Postoperative | p-value |
|---|---|---|---|
| Cobb angle (°) | 3.95 ± 10.93 | 10.72 ± 10.27 | 0.019* |
| T1 Slope (°) | 21.73 ± 7.63 | 25.05 ± 6.52 | 0.007* |
| C1–7 SVA (mm) | 31.33 ± 12.60 | 32.14 ± 15.07 | 0.731 |
| C2–7 SVA (mm) | 18.91 ± 10.17 | 15.47 ± 10.82 | 0.057 |
| CGH–C7 SVA (mm) | 15.92 ± 14.87 | 9.81 ± 17.03 | 0.026* |
Table 4.
The scores of the Selected Brief-Michigan Hand Questionnaire (SB-MHQ) domains preoperatively and at follow-up
| SB-MHQ domains | Preoperative | Final follow-up | p-value |
|---|---|---|---|
| Function | 2.01 ± 0.89 | 3.15 ± 0.97 | < 0.001* |
| Activities of daily living | 5.21 ± 1.09 | 5.91 ± 1.24 | < 0.001* |
| Work | 4.65 ± 1.34 | 5.99 ± 1.56 | < 0.001* |
| Satisfaction | 5.17 ± 1.35 | 5.42 ± 1.45 | < 0.001* |
| Total | 17.04 ± 4.32 | 20.47 ± 4.98 | < 0.001* |
Table 5.
Comparison of EMG before and after operation between the ideal improvement and poor improvement groups
| Variable | Ideal development | Poor development | p-value |
|---|---|---|---|
| Preoperative | |||
| CMAP (median nerve) | 9.38 ± 2.84 | 9.50 ± 2.81 | 0.862 |
| CMAP (ulnar nerve) | 5.30 ± 2.97 | 4.10 ± 3.01 | 0.108 |
| Amount of fibrillation and positive sharp wave (APB) | 0.74 ± 0.71 | 1.07 ± 0.80 | 0.087 |
| Amount of fibrillation and positive sharp wave (ADM) | 1.11 ± 0.89 | 0.90 ± 0.84 | 0.338 |
| Postoperative | |||
| CMAP (median nerve) | 13.71 ± 3.38 | 11.64 ± 2.92 | 0.009* |
| CMAP (ulnar nerve) | 8.65 ± 3.36 | 6.10 ± 2.91 | 0.001* |
| Amount of fibrillation and positive sharp wave (APB) | 0.25 ± 0.44 | 0.61 ± 0.49 | 0.003* |
| Amount of fibrillation and positive sharp wave (ADM) | 0.40 ± 0.50 | 0.47 ± 0.50 | 0.582 |
Table 6.
Comparison of pre- and postoperative factors and clinical outcomes in the ideal improvement and poor improvement groups
| Variable | Ideal improvement (n = 27) | Poor improvement (n = 42) | p-value |
|---|---|---|---|
| Age (yr) | 19.45 ± 4.02 | 18.77 ± 4.97 | 0.537 |
| No. of operative segments | |||
| 2 | 33 | 33 | 0.572 |
| 3 | 2 | 1 | |
| Follow-up time (yr) | 5.22 ± 1.14 | 5.71 ± 2.42 | 0.258 |
| Preoperative imaging parameters | |||
| Cobb angle (°) | 8.52 ± 9.68 | 2.94 ± 11.66 | 0.042* |
| T1 slope (°) | 21.54 ± 8.73 | 22.02 ± 19.79 | 0.801 |
| C1–7 SVA (mm) | 29.27 ± 12.53 | 34.54 ± 12.24 | 0.090 |
| C2–7 SVA (mm) | 17.23 ± 10.09 | 21.53 ± 9.91 | 0.087 |
| CGH–C7 SVA (mm) | 11.86 ± 15.21 | 22.23 ± 12.05 | 0.004* |
| ROM (°) | 70.19 ± 16.26 | 68.08 ± 19.14 | 0.625 |
| Postoperative imaging parameters | |||
| Cobb angle (°) | -1.22 ± 9.88 | 1.20 ± 10.36 | 0.332 |
| T1 slope (°) | 24.23 ± 6.58 | 26.32 ± 6.33 | 0.195 |
| C1–7 SVA (mm) | 27.76 ± 15.56 | 35.21 ± 14.00 | 0.047* |
| C2–7 SVA (mm) | 14.59 ± 10.65 | 16.85 ± 11.14 | 0.399 |
| CGH–C7 SVA (mm) | 8.89 ± 19.00 | 11.24 ± 13.63 | 0.580 |
Table 7.
Multivariate logistic regression results of risk factors
| Parameter | Odds ratio | 95% Confidence interval | p-value |
|---|---|---|---|
| Preoperative | |||
| Cobb angle | 1.096 | 1.005–1.196 | 0.037* |
| T1 slope | 0.911 | 0.811–1.024 | 0.117 |
| C1–7 SVA | 1.012 | 0.869–1.177 | 0.879 |
| C2–7 SVA | 0.972 | 0.821–1.151 | 0.743 |
| CGH–C7 SVA | 1.098 | 1.026–1.175 | 0.007* |
| ROM | 0.985 | 0.953–1.017 | 0.351 |
| Postoperative | |||
| Cobb angle | 1.021 | -0.053 to 0.096 | 0.570 |
| T1 slope | 1.034 | -0.079 to 0.147 | 0.560 |
| C1–7 SVA | 1.067 | -0.014 to 0.144 | 0.110 |
| C2–7 SVA | 0.990 | -0.070 to 0.050 | 0.757 |
| CGH–C7 SVA | 0.968 | -0.094 to 0.030 | 0.319 |
REFERENCES
1. Hirayama K, Tsubaki T, Toyokura Y, et al. Juvenile muscular atrophy of unilateral upper extremity. Neurology 1963 13:373-80.


2. Hirayama K. Juvenile muscular atrophy of unilateral upper extremity (Hirayama disease)--half-century progress and establishment since its discovery. Brain Nerve 2008 60:17-29.

4. Waljee JF, Kim HM, Burns PB, et al. Development of a brief, 12-item version of the Michigan Hand Questionnaire. Plast Reconstr Surg 2011 128:208-20.



5. Kameyama T, Ando T, Mimatsu K, et al. Delayed exacerbation of cervical myelopathy in a case of juvenile muscular atrophy of unilateral distal upper extremity. Rinsho Shinkeigaku 1997 37:60-3.

6. Kohno M, Takahashi H, Ide K, et al. Surgical treatment for patients with cervical flexion myelopathy. J Neurosurg 1999 91(1 Suppl):33-42.


7. Lee SH, Kim KT, Seo EM, et al. The influence of thoracic inlet alignment on the craniocervical sagittal balance in asymptomatic adults. J Spinal Disord Tech 2012 25:E41-7.


8. Xu C, Zhang Y, Dong M, et al. The relationship between preoperative cervical sagittal balance and clinical outcome of laminoplasty treated cervical ossification of the posterior longitudinal ligament patients. Spine J 2020 20:1422-9.


9. Song J, Cui ZY, Chen ZH, et al. Analysis of the effect of surgical treatment for the patients with Hirayama disease from the perspective of cervical spine sagittal alignment. World Neurosurg 2020 133:e342-7.


10. Song J, Wang HL, Zheng CJ, et al. Risk factors for surgical results of Hirayama disease: a retrospective analysis of a large cohort. World Neurosurg 2017 105:69-77.


12. Jin X, Jiang JY, Lu FZ, et al. Electrophysiological differences between Hirayama disease, amyotrophic lateral sclerosis and cervical spondylotic amyotrophy. BMC Musculoskelet Disord 2014 15:349.



13. Hirayama K, Tokumaru Y. Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology 2000 54:1922-6.


14. Toma S, Shiozawa Z. Amyotrophic cervical myelopathy in adolescence. J Neurol Neurosurg Psychiatry 1995 58:56-64.



15. Gamez J, Also E, Alias L, et al. Investigation of the role of SMN1 and SMN2 haploinsufficiency as a risk factor for Hirayama’s disease: clinical, neurophysiological and genetic characteristics in a Spanish series of 13 patients. Clin Neurol Neurosurg 2007 109:844-8.


16. Kira J, Ochi H. Juvenile muscular atrophy of the distal upper limb (Hirayama disease) associated with atopy. J Neurol Neurosurg Psychiatry 2001 70:798-801.



17. Konno S, Goto S, Murakami M, et al. Juvenile amyotrophy of the distal upper extremity: pathologic findings of the dura mater and surgical management. Spine (Phila Pa 1976) 1997 22:486-92.


19. Xu X, Han H, Gao H, et al. The increased range of cervical flexed motion detected by radiographs in Hirayama disease. Eur J Radiol 2011 78:82-6.


20. Tokumaru Y, Hirayama K. Cervical collar therapy for juvenile muscular atrophy of distal upper extremity (Hirayama disease): results from 38 cases. Rinsho Shinkeigaku 2001 41:173-8.

21. Zhou B, Chen L, Fan D, et al. Clinical features of Hirayama disease in mainland China. Amyotroph Lateral Scler 2010 11:133-9.


22. Kuo YH, Kuo CH, Huang WC, et al. Anterior cervical discectomy and fusion for Hirayama disease: a case report and literature review. Neurospine 2019 16:626-30.



23. Zhang H, Wang S, Li Z, et al. Anterior cervical surgery for the treatment of Hirayama disease. World Neurosurg 2019 127:e910-8.


24. Wu W, Wang S, Lin J. A 34-year-old female patient with hirayama disease complicated by severe spinal cord injury. World Neurosurg 2019 130:84-8.


25. Paredes I, Esteban J, Ramos A, et al. A severe case of Hirayama disease successfully treated by anterior cervical fusion. J Neurosurg Spine 2014 20:191-5.


26. Wang H, Sun C, Yang S, et al. Dynamic cervical radiographs in patients with Hirayama disease: an unneglectable factor on the choice of surgery options. World Neurosurg 2018 114:e433-40.


27. Tang C, Sun Y, Pan S. Variation of disc-facet orientation between Hirayama disease and non-Hirayama disease. Chin J Spine Spinal Cord 2013 23:577-81.
28. Tang C, Sun Y, Pan S. The CT morphological difference of Luschka joint between Hirayama disease patients and non-Hirayama disease patients. Chin J Spine Spinal Cord 2014 24:13-9.
29. Li X, Sun X, Lu S. Effects of cervical sagittal alignment on the cervical decompression surgery outcomes of cervical spondylotic myelopathy. Chin J Bone Joint 2018 7:867-72.
30. Jouibari MF, Le Huec JC, Ranjbar Hameghavandi MH, et al. Comparison of cervical sagittal parameters among patients with neck pain and healthy controls: a comparative cross-sectional study. Eur Spine J 2019 28:2319-24.


31. Mizutani J, Verma K, Endo K, et al. Global spinal alignment in cervical kyphotic deformity: the importance of head position and thoracolumbar alignment in the compensatory mechanism. Neurosurgery 2018 82:686-94.


32. Yagi M, Takeda K, Machida M, et al. Discordance of gravity line and C7PL in patient with adult spinal deformity--factors affecting the occiput-trunk sagittal discordance. Spine J 2015 15:213-21.


33. Cobb J, Cobb JL, Cobb JR. Outline for study of scoliosis. Instr Course Lect 1948 5:261-75.

- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























