Risk Factors for Pulmonary Cement Embolism (PCE) After Polymethylmethacrylate Augmentation: Analysis of 32 PCE Cases
Article information
Abstract
Objective
Pulmonary cement embolism (PCE) is an underestimated but potentially fatal complication after cement augmentation. Although the treatment and follow-up of PCE have been reported in the literature, the risk factors for PCE are so far less investigated. This study aims to identify the preoperative and intraoperative risk factors for the development of PCE.
Methods
A total of 1,373 patients treated with the polymethylmethacrylate (PMMA) augmentation technique were retrospectively included. Patients with PCE were divided into vertebral augmentation group and screw augmentation group. Possible risk factors were collected as follows: age, sex, bone mineral density, body mass index, diagnosis, comorbidity, surgical procedure, type of screw, augmented level, number of augmented vertebrae, fracture severity, presence of intravertebral cleft, cement volume, marked leakage in the paravertebral venous plexus, and periods of surgery. Binary logistic regression analyses were used to analyze independent risk factors for PCE.
Results
PCE was identified in 32 patients, with an incidence rate of 2.33% (32 of 1,373). For patients who had undergone vertebral augmentation, marked leakage in the paravertebral venous plexus (odds ratio [OR], 1.2; 95% confidence interval [CI], 0.1–10.3; p=0.000) and previous surgery (OR, 16.1; 95% CI, 4.2–61.0; p=0.007) were independent risk factors for PCE. Regarding patients who had undergone screw augmentation, the marked leakage in the paravertebral venous plexus (OR, 4.2; 95% CI, 0.5–37.3; p=0.004) was the main risk factor.
Conclusion
Marked leakage in the paravertebral venous plexus and previous surgery were significant risk factors related to PCE. Paravertebral leakage and operator experience should be concerned when performing PMMA augmentation.
INTRODUCTION
Since Galibert and Deramond first described the use of percutaneous vertebroplasty (PVP) for the treatment of symptomatic vertebral hemangioma in 1984 [1], cement augmentation with polymethylmethacrylate (PMMA) has been widely used in the treatment of painful vertebral fractures and osteoporosis‐related degenerative spine disorders [2]. PMMA bone cement is heavily used in PVP, percutaneous kyphoplasty (PKP), and cementaugmented pedicle screw instrumentation (CAPSI) owing to the rapid and durable stability improvements of the fracture area and bone-screw interface [3,4]. However, these surgical procedures also bear a high risk of cement leakage into the vertebral venous system and fracture gaps [5]. The incidence of cement leakage is as high as 38.3%–93.6% in PVP/PKP [6-8] and 12.5%–82.4% in CAPSI [5,6,9-11]. Similarly, the same high incidence of cement leakage after CAPSI (81.68%) was also found in our inpatients [5]. Cement leakage may lead to serious complications such as nerve root injury [8], spinal cord compression [5], pulmonary cement embolism (PCE) [12,13], cardioembolism [14,15], anaphylactic shock [16], and even death [16,17]. Among the abovementioned complications, PCE is one of the most severe complications.
PCE is caused by cement leakage into the vertebral venous plexus and reaches the pulmonary arteries through the azygous/hemiazygos system and cava vein. According to previous reports in the literature, PCE was detected in 1.0%–28.6% of patients by postoperative chest radiographs and/or computed tomography (CT) [18-21]. Although most PCE patients are asymptomatic or present mild pulmonary symptoms [22], a portion of PCE cases may develop aggravated hemodynamic repercussions or result in death [16,17]. Therefore, it is important to identify the risk factors for the development of PCE.
Whereas several case reports and case series have investigated the treatment and follow-up of PCE [5,18,23-25], studies on the risk factors for PCE are lacking. To our knowledge, only 3 studies have investigated the risk factors for PCE after cement augmentation [20,26,27]. This study aims to explore the risk factors for PCE and has collected the largest sample size to date.
MATERIAL AND METHODS
1. Patient Population
The study involving human participants were reviewed and approved by the Ethics Committee of The First Affiliated Hospital of Guangzhou University of Chinese Medicine (No. ZYYECK[2019]186). This study has been performed following the ethical standards in an appropriate version of the 1964 Declaration of Helsinki. Informed consent was waived by the Ethics Committee. Patients who underwent PVP, PKP, or CAPSI between January 2006 and December 2019 were reviewed. A total of 1,838 patients with osteoporotic vertebral fractures, spinal tumors, and degenerative spine diseases were initially retrieved from the hospital database. Patients without postoperative chest radiographs or chest CT were excluded. Finally, a total of 1,373 patients were included for further analyses.
The demographic and clinical information of patients was collected, including sex, age at operation, spinal bone mineral density (BMD), diagnosis, body mass index (BMI), comorbidities, date of surgery, surgical procedure, augmented level, type of screw, cement volume per level, and viscosity of bone cement. Patients with PCE were divided into vertebral augmentation group and screw augmentation group to conduct a subgroup analysis. For no obvious cement leakage occurred in vertebral augmentation level, patients who had undergone CAPSI+PVP were classified as screw augmentation group.
PMMA-based cement materials were used for all patients. Low-viscosity PMMA (Tecres S.P.A., Sommacampagna, Italy) was used in most patients. In contrast, part of patients who received vertebral augmentation was used high-viscosity PMMA (Confidence spinal cement system, Medtronic, Minneapolis, MN, USA) at the discretion of the attending surgery. In general, we injected PMMA with every 0.1-mL increment when it had a toothpaste-like viscosity (Mixing the low-viscosity bone cement for 30 seconds and waiting for 390 seconds, then the consistency of cement will change from liquid to toothpaste-like). The cement volume in the thoracic and lumbar vertebrae was about 2.5–6 mL and 3–8 mL. All vertebral augmentation procedures were performed bilaterally. According to different screw augmentation techniques, cement-augmented pedicle screws were divided into fenestrated and solid screws. The periods of surgery were classified equally into 2 periods. Because vertebral augmentation technique and screw augmentation technique were successively carried out in our department, time division of surgical periods between vertebral augmentation group (2006–2012 and 2013–2019) and screw augmentation group (2008–2013 and 2014–2019) was a little different.
2. Radiographic Analysis
Generally, the chest x-ray was reviewed routinely after the operation. If the patients complain of pulmonary problems, they will receive additional thoracic CT. To distinguish vascular calcification or calcified granuloma from PCE, a comparison between preoperative and postoperative chest radiographs (or chest CT scans, if possible) was performed. PCE was defined as postoperative emerging, solitary, or multiple branching linear density along the pulmonary vessel. If the distinction between vascular calcification and PCE was sometimes difficult in x-ray, a high-density branching with an attenuation greater than 500 HU in CT was judged as PCE [19]. Cement embolism sites were categorized as right lung, left lung, or bilateral lungs according to the location of the PCE in postoperative imaging (x-ray or CT scan). The length of PCE was measured in anterior-posterior or lateral chest x-rays by the Picture Archiving and Communication System. According to the semiquantitative classification of Genant et al. [28] fracture severity was classified as mild-moderate (20%–40%) or severe (> 40%). The presence of an intravertebral cleft was identified by a linear-like or irregular shape of very low density within a compressed vertebral body on CT and/or x-rays of the spine [29]. Evaluation of fracture severity and the presence of intravertebral cleft was only performed for cases with vertebral fractures. A significant leakage in the paravertebral venous plexus was defined as a linear-shaped, high-density leakage with a minimum length of 1 cm in the anterior or lateral region of the vertebral body. All radiographic analyses were performed by 2 experienced observers independently.
3. Selection of the Control Group
In general, PCE and non-PCE cases were matched in a 1:4 ratio to provide sufficient statistical power [30]. Non-PCE patients were selected by random sampling. The sampling strategy consisted of the following: Randomization was performed with computer-generated random numbers on the website www.randomizer.org. Depending on the sample size of vertebral augmentation group and screw augmentation group, a certain number of numbers were randomly generated to constitute the control group. This selection method made the control group consistent with non-PCE patients to the greatest possible degree.
4. Statistical Methods
Statistical analyses were performed using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). Measurement data were compared by using independent-sample t-tests. For counting data, Fisher exact probability tests were used. Multivariate logistic regression analysis was further analyzed risk factors with a significant difference for multivariate analysis. A p<0.05 was regarded to be significantly different.
RESULTS
1. Characteristics of PCE Patients
Thirty-two patients (3 males, 29 females) with PCE were identified. The overall incidence rate of PCE was 2.33% (32 of 1,373). The PCE sample had an average age of 71.52 years (range, 53–92) and an average T score of -3.75 SD (range, -6.6 to -1.3). The demographics of the PCE and non-PCE patients are shown in Table 1.
All emboli were found in subsegment pulmonary arteries classified as peripheral PCE. There were no perioperative deaths due to PCE. Most embolisms appeared in the right lung (68.75%, n=22), with some appearing bilaterally in the lungs (31.25%, n=10). Of the 32 patients with PCE, 5 patients (15.63%) experienced transient symptoms or hemodynamic repercussions and received symptomatic or anticoagulation therapy, and the remaining 27 patients (84.38%) did not demonstrate clinical syndromes during the perioperative period. No patient required further surgery to remove the cement emboli. After discharge, 3 patients still took aspirin or warfarin due to cardiovascular disease, while the remaining patients did not receive long-term anticoagulation therapy. Demographic and clinical information of PCE patients is presented in Table 2.
2. Risk Factor Analysis
From non-PCE patients, 88 patients who had undergone vertebral augmentation (PVP or PKP) and 40 patients who had undergone screw augmentation were randomly selected as the control group 1 and 2. For patients who had undergone vertebral augmentation, the incidence of PCE was significantly more frequent in cases with a larger number of augmented vertebrae (p<0.05), marked leakage in the paravertebral venous plexus (p<0.001), and previous surgery (p<0.05) (Table 3). For patients who had undergone screw augmentation, marked leakage in the paravertebral venous plexus (p<0.05) is a significant risk factor for the occurrence of PCE (Table 4). These risk factors with significant differences were further compared by a multivariate logistic regression model. For patients who had undergone vertebral augmentation, the regression analysis revealed that marked leakage in the paravertebral venous plexus (OR, 1.2; 95% CI, 0.1–10.3; p=0.000) and previous surgery (OR, 16.1; 95% CI, 4.2–61.0; p=0.007) were independent risk factors for PCE. Regarding patients who had undergone screw augmentation, the marked leakage in the paravertebral venous plexus (OR, 4.2; 95% CI, 0.5–37.3; p=0.004) was the main risk factor (Table 5). Representative cases of PCE patients undergoing PVP and CAPSI are shown in Figs. 1 and 2, respectively.
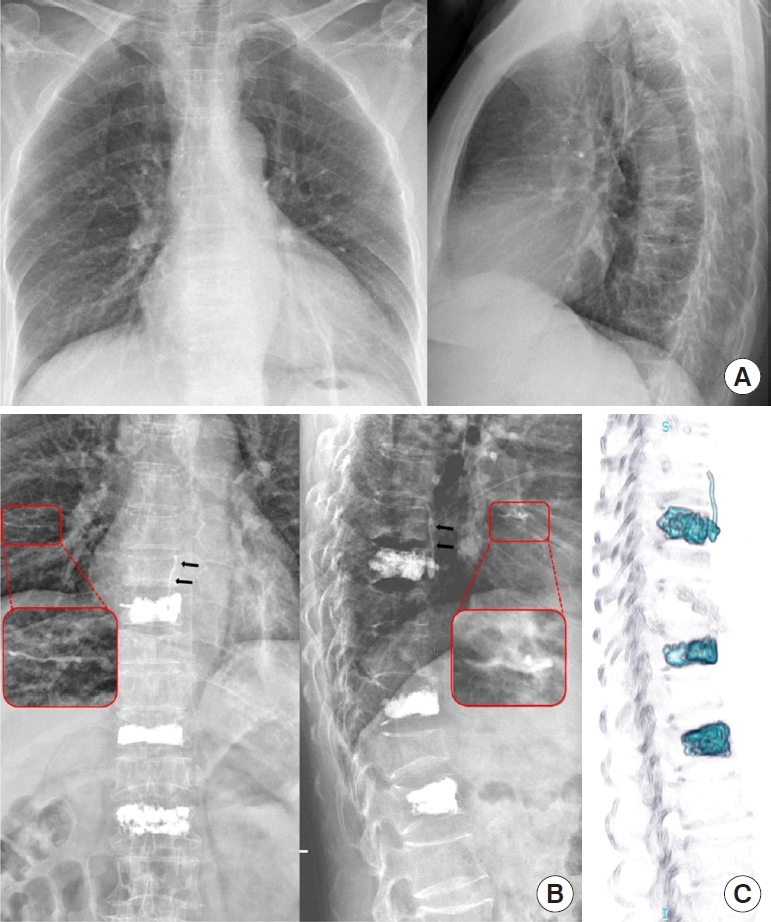
A 56-year-old female developed postoperative pulmonary cement embolism after vertebroplasty at the T8, T11, and L1 levels (case No. 17). (A) Chest radiography findings were normal before surgery. (B) Postoperative x-ray showed significant leakage of the paravertebral venous plexus (black arrow) at the T8 level and a tubular-shaped, high-density embolism in the right lung (red box: zoomed region). (C) The 3-dimensional computed tomography reconstruction provided more accurate and clearer views of cement leaks.

A 73-year-old female developed postoperative pulmonary cement embolism after cement-augmented pedicle screw instrumentation at the L2–5 level (case No. 28). (A) Anteroposterior and lateral digital radiographs showed curvilinear cement in the paravertebral venous plexus (black arrow). (B) Postoperative chest x-rays showed a linear-like, hyperdense cement embolism in the right lung (red arrow). (C) The zoomed region of the red box shows cement leakage into the paravertebral venous plexus at the L5 level. In addition, a cement embolism was found in the right pulmonary vascular tree (red arrows).
DISCUSSION
PCE after vertebral cement augmentation procedures was first reported in 1999 by Padovani et al. [31]. In 2011, Luetmer et al. [26] described the largest PCE case series (n=23) thus far. Subsequently, El Saman et al. [21] reported the highest incidence of PCE (28.6%, 12 of 42) through routine postoperative chest imaging in 2013. The clinical importance of PCE is underestimated since previous studies showed that most cases were asymptomatic or had slight discomfort. However, a growing number of severe cases have been reported involving acute respiratory distress syndrome [16,32-34] and even death [16,17]. Therefore, surgeons must be aware of this potentially fatal complication following PMMA augmentation. Identifying risk factors for PCE can help surgeons improve preoperative planning and reduce the incidence.
This study revealed that marked leakage in the paravertebral venous plexus and previous surgery had a statistically significant relationship with the development of PCE. Anatomically, the vertebral venous plexus consists of 3 interconnected veins: the internal vertebral venous plexuses, the external vertebral venous plexuses, and the basivertebral veins [35]. The external vertebral venous plexuses were connected with the superior and inferior vena cava through intercommunication with the azygos venous system and the lumbar veins, respectively [35]. When the vertebral venous plexus was injured by vertebral compression fracture, paracentesis procedures, or screw insertion, liquid PMMA bone cement can easily escape into the ruptured venous system. Then, through the valveless vertebral venous plexus and vena cava, the leaked cement can drain into the pulmonary circulation [36]. Thus, paravertebral venous leakage can be recognized as an early sign of PCE. When significant leakage in the paravertebral venous plexus occurs intraoperatively, the surgeon should alert the high risk of developing PCE and stop the injection immediately.
Factors related to paravertebral venous plexus leakage have been reported in the literature [3-7,21,37], including large-volume injection of bone cement, multilevel cement augmentation, the low viscosity of cement, early injection when bone cement is in dilute viscosity, high application pressure, the tip of screw/puncture needle adjacent to the mid-part of the vertebral body, less experienced surgeons, and inadequate fluoroscopic confirmation. Knowing these risk factors could be useful to develop preventive strategies for paravertebral venous plexus leakage and therefore decrease the incidence of PCE.
Although the period of surgery was not an independent risk factor in screw augmentation group (probably due to the small sample size), previous surgery bears a significantly higher risk of PCE than subsequent surgery. This result suggests that surgical experience and awareness about PCE have important implications for the occurrence of this complication. With the improvement of surgical techniques and the understanding of PCE, surgeons have been more careful in filling the vertebral body and have paid more attention to paravertebral cement leakage [38]. Based on the above risk factors and our experience, we recommend injecting cement with a toothpaste-like viscosity in small doses multiple times (0.1 mL–0.2 mL for each injection) and under continuous fluoroscopic monitoring [2,39,40]. Some measures, including keeping the tip of screw/puncture needle away from the midline of the vertebral body [5], reduce the volume of cement [13], and limit the number of augmented vertebral body [40], could also be useful to prevent cement leakage and to contribute to reductions in the incidence of PCE.
Additional risk factors for PCE have also been reported in the literature. Kim et al. [20] found that cement leakage into the inferior vena cava is a significant risk factor for PCE, which agrees with the present study. According to Luetmer et al. [26] and Hsieh et al. [27] those with PCE were significantly younger, treated with more total levels, and injected with a larger volume of cement. The author did not have a definitive explanation for age differences. Concerning more augmented levels and larger volumes of cement, it is more likely to invade the basivertebral system [37,41]. Although not statistically significant, our study also reveals that more augmented vertebrae may be a risk factor for PCE. Besides, a lower frequency of PCE was noted for the presence of intravertebral clefts (p=0.068), which may be related to the low injection pressure and avascular necrosis of the vertebral body.
The primary limitation is that some patients did not undergo postoperative chest radiography or chest CT, which may underestimate asymptomatic patients. A secondary limitation is that although operator variability and their manipulation may play a pivotal role in the development of PCE, we did not analyze the different operators or their operative habits. A final limitation is that some patients underwent PVP twice or more, so these patients were included multiple times.
CONCLUSION
PCE was not a very rare complication after PMMA augmentation. Significant leakage in paravertebral venous plexus and previous surgery were significant risk factors related to PCE. Paravertebral leakage and operator experience should be concerned when performing PMMA augmentation technique.
Notes
The authors have nothing to disclose.
Acknowledgements
This study was funded by the innovation and strength project of The First Affiliated Hospital of Guangzhou University of Chinese Medicine (2019IIT32).





