Improvement in Neurogenic Bowel and Bladder Dysfunction Following Posterior Decompression Surgery for Cauda Equina Syndrome: A Prospective Cohort Study
Article information
Abstract
Objective
The mechanisms of neurogenic bowel dysfunction (NBD) and neurogenic bladder (NB), which are major consequences of spinal cord injury and occasionally degenerative lumbar disease. The following in patients with cauda equina syndrome who underwent posterior decompression surgery was investigated: (1) the preoperative prevalence of NBD and NB, measured using the Constipation Scoring System (CSS) and International Prostate Symptoms Score (IPSS); (2) the degree and timing of postoperative improvement of NBD and NB.
Methods
We administered the CSS and IPSS in 93 patients before surgery and at 1, 3, 6, and 12 months postoperatively. We prospectively examined patient characteristics, Japanese Orthopaedic Association (JOA) score, and postoperative improvements in each score.
Results
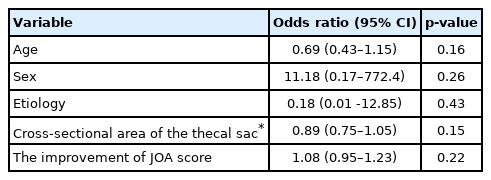
The prevalence of symptomatic defecation and urinary symptoms at admission were 37 patients (38.1%) and 31 patients (33.3%), respectively. Among the symptomatic patients with defecation problems, 12 patients had improved at 1 month, 13 at 3 months, 14 at 6 months, and 13 at 12 months postoperatively. Among the symptomatic patients with urinary problems, 5 patients improved at 1 month, 11 at 3 months, 6 at 6 months, and 10 at 1 year postoperatively. Comparing patients with improved versus unimproved in CSS, the degree of JOA score improvement was a significant prognosis factor (p<0.05; odds ratio, 1.05).
Conclusion
The prevalence of symptomatic defecation and urinary symptoms in patients with cauda equina syndrome was 38.1% and 33.3%, respectively. Decompression surgery improved symptoms in 30%–50%. These effects were first observed 1 month after the operation and persisted up to 1 year.
INTRODUCTION
Neurogenic bowel dysfunction (NBD) and neurogenic bladder (NB) are 2 major complications of spinal cord or nerve root injury. Although neurologic damage secondary to spinal cord injury differs among patients depending on the neurologic level and severity of the lesion, the changes in bowel motility, sphincter control, and gross motor dexterity interact to make bowel management a major life-style problem limiting quality of life. Hanson and Franklin [1] reported that 80% of male paraplegics and 46% of male tetraplegics would rank bowel and bladder as their greatest functional loss of mobility.
Whereas symptomatic lumbar degenerative disease causes spinal claudication as well as back and radicular leg pain. In severe cases, this can lead to loss of bladder and bowel control, resulting in constipation, frequent urination, and functional obstruction. However, the mechanisms of NBD and NB have not been clearly investigated and few studies have comprehensively evaluated pre-/postoperative NB [2-6].
In this study, we investigated the following matters in patients with cauda equina syndrome who underwent posterior decompression surgery with or without lumbar fusion: (1) the preoperative prevalence of NBD and NB, evaluated using the Constipation Scoring System (CSS) and International Prostate Symptoms Score (IPSS); (2) the degree and timing of postoperative improvement of NBD and NB.
MATRIALS AND METHODS
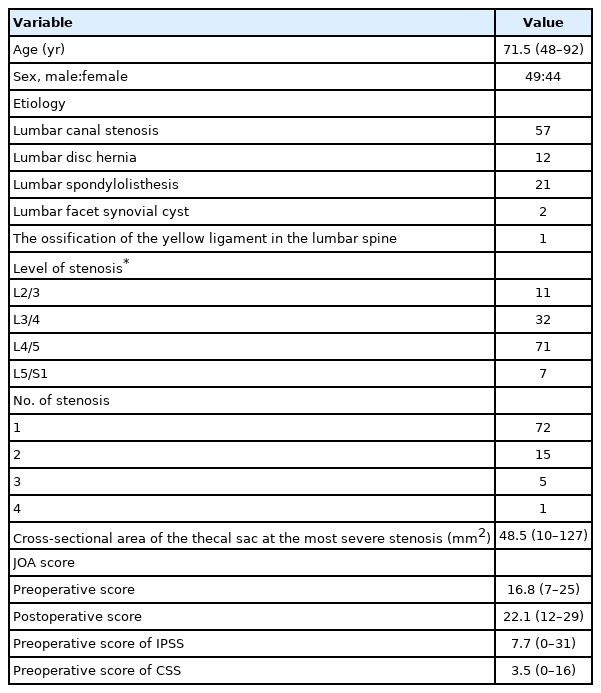
We prospectively analyzed data collected from February 2018 to March 2020 for 93 patients who underwent lumbar spinal decompression surgery with or without fusion for the treatment of lumbar degenerative disease. The patient inclusion criteria were as follows: (1) claudication resistant to repeated conservative treatment, (2) neurological deficit such as reduced tendon reflex in the bilateral lower extremities and pain/numbness in the bilateral lower extremities compatible with cauda equina syndrome, (3) central canal stenosis revealed via magnetic resonance imaging and defects and/or blocking of contrast media in the cauda equina revealed via myelography. The exclusion criteria were as follows: (1) lower urinary tract lesions such as those associated with prostatic hypertrophy, (2) coincident cervical or thoracic lesions, (3) history of spinal, urological, or gynecological surgery, (4) new medication regiment for treatment of digestive organs, bladder, or prostate problems, (5) medication regiment including opioids that could influence bowel function. All patients underwent urologic evaluation by a urologist to rule out lower urinary tract lesions. Detailed micturition and defecation histories were obtained from each patient using the CSS and IPSS on admission. All of these parameters were evaluated at 1, 3, 6, and 12 months postoperatively. Pre- and postoperative clinical symptoms were assessed using the CSS, IPSS, and the Japanese Orthopaedic Association (JOA) score. Additionally, we measured cross-sectional area of the thecal sac at the spinal segment exhibiting most severe stenosis using the Image J software program [7]. Regarding defecation and urinary symptoms, we prospectively examined data regarding patient age, sex, disease status, lesion level, and changes in each score, and investigated prognostic factors contributing to the improvement of the defecation and urinary symptoms comparing improved patients with unimproved patients. This study was approved by the Institutional Review Board of Fujieda Heisei Memorial Hospital (FHR 2020-1).
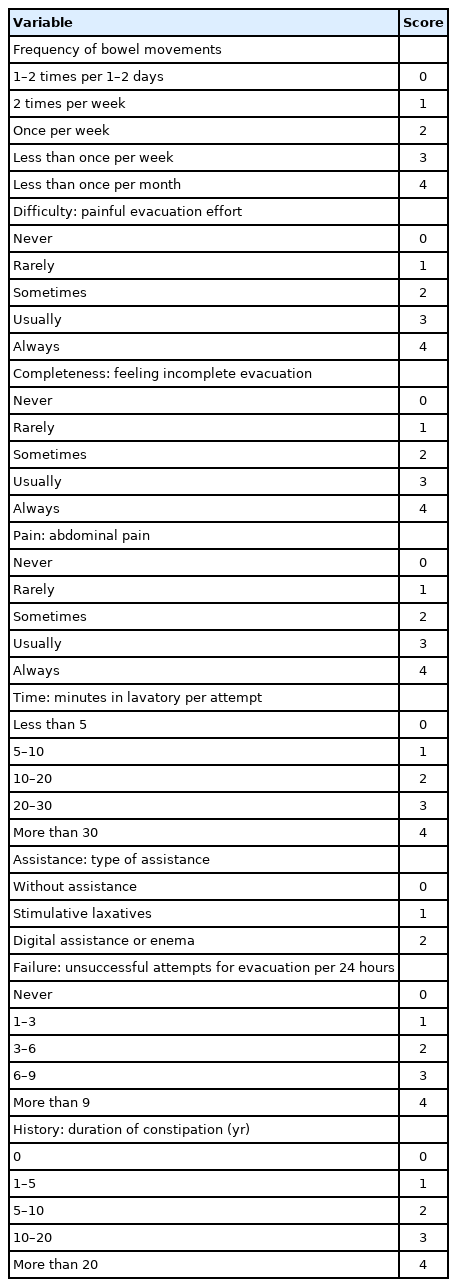
1. Constipation Scoring System
The CSS is based on 8 questions concerning evacuation [8]. For each question, the patient chooses 1 out of 8 options indicating the severity of the particular symptom. The answers are assigned points from 0 to 4. The total score can therefore range from 0 to 30. The questions refer to the following evacuation symptoms: (1) Frequency of bowel movements, (2) Difficulty: painful evacuation efforts, (3) Completeness: feeling of incomplete evacuation, (4) Pain: abdominal pain, (5) Time: minutes in lavatory per attempt, (6) Assistance: type of assistance, (7) Failure: unsuccessful attempts at evacuation per 24 hours, (8) History: duration of constipation (Table 1). In this study, a score of more than 4 was defined as symptomatic NBD. Patients who varied from more than 4 preoperatively to less than 3 postoperatively were defined as having experienced “significant improvement” in CSS.
2. International Prostate Symptoms Score
The IPSS is based on the answers to 7 questions concerning urinary symptoms and 1 question concerning quality of life [9]. For each question concerning urinary symptoms, the patient is asked to choose 1 out of 6 answers indicating the severity of that particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (asymptomatic to very symptomatic). The questions refer to the following urinary symptoms: (1) incomplete emptying, (2) frequency, (3) intermittency, (4) urgency, (5) weak stream, (6) straining, (7) nocturia (Table 2). In this study, a score of more than 8 was defined as indicating “symptomatic” NB. Patients who varied from more than 8 preoperatively to less than 7 postoperatively were defined as having experienced “significant improvement” in IPSS.
3. Statistical Analysis
Statistical analyses were performed using JMP statistical software ver. 13 (SAS Institute Inc., Cary, NC, USA). The chi-square test, Mann-Whitney U-test, and Fisher exact probability test were applied for all of statistical assessments. Logistic regression analysis was used for multivariate analysis. Significance of the obtained results was judged at the 5% level.
RESULTS
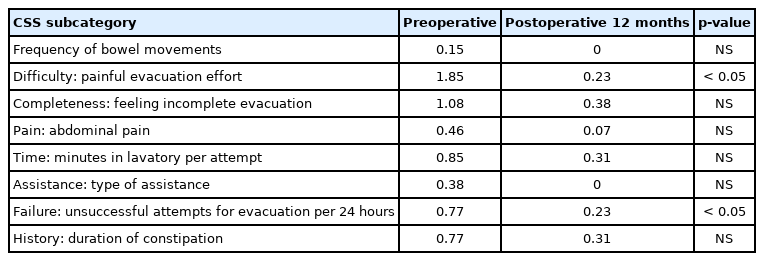
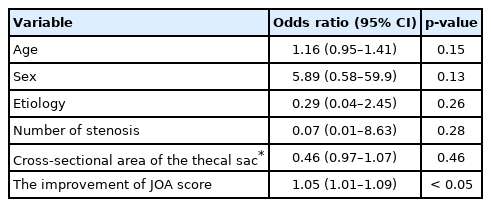
The patient group constituted 49 men and 44 women with an average age of 71.5 years (range, 48–92 years). The patient diagnoses were as follows: 57 patients had lumbar canal stenosis, 12 had a lumbar disc hernia, 21 had lumbar spondylolisthesis, 2 had a lumbar facet synovial cyst, and 1 had ossification of the yellow ligament in the lumbar spine. The level of stenosis was 11 at L2/3, 32 at L3/4, 71 at L4/5, and 7 at L5/S1 (The total does not add up because of multiple stenoses). The clinical findings are summarized in Table 3. Seventy-two patients received only decompression surgery and 21 patients underwent decompression with interbody fusion. Symptomatic defecation and urinary symptoms were present in 37 (38.1%) and 31 patients (33.3%), respectively. Among the symptomatic patients with defecation problems, 2 were excluded because they began taking new medication for constipation postoperatively. Of the 35 (20 males, 15 females) patients, 12 (5 males, 7 females) improved at 1 month, 13 (6 males, 7 females) improved at 3 months, 14 (7 males, 7 females) improved at 6 months, and 13 (6 males, 7 females) improved at 12 months postoperatively (Fig. 1). In the 13 patients who showed improvement at 12 months postoperatively, the index of “difficulty: painful evacuation efforts” and “failure: unsuccessful attempts at evacuation per 24 hours” ratios had especially improved (Table 4). Among these 13 patients with improved defecation symptoms one year after the operation, 6 patients had preoperative urinary symptoms. Of these 6 patients, 3 did not show improvements in urinary symptoms. Among the symptomatic patients with urinary symptoms, 11 were excluded from the study because they began taking new medication for urinary symptoms postoperatively. Of the remaining 20 (14 males, 6 females) patients, 5 (4 males, 1 female) improved at 1 month, 11 (7 males, 4 females) improved at 3 months, 6 (4 males, 2 females) improved at 6 months, and 10 (7 males, 3 females) improved at 12 months postoperatively (Fig. 2). Among the 10 patients whose urinary symptoms improved at one year postoperatively, 6 patients had defecation symptoms. Of this group, 2 patients did not show improvement of defecation symptoms. The improvement rate of CSS and IPSS at 1 year postoperatively were 30%, 50% in male and 46.7%, 50% in female, respectively. Comparing patients with improved versus unimproved in CSS, the degree of JOA score improvement was a significant prognosis factor (p<0.05; odds ratio, 1.05) (Table 5). Comparing patients with improved versus unimproved symptoms in IPSS revealed no significant difference in each factor (Table 6). Among 18 patients with both preoperative defecation and urinary symptoms, 5 patients (27.8%) showed postoperative improvements in both types of symptoms. However, in the other 13 patients, only either defecation or urinary symptoms improved.
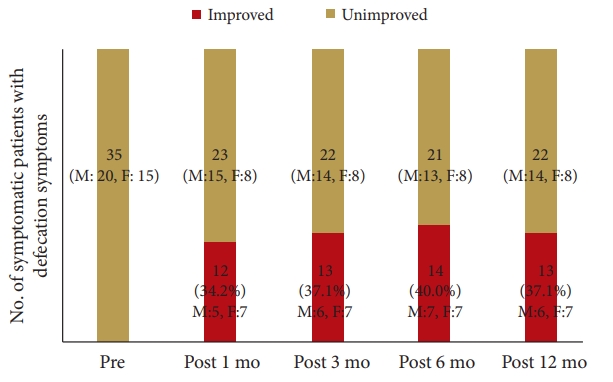
Graph shows postoperative improvement of Constipation Scoring System in patients with defecation symptoms on admission and at 1, 3, 6, and 12 months postoperatively.
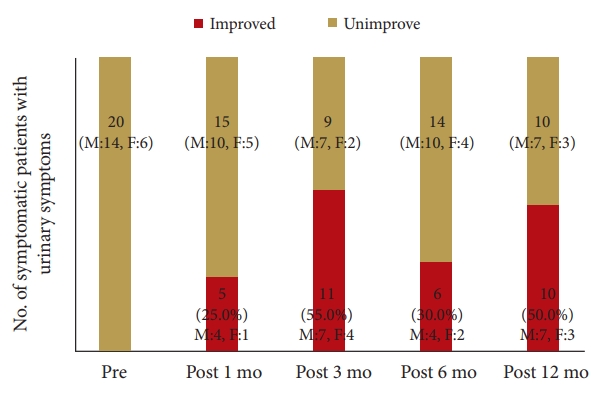
Graph shows postoperative improvement of International Prostate Symptoms Score in patients with urinary symptoms on admission and at 1, 3, 6, and 12 months postoperatively.
DISCUSSION
NBD following spinal cord injury is increasingly recognized as an area of major physical and psychological difficulty for spinal cord injury patients [10]. NB results from compression of the parasympathetic fibers of the S2–4 nerve roots innervating the bladder, resulting in areflexic or acontractile detrusor. Some researchers have reported that urinary symptoms due to lumbar degenerative diseases improved following decompression surgery [2-6]. Estimates of the prevalence of lower urinary tract symptoms associated with lumbar degenerative disease vary widely throughout the literature, ranging from 10% to 70%, due to differences in definitions and diagnostic modalities [2-6]. Further, no reports have examined both NB and NBD in patients with cauda equina syndrome.
In the present study, we meticulously examined defecation and urinary symptoms in patients with lumbar degenerative diseases using the CSS and IPSS, and conducted follow-up assessments until 1 year postoperatively. Our results indicate that about 30%–40% of patients with cauda equina syndrome had symptomatic defecation and urinary symptoms. Improvements in symptoms were found in 30%–50% of these patients following decompression surgery at just 1 month postoperatively, and these effects persisted for at least for 1 year.
Little is known about the pathophysiology of bowel dysfunction in patients with cauda equina syndrome. Stimulatory sympathetic innervation for the bowel orally to the splenic flexure is derived from the vagal nerves, while parasympathetic innervation of the distal colon and rectum is transmitted via the second to forth sacral roots (S2–4) [11]. Unlike in the urinary tract, the gut wall contains intrinsic pacemakers and a neural network that is part of the sympathetic and parasympathetic chain that programs the activity of the smooth muscles that are necessary to expel waste material [11]. Krogh et al. [12] reported that chronic conal or caudal equina lesions in patients with spinal cord injury significantly prolonged transit times, not only at the transverse and descending clonic segments, but also at the rectosigmoid region. In our study, BSS, which means the parameter of transit time, did not vary. However, the indices of “difficulty: painful evacuation effort” and “failure: unsuccessful attempts at evacuation per 24 hours” in the CSS had significantly improved. To the best of our knowledge, no studies have described the relationship between constipation and cauda equina syndrome. We speculated that a reduction in the frequency of bowel movements might be caused by compression of the cauda equina, resulting in constipation.
Previous reports on treatments for bladder dysfunction have demonstrated improvements in 56.5% to 75% of patients [3-6]. This variation in the degree of improvement may be influenced by differences not only in the modality used to treat urinary symptoms but also in the definition of impairment. Although the muscles responsible for bowel movements and bladder function are innervated by the S2–4 spinal nerves, discrepancies in postoperative improvement have been observed between defecation and urinary function, as shown in the present study. The mechanisms underlying this discrepancy have not been comprehensively investigated. The difference in the storage volume of stool and urine may be one important factor.
Male and female have comparable anorectal anatomy, yet they differ significantly in gross anatomy and physiology of the lower urinary tract [13]. In this study, the improvement rate of CSS and IPSS at 1 year postoperatively were 30%, 50% in male and 46.7%, 50% in female, respectively. There was no significant difference about the improvement of CSS and IPSS. We could not analyze prognostic factors because of small numbers in each group, which could be one of limitations.
The present study has several other limitations. First, the causes of constipation were varied and may be multifactorial to include mental status, meal contents, and activities of daily living. Particularly, the relationship between leg pain and constipation should be taken into consideration. It is well known that pain due to sympathetic hyperactivity can cause micturition or defecation difficulties [14]. Second, the cutoff value of the CSS for symptomatic patients was not absolute. Although we used a CSS score cutoff of “4,” the rate of symptomatic patients with defecation could have varied further. Despite these limitations, we believe that the current article provides important information regarding defecation and urinary symptoms in patients with cauda equina syndrome.
CONCLUSION
The prevalence of symptomatic defecation and urinary symptoms in patients with lumbar degenerative diseases were 38.1% and 33.3%, respectively. Decompression surgery could improve symptoms in 30%–50% of patients, and the positive effects emerged at one month after the operation and persisted for at least one year.
Notes
The authors have nothing to disclose.