Percutaneous Endoscopic Interbody Debridement and Fusion for Pyogenic Lumbar Spondylodiskitis: Surgical Technique and the Comparison With Percutaneous Endoscopic Drainage and Debridement
Article information
Abstract
Objective
Surgical treatment of severe infectious spondylodiskitis remains challenging. Although minimally invasive percutaneous endoscopic drainage and debridement (PEDD) may yield good results in complicated cases, outcomes of patients with extensive structural damage and mechanical instability may be unsatisfactory. To address severe infectious spondylodiskitis, we have developed a surgical technique called percutaneous endoscopic interbody debridement and fusion (PEIDF), which comprises endoscopic debridement, bonegraft interbody fusion, and percutaneous posterior instrumentation.
Methods
Outcomes of PEIDF in 12 patients and PEDD in 15 patients with infectious spondylodiskitis from April 2014 to July 2018 were reviewed retrospectively. Outcome were compared between 2 kinds of surgical procedures.
Results
Patients in PEIDF group had significantly lower rate of revision surgery (8.3% vs. 58.3%), better kyphosis angle (-5.73°±8.74 vs. 1.07°±2.70 in postoperative; 7.09°±7.23 vs. 0.79°±4.08 in kyphosis correction at 1 year), and higher fusion rate (83.3% vs. 46.7%) than those who received PEDD.
Conclusion
PEIDF is an effective approach for treating infectious spondylodiskitis, especially in patients with spinal instability and multiple medical comorbidities.
INTRODUCTION
Infectious spondylodiskitis presents as severe infection that may lead to disability and death [1], and is the third most common form of osteomyelitis in persons aged over 50 years [2]. Sometimes it presents as a postoperative complication, or as severe endplate destruction or massive paraspinal abscess in patients who have not undergone previous spine surgery [3-5]. Patients with identified pathogen treated with appropriate antibiotics are effective [1]. However, surgical treatment is indicated when patients are unresponsive to antibiotics, have progressive spinal deformity or instability, and when acute neurological impairment occurs [6].
Because the focus of infection is at the anterior column of vertebrae, direct and radical surgical debridement direct to anterior infectious foci is considered as the effective surgical treatment [3]. Percutaneous endoscopic drainage and debridement (PEDD) was the first endoscopic procedure developed to treat infectious spondylodiskitis and it reportedly provides effective infection control, except in the presence of extensive infection and structural instability [7-10]. However, anterior instrumentation may be tenuous because the concomitant osteoporosis associated with infection renders the vertebrae structurally weak and may prevent adequate fixation [11,12]. Stable posterior fixation is considered a major factor for successful infection control and satisfactory outcomes [13,14]. Particularly, patients with advanced infectious spondylodiskitis leading to mechanical instability should still receive anterior debridement and stable fixation with autograft interbody fusion surgery [9]. A combined anterior open surgery plus posterior instrumentation helps to overcome stability-related drawback of anterior approach alone [4,15]. By contrast, it entails 2 surgeries with additional morbidity [16,17].
To address the complex issues of patients with infectious spondylodiskitis and to ultimately provide more effective treatment, the surgical technique, percutaneous endoscopic interbody debridement and fusion (PEIDF), was developed. The procedure comprises endoscopic debridement, bone-graft interbody fusion and posterior instrumentation. We evaluated the efficacy of the PEIDF technique in treating patients with infectious spondylodiskitis and compared them with those receiving PEDD. The hypothesis of this work is the efficiency of PEIDF would be at least equal or even better than PEDD, especially in the reoperation rate and kyphosis correction.
MATERIALS AND METHODS
1. Patients
Patients with infectious lumbar spondylodiskitis who received either PEIDF or PEDD at our hospital from April 2014 to July 2018 were recruited. They were selected to undergo PEIDF or PEDD due to poor overall health status with severe comorbidities for whom open surgeries were considered to be relatively contraindicated by orthopedic surgeons and infectious disease specialists. Infectious spondylodiskitis was diagnosed based on clinical symptoms, elevated inflammatory markers, and imaging results. Patients enrolled in our study were all transferred from Department of Internal Medicine at our institute, a 3,700-bed tertiary referral center. In our clinical practice, empirical antibiotics were started since initial diagnosis of infectious spondylodiskitis. Most patients suffered from 2 to 4weeks of medical treatment course without clinical improvement, and all of them presented with intractable back pain and required narcotics for pain control preoperatively. PEIDF and PEDD were performed by 2 separate spine surgeons and the type of procedures was decided by the patient’s preference after thorough preoperative discussion and explanation. Surgical indications were infectious lumbar spondylodiskitis with intractable back pain, unknown pathogen, and poor response to medical treatment. Exclusion criteria were follow-up of less than one year, fungal or mycobacterial infection, neurologic deficits, multilevel spine infection, and complications from previous spine surgeries. In addition, patients with critically unstable status compromising the basic cardiac and pulmonary function were excluded, including recent ischemic heart disease with percutaneous coronary intervention, severe cardiac valvular disease, congestive heart failure, pulmonary hypertension, acute exacerbation of chronic obstructive pulmonary disease, and recent stroke. This study was approved by the Institutional Review Board (IRB) of Chang Gung Medical Foundation (IRB No. 201801219B0) and waived the requirement for informed consent due to the retrospective nature of the study.
Intravenous antibiotics were administered immediately after surgeries based on the culture reports and suggestions from the infectious disease specialists for 3–4 weeks according to the improvement of inflammatory markers, and then followed by oral antibiotics until at least 3 months of total antibiotic therapy (intravenous and oral antibiotics). All patients were encouraged to wear a brace while ambulating for at least 3 months, and they were under outpatient followed-up postoperatively at 3, 6 months, and then annually.
2. Surgical Procedure of PEIDF
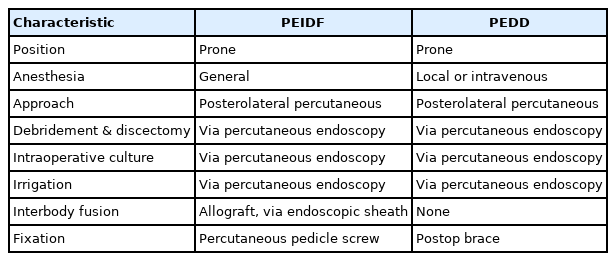
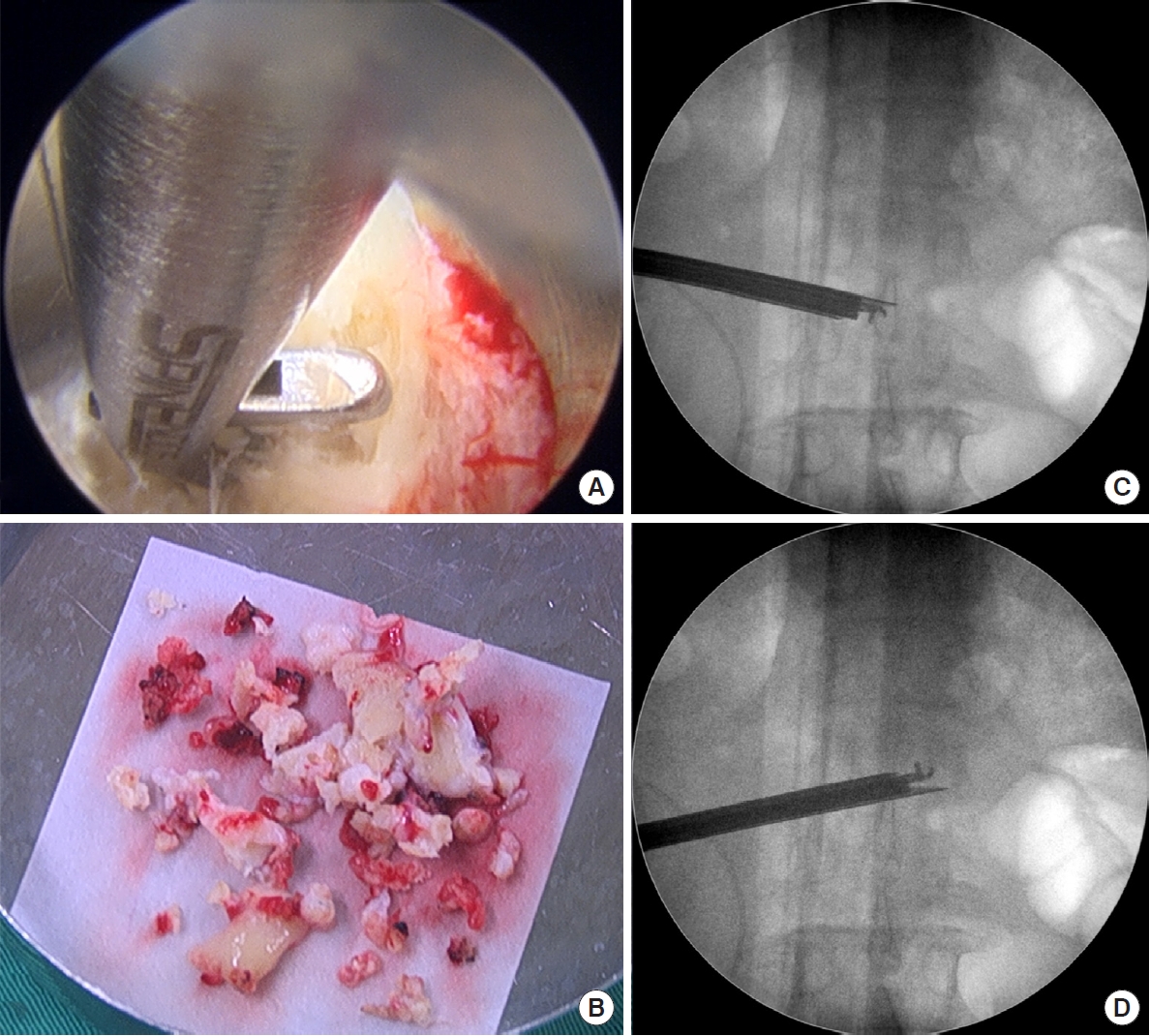
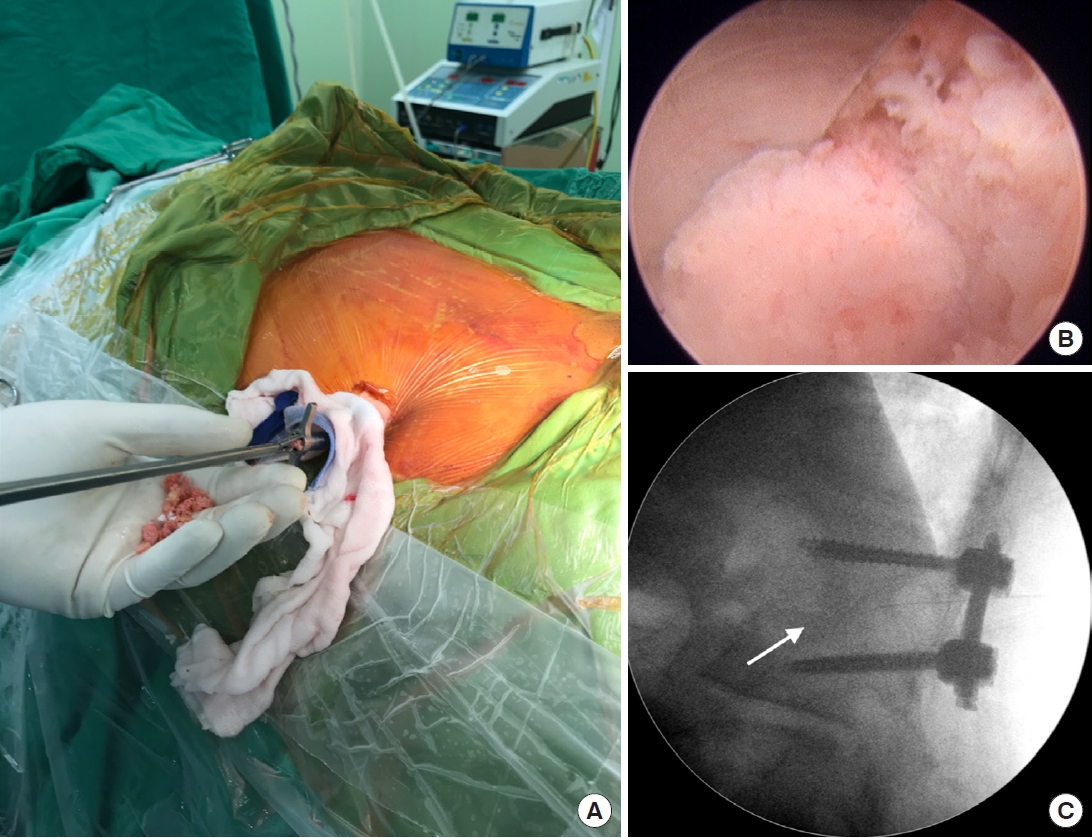
Patients undergoing PEIDF were positioned prone with general anesthesia on a radiolucent table and intraoperative neuromonitor was used to prevent nerve roots injury. After sterile preparation, the spinal needle was inserted directly into the target infected disc from the posterolateral side of the waist under the guidance of fluoroscopy. A guidewire was introduced through the spinal needle, and the needle was subsequently withdrawn. Dilators followed to create a larger space for the insertion of a working sleeve. Once the working portal was established and confirmed by fluoroscopy, necrotic tissue from the infected disc space was obtained for culture prior to any normal saline irrigation. Debridement of the infected disc was performed using grasping forceps, Kerrison punches, and endoscopic scissors. Multidirectional debridement was performed by adjusting the direction and depth of the working sleeve (Fig. 1). Disc space reconstruction and intervertebral fusion were performed after radical debridement of infected disc foci and more than 1,000-mL normal saline irrigation. We filled up the hollow intervertebral space defect with morselized femoral-head-bone allograft. The allograft bone chips were placed into the disc space through the working sleeve, which was then compressed with an endoscopic impactor to increase the density of the allograft (Fig. 2). Posterior percutaneous screws insertion at 1 level above and below was performed under the guidance of fluoroscopy to avoid back muscle dissection and soft tissue injury (Fig. 3). Posterior instrumentation provides posterior support, and anterior bone grafting provides anterior column stability and interbody fusion. The combination provides a 3-dimensional stable construct for infection control and bone fusion. The comparison between PEIDF and PEDD procedures were shown in Table 1.
(A) Discectomy and debridement of the infected disc were performed using grasping forceps, Kerrison punches, and endoscopic scissors under fluoroscopic guidance. (B) Necrotic tissues from the infected disc space were sent for histopathological and microbiological studies. (C, D) Multidirectional debridement was performed by adjusting the direction and the depth of the working portal.
(A, B) Morselized bone graft was put into the disc space defect through the working portal, and was then compressed with an endoscopic impactor to make it denser. (C) Fluoroscopy image of impacted morselized bone graft in the disc space (white arrow).
(A) Preoperative lumbar spine lateral plain film revealed L1–2 discs destruction and endplate erosion with local kyphosis (white lines). (B) Posterior percutaneous pedicle screw insertion at 1 level above and below the index level. Percutaneous insertion avoided back muscle dissection and soft tissue injury, and only required 4 small skin incisions. (C, D) Postoperative lumbar plain films showed that kyphosis correction was maintained (black lines) by anterior defect reconstruction and posterior percutaneous instrumentation.
3. Surgical Procedure of PEDD
Like PEIDF, patients receiving PEDD were under a prone position via a posterolateral percutaneous approach. PEDD was performed under local or intravenous general anesthesia. After sterile preparation, the spinal needle was inserted directly into the target infected disc under the guidance of fluoroscopy. A guidewire was introduced through the spinal needle, and the needle was subsequently withdrawn. Dilators followed to create a larger space for the insertion of a working sleeve. Once the working portal was established and confirmed by fluoroscopy, necrotic tissue from the infected disc space was obtained for culture prior to any interference, including normal saline irrigation. Debridement of the infected disc was performed using grasping forceps, Kerrison punches, and endoscopic scissors. Multidirectional debridement was performed by adjusting the direction and depth of the working sleeve into the intervertebral space. The surgical endpoint was defined when all necrotic tissues were removed with healthy cancellous bone bleeding seen. After radical debridement, more than 1,000-mL normal saline irrigation was performed to decrease the bacteria loading and wash out necrotic debris as much as possible. Finally, a drainage catheter connecting with a negative-pressure Hemovac was inserted into the infectious intervertebral space through the working sleeve.
4. Clinical Evaluation
Data collected and analyzed from all patients included demographic data, clinical images, laboratory data, and culture reports. Preoperative magnetic resonance imaging was assessed for diagnosis, and plain radiographs of the lumbar spine before surgery, after surgery, and at follow-up were assessed for kyphosis correction. The segmental kyphosis angle of the lesion sites was measured using the superior endplate of the infected vertebral body above and inferior endplate of the infected vertebra below as reference points. Computed tomography (CT) was obtained one year after surgery to examine fusion status. Each intraoperative specimen was submitted immediately and was examined for microorganisms. Bone-graft fusion status was classified by Eck fusion criteria [18]. The functional outcomes between 2 groups were investigated with visual analogue scale (VAS) to assess lower back pain and Oswestry Disability Index (ODI) to evaluate the level of disability. The VAS evaluations were done at preoperation, postoperation (1 week after surgery), and postoperative 3-month follow-up. And the ODI was assessed at preoperation and postoperative 3-month follow-up. We excluded the patients who died or received reoperations to truly evaluate the functional outcomes between the 2 procedures.
5. Statistical Analysis
All statistical calculations were performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). Quantitative variables are expressed as mean±standard deviation. Changes in variables at different time points were examined using the t-test, Mann-Whitney U-test for continuous variables, and Fisher exact test for categorical variables. The threshold for statistical significance was set at p<0.05.
RESULTS
Twelve patients underwent PEIDF and 15 received PEDD during the period from April 2014 to July 2018. The mean age of patients in PEIDF group was 68.17±14.56 with 7 males and 5 females; PEDD group had the mean age, 63.07±14.95, comprised of 12 males and 3 females. The most common infectious levels were L3–4 and L4–5 in all patients. Demographic data of the 2 groups were summarized in Table 2 and there was no statistical significance in age, sex, infectious level, and comorbidity between the 2 groups.
Two patients in PEIDF group did not have satisfactory outcome. One patient experienced a recurrent spine infection with septic nonunion and adjacent level spondylodiskitis. The patient was seen in the outpatient clinic at 2 months after discharge and readmitted due to elevated C-reactive protein (CRP) level and obvious posterior pedicle screws loosening. Six weeks of intravenous antibiotics did not control the recurrent spine infection, and she received the anterior open surgery. The other patient had the preoperative traumatic subdural hemorrhage. This patient had an excellent CRP level decrease at 1 and 3 weeks after surgery, and intraoperative tissue culture showed Staphylococcus aureus infection. However, this patient experienced a sudden onset subarachnoid hemorrhage in the intensive care unit at 1 month postoperatively. Compared with PEIDF group, there were 2 patients in PEDD group underwent revision surgeries for further debridement and posterior instrumentation within postoperative 3 months because of unrelieved back pain and infection control. Five patients in PEDD group suffered from residual back pain and soreness and then received fusion surgeries within postoperative 1 year. One PEDD patient’s clinical outcome was complicated by septic shock and he expired during hemodialysis after 2 weeks of PEDD.
1. Clinical and Functional Results
Both groups reported immediate back pain relief postoperatively, and CRP improvement was obvious at postoperative 1 and 3 weeks. Although the infection control was similarly effective between PEIDF and PEDD group, there were 7 patients in PEDD group undergoing revised open fusion surgeries due to persistent back pain and residual infection within postoperative 1 year, significantly more than that in PEIDF group (p=0.030) (Table 2). The surgical time of PEIDF group was significantly longer than that of PEDD group (p=0.047), but the surgical blood loss was both minimal, less than 50 mL because all procedures in PEIDF and PEDD were minimally invasive. The functional outcomes were assessed by VAS (preoperative, postoperative 1 week and 3 months) and ODI (preoperative and postoperative 3 months). We excluded 2 patients from PEIDF group and 8 from PEDD group because of either death or reoperation. Between PEIDF and PEDD patients without death and reoperation, 2 procedures had the same postoperative back pain relief and functional recovery (Table 2). There was no surgery-related major complication. However, 2 patients in PEIDF group (2 of 12, 16.7%) and 1 in PEDD group (1 of 15, 6.7%) complained about transient leg paresthesia. The symptoms were mild and disappeared within 1 month.
2. Pathogens and Radiographic Outcomes
Causative bacteria were successfully identified in 10 patients of PEIDF group (the culture-positive rate, 83.3%) and 12 of PEDD group (80%) (Table 3). There was no significant difference about the culture-positive rate between the 2 groups (p=0.825). Among those 22 patients, S. aureus was the predominant pathogen and 11 patients (6 methicillin-resistant and 5 methicillin-sensitive) were infected with it. The other causative microorganisms included Escherichia coli (3), Streptococcus spp. (4), Klebsiella pneumoniae (2), Enterococcus spp. (1), and Pseudomonas aeruginosa (2).
In follow-up radiographs, bone-graft interbody fusion of the infected segments was successful in most patients of PEIDF group, with the fusion rate was 83.3%. CT at postoperative one year showed, 7 had partial fusion (Fig. 4) and 3 solid fusion (Fig. 5). In PEIDF group, only 2 patients had no radiographic evidence of partial or solid fusion. One underwent anterior debridement and reconstruction with a struct allograft due to pseudoarthrosis, and the other died during hospitalization at 1 month postoperatively. Intraoperative interbody fusion with disc height restoration by allograft and posterior instrumentation of PEIDF supported the patients to get better postoperative segmental kyphosis angle (-5.73±8.74 in PEIDF vs. 1.07±2.70 in PEDD, p=0.004) (Table 3) and kyphosis angle correction at postoperative 1 year (7.09±7.23 in PEIDF vs. 0.79±4.08 in PEDD, p=0.049).
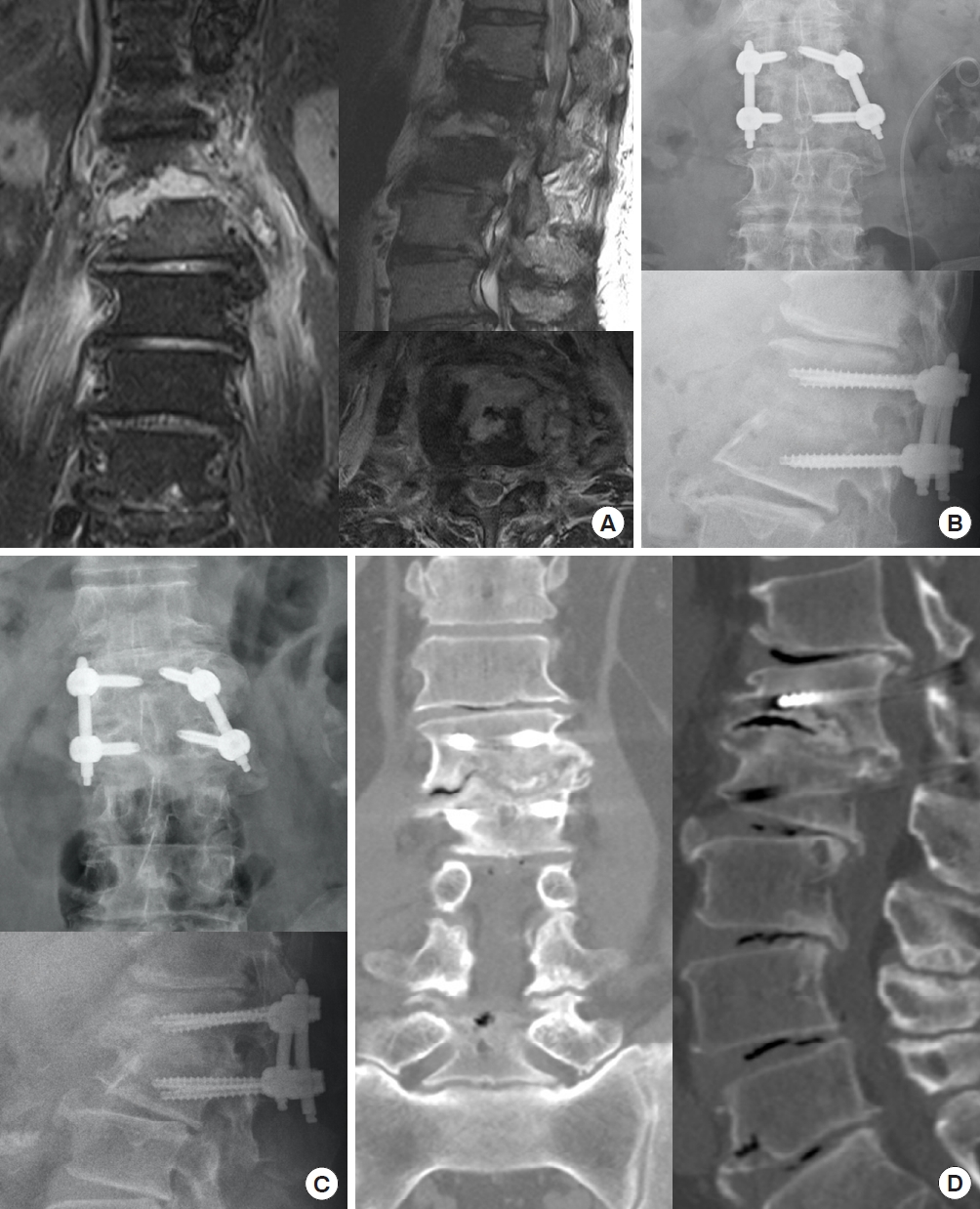
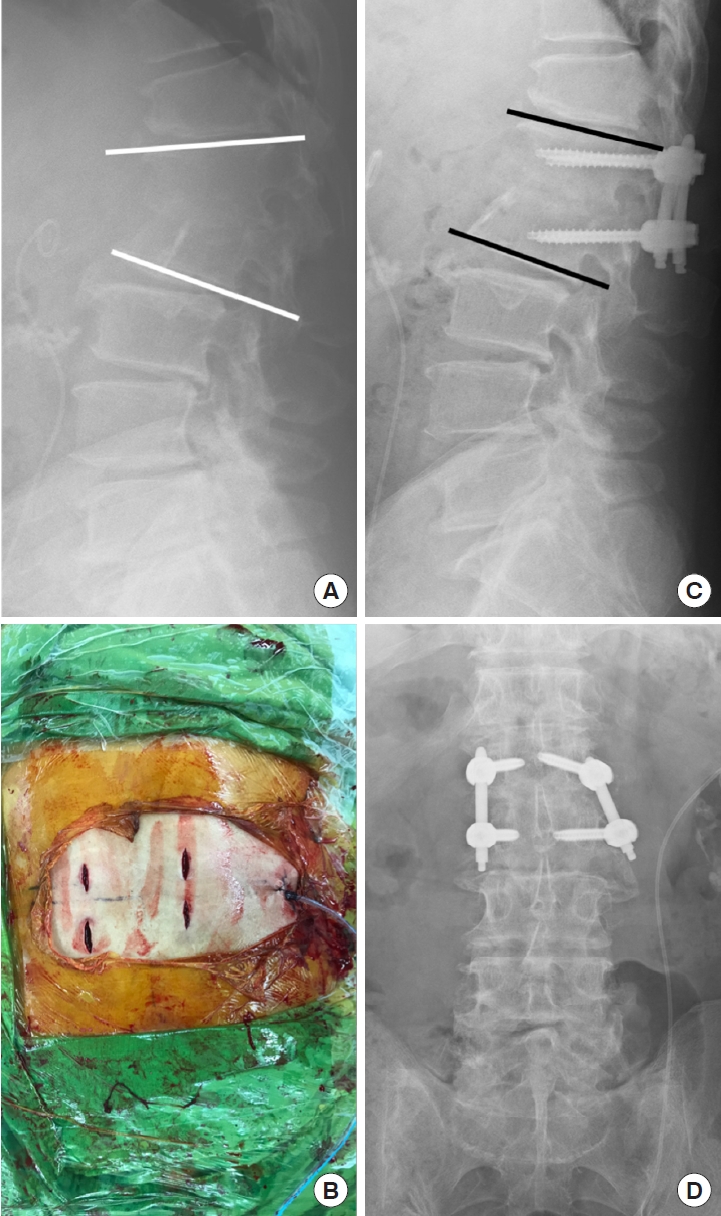
A 55-year-old man with L1–2 infectious spondylodiskitis (Escherichia coli) underwent percutaneous endoscopic interbody debridement and fusion treatment. (A) Preoperative magnetic resonance imaging revealed L1–2 infectious spondylodiskitis with severe disc destruction and large bony defect. (B) Postoperative radiography showed kyphosis correction fixed with posterior instrumentation and bony defect reconstruction filled up with cancellous allograft bone chips. (C) Bony bridges were found at plain films at postoperative 1 year and (D) only mild defect was noted without fusion in computed tomography scan.
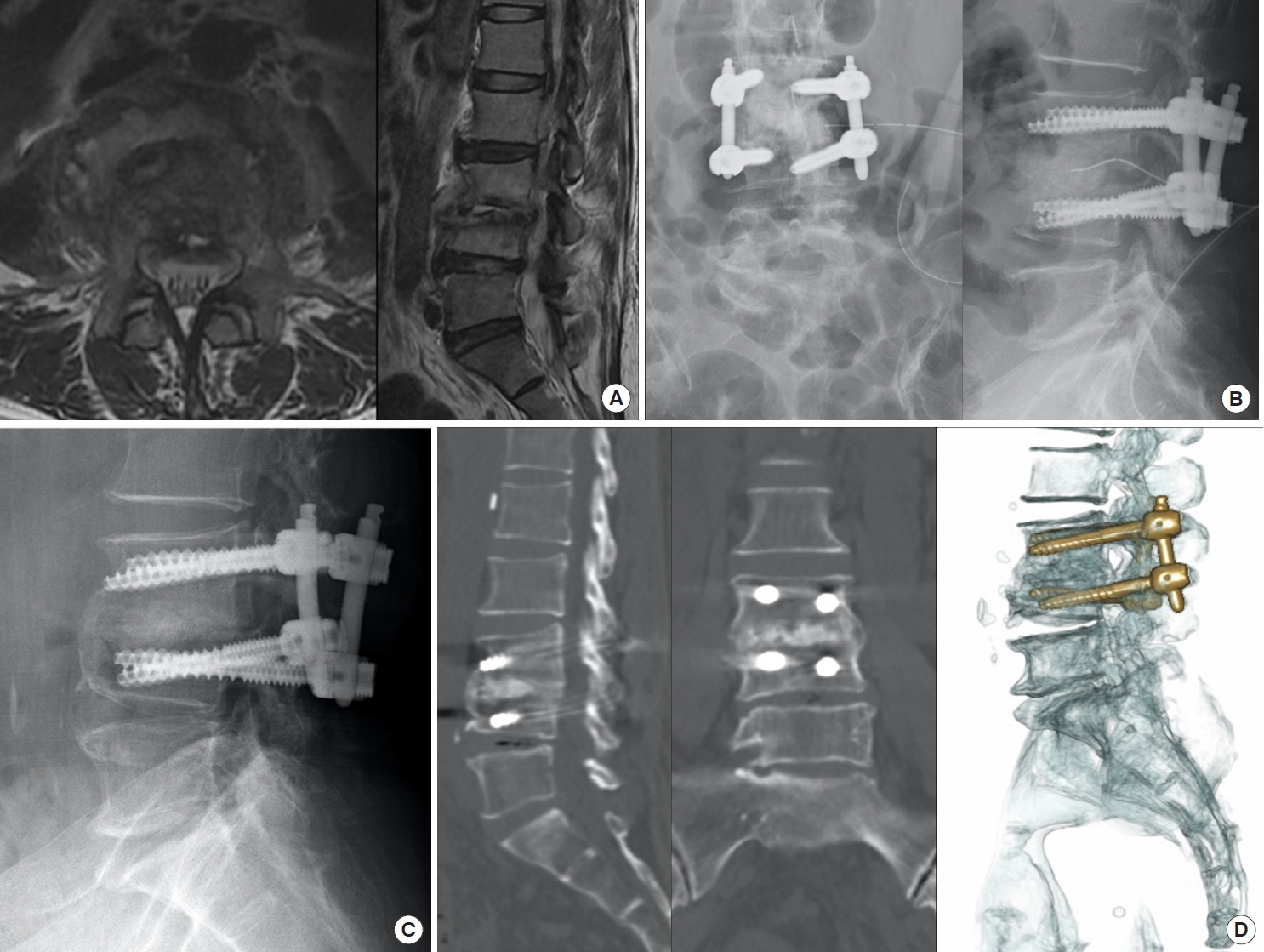
A 58-year-old woman had liver cirrhosis and renal function impairment. (A) She suffered from L3–4 Staphylococcus caprae spondylodiskitis with severe back pain and massive paraspinal abscess. (B) After percutaneous endoscopic interbody debridement and fusion treatment, she got back pain relief and postoperative radiography showed disc defect filled up with allograft bone chips for interbody fusion. (C) Solid bony bridge and interbody fusion were obvious in radiography after postoperative 1 year, (D) especially in 3-dimensional computed tomography scans.
DISCUSSION
Our study is the first one to investigate the efficiency of 2 kinds of endoscopic procedures to treat infectious spondylodiskitis. PEIDF was developed under the basis of PEDD with bonegraft interbody fusion and percutaneous posterior instrumentation to enhance infection control, kyphosis correction, and spinal stability. According to our preliminary results, PEIDF had many similar advantages as PEDD in minimally invasion, well infection control, high pathogen identification rate, and back pain relief. Furthermore, PEIDF performed better kyphosis correction, less reoperation rate, and higher fusion rate than PEDD. Mechanical instability and kyphosis caused by vertebrae destruction of infectious spondylodiskitis would be solved by intervertebral bone grafting and percutaneous posterior instrumentation in PEIDF. In our point of view, PEIDF could be widely applicated in treating infectious spondylodiskitis of patients without neurologic deficits.
For infectious spondylodiskitis, conservative treatment with pathogen-sensitive antibiotics is adequate for most patients. However, there is a high rate of treatment failure for nonsurgical methods if the pathogen is not detected at an early stage. Rezai et al. [19] reported that 25% of patients initially treated medically would eventually need surgical treatment. Common surgical indications for infectious spondylodiskitis include failed antibiotic therapy, intractable back pain, neurological deficits, epidural abscesses, extensive spine instability, and kyphotic deformity. In cases with severe spine instability or deformity, anterior debridement and reconstruction with or without posterior instrumentation is considered the mainstream surgical treatment, and results in satisfactory outcomes. Nonetheless, open anterior debridement surgery is associated with a high complication and mortality rate [20]. Many patients with infectious spondylodiskitis are immunocompromised patients with multiple comorbidities, which make them poor candidates for open anterior surgeries. Prior studies have shown that the 1-stage posterior transforaminal approach with posterior instrumentation for infectious thoracic and lumbar spondylodiskitis can reduce the rate of complications in open anterior surgeries and is associated with decreased blood loss, a good fusion rate, a satisfactory culture rate, and early postoperative ambulation with better functional outcomes [13,21]. Nevertheless, there are still concerns with the 1-stage posterior procedure such as infectious contamination from anterior infectious foci to posterior virgin site, less visibility of the infected disc, posterior ligamentous complex injury, and the risks associated with manipulation of the spinal cord.
Some minimally invasive methods for treating spine infections have been described by other authors. CT-guided drainage had long been an alternative treatment for patients whose pathogen was not identified or who failed medical treatment [22-24]. As an extension of percutaneous abscess drainage, first PEDD was described by Yang et al. [25] in 2007. The procedure yielded a high rate of pathogen identification and infection control using only 1 or 2 small incisions. Subsequently, many similar methods were then described including different endoscope approaches, bilateral portal use, and irrigation with diluted beta iodine solution [9,26,27]. The indications of PEDD were also extended to treat some cases of complicated infectious spondylodiskitis. According to Yang et al. [9] patients with large paraspinal abscesses or postoperative recurrent infection could be treated by PEDD. However, in the same study, they reported failure of PEDD in patients with severe endplate destruction and spinal instability. The authors suggested that debridement alone could not address mechanical problems, and mechanical instability of spine was one of the main reasons for revision surgeries. In our study, we did not exclude the patients with spinal instability, severe local kyphosis, and extensive bony defects preoperatively. Although, most of patients in PEDD group got immediate relief of back pain and infection control after the surgeries, 7 of them suffered from residual back pain during ambulation within postoperative 3 months and one year. Unrelieved motion pain with mechanical instability was the major factor which contributed to more subsequent revision fusion surgeries in PEDD group than that in PEIDF group (58.3% vs. 8.3%, p=0.030). Firm fixation could also rapidly reduce back pain and consolidate infection control, even in the absence of debridement of infected tissue [14]. Yang et al. [25] also demonstrated that surgical stabilization for infectious spondylodiskitis was associated with faster recovery, lower pain scores, and improved quality of life.
Surgical treatment for infectious spondylodiskitis remains challenging, especially for patients with comorbidities such as diabetes mellitus, immunosuppression, heart failure, liver cirrhosis, and renal failure [28]. In this study, we introduced a newly developed surgical technique, PEIDF that simultaneously comprises anterior endoscopic debridement, reconstruction, bone-grafting interbody fusion, and posterior instrumentation. The goals of surgical treatment of spinal infections are to debride the infected tissue, identify the pathogen, and restabilize the deformed spine. There were 2 major parts of our PEIDF procedure. One was anterior procedure, infectious foci debridement and reconstruction, and the other was posterior procedure, stabilization via posterior instrumentation. Both anterior and posterior procedures could be done under prone position. In the anterior procedure of our PEIDF, infectious foci debridement of the intervertebral space, which was traditionally conducted via anterior open surgery, were performed through the endoscopic working sleeve via a 1-cm incision at the posterolateral side of the waist, as in Fig. 2. Endoscopy and fluoroscopy provided a clear and detailed view of the surgical fields, and they allowed us to do radical sequestrectomy and debridement of all necrotic disc tissue with minimal soft tissue damage and blood loss, as in Fig. 1. There was no concern for posterior contamination due to the intact fascia and posterior longitudinal ligament, which acted as a natural barrier to prevent infection spreading. Furthermore, intervertebral reconstruction and fusion via impacted cancellous allograft bone chips through the endoscopic working sleeve would decrease the postoperative instability and back pain. Posterior instrumentation with percutaneous pedicle screw insertion comprised the posterior procedure of PEIDF, along with the benefits of preserving back erector muscles, facet joints, and bony structures. From reviewing the literature, posterior transforaminal debridement and fusion via impacted cancellous bone chips with pedicle screw fixation was proven effective and offered good clinical outcome for the treatment of infectious spondylodiskitis in lumbar and thoracic spine [13,21,29,30]. Under this basis, PEIDF provides the same qualification for spinal fusion and stability, alongside the preservation of back erector muscles, facet joints, and posterior bony structures. In summary, our PEIDF procedure would be composed of anterior radical debridement of infectious foci and restabilization via anterior bone-grafting reconstruction and posterior percutaneous instrumentation, with only five 1-cm skin incisions.
Percutaneous endoscopic lumbar discectomy and interbody fusion had been described in recent years, with good outcomes in patients without infections [31-37]. In our review of the literature, we found no reports about the application of endoscopic technique for infectious spine debridement, reconstruction, and fusion. In our preliminary results of PEIDF group, the infection control and fusion rates were satisfactory. Only 1 patient required revision surgery due to recurrent infection and implant loosening 2 months after discharge. However, this patient had end-stage renal failure and right septic knee. Three patients achieved solid fusion, and 7 partial union at 1 year after surgery, with an overall fusion rate of 83.3%. Spine sagittal alignment was also improved after surgery, and postoperative kyphosis angle and correction at 1 year were significantly better in PEIDF group. Late kyphosis is often observed to some degree after spine infection, especially in patients treated conservatively. Chronic back pain, muscle fatigue, and disability are related to spine malalignment, including pathologic kyphosis [38]. Loss of lordosis causes a predisposition to adjacent disc degeneration and physiological discomfort. Rajnics et al. [39] showed that people with predisposing disc degeneration and low back pain had a relatively kyphotic spine in the sagittal plane.
Antibiotic treatment guided by culture results remains the mainstay treatment of pyogenic spondylodiskitis [40]. Microbiological diagnosis is essential to enable targeted antibiotic treatment, and could be obtained through blood samples or deep necrotic tissue from CT-guided biopsy or surgery. In all patients of this study, abscess and necrotic tissue from the infected disc were sent for histopathological and microbiological studies. We performed this procedure as the first step, prior to any normal saline irrigation during both PEIDF and PEDD. Then, positive pathogen identification was 10 in PEIDF group (83.3%) and 12 in PEDD group (80%) and the main pathogen identified in our study was S. aureus (11 of 22, 50%), which were similar with other studies [41].
The innovative surgical procedure, PEIDF is composed of the advantage of minimally invasive approach for infectious foci debridement in PEDD, and the spinal restabilization through bone-grafting reconstruction and percutaneous posterior instrumentation. This procedure supports minimally invasive surgical treatment for infected patients with multiple comorbidities, machinal back pain, spinal instability, large bone defect, and kyphosis. There are some limitations of this study. The study was performed at one single medical center, and the number of patients was small. The retrospective nature of the study makes control of bias and confounders difficult. Some selection bias would result from our narrow inclusion criteria and exclusion criteria. Lastly, the follow-up period was short; longer follow-up time is necessary to determine the long-term outcomes of the PEIDF procedure.
CONCLUSION
PEIDF is an effective procedure for the treatment of pyogenic lumbar spondylodiskitis, especially in patients with an unstable spine and medical comorbidities. It is minimally invasive, allows rapid ambulation, and is associated with good clinical and functional outcomes. Based on the results of this study, future studies with larger numbers of patients and longer follow-up to further evaluate the procedure are warranted.
Notes
The authors have nothing to disclose.
Acknowledgements
This work is supported by Chang Gung Memorial Hospital (CMRPG3K0631).