Cost-Utility Analysis Compared Between Radiotherapy Alone and Combined Surgery and Radiotherapy for Symptomatic Spinal Metastases in Thailand
Article information
Abstract
Objective
To investigate the patient quality of life and cost-utility compared between radiotherapy alone and combined surgery and radiotherapy for spinal metastasis (SM) in Thailand.
Methods
Patients with SM with an indication for surgery during 2018–2020 were prospectively recruited. Patients were assigned to either the combination surgery and radiotherapy group or the radiotherapy alone group. Quality of life was assessed by EuroQol-5D-5L (EQ-5D-5L) questionnaire, and relevant healthcare costs were collected pretreatment, and at 3-month and 6-month posttreatment. Total lifetime cost and quality-adjusted life-years (QALYs) were estimated for each group.
Results
Twenty-four SM patients (18 females, 6 males) were included. Of those, 12 patients underwent combination treatment, and 12 underwent radiotherapy alone. At 6-month posttreatment, 10 patients in the surgery group, and 11 patients in the nonsurgery group remained alive for a survival rate of 83.3% and 91.7%, retrospectively. At 6-month posttreatment, the mean utility in the combination treatment group was significantly better than in the radiotherapy alone group (0.804 ± 0.264 vs. 0.518 ± 0.282, respectively; p = 0.011). Total lifetime costs were 59,863.14 United States dollar (USD) in the combination treatment group and 24,526.97 USD in the radiation-only group. The incremental cost-effectiveness ratio using 6-month follow-up data was 57,074.01 USD per QALY gained.
Conclusion
Surgical treatment combined with radiotherapy to treat SM significantly improved patient quality of life compared to radiotherapy alone during the 6-month posttreatment period. However, combination treatment was found not to be cost-effective compared to radiotherapy alone for SM at the Thailand willingness-to-pay threshold of 5,113 USD/QALY.
INTRODUCTION
Spinal metastasis results from cancer cells that spread from the primary tumor, which is located somewhere else in the body. Spinal metastasis can cause severe pain, impaired ambulation, and neurological deficit–all of which significantly adversely affect patient quality of life. Several studies reported favorable results of palliative surgery combined with radiotherapy for improving pain, ambulation, and quality of life compared to radiotherapy alone [1-5]. As such, palliative surgery plays an important role in the multidisciplinary management of spinal metastasis.
However, the additional cost of surgery combined with radiotherapy makes combination therapy for spinal metastasis much more expensive than radiotherapy alone. From a study conducted in Denmark, Tipsmark et al. [6] reported the cost of radiotherapy alone to be 36,616 euro (EUR), whereas the cost of surgery with decompression, instrumentation, and reconstruction was 87,814 EUR.
Cost-utility analysis (CUA), which evaluates both clinical and economic outcomes, provides important evidence-based information that helps clinicians and policymakers in decision-making relative treatment strategy. CUA studies that compared surgical treatment and radiotherapy alone for the treatment of spinal metastasis have been reported in Japan, the United Kingdom, Belgium, Canada, and the United States. The results of those studies showed combination surgery and radiotherapy to be cost-effective compared to radiotherapy alone for treating spinal metastasis in developed countries [2,7-10].
Studies in the cost-effectiveness of surgery for spinal metastasis patients in developing countries, such as Thailand, are limited. Accordingly, the aim of this study was to investigate the patient quality of life and cost-utility compared between radiotherapy alone and combined surgery and radiotherapy for spinal metastasis in Thailand.
MATERIALS AND METHODS
This prospective cohort study was conducted at the Faculty of Medicine Siriraj Hospital, Mahidol University–Thailand’s largest medical school and national tertiary referral center. The protocol for this study received approval from the Siriraj Institutional Review Board (protocol number: 395/2561[EC3]), and written informed consent was obtained from each enrolled study patient.
1. Subjects
Patients aged 18 years or older with spinal metastasis with an indication for surgery during 2018–2020 were prospectively recruited. Diagnosis of spinal metastasis was made by radiological or pathological methods. Indications for surgery included intractable pain, spinal instability, and neurological symptom. Patients having one or more of the following were excluded: (1) curative surgery, (2) posterior instrumentation more than 10 levels, (3) previous history of radiotherapy at the affected spine level, and/or (4) impaired consciousness that prevented completion of the study questionnaire.
2. Study Procedures
Eligible patients that accepted our invitation to join the study were educated about the study objective and protocol. The spinal instability neoplastic score was used to assess the severity of spinal instability. All patients underwent intensive adjuvant treatments, radiotherapy, rehabilitation, and palliative care. All patients were offered the opportunity to undergo surgical treatment, and the patient made the final decision. The patients who decided to undergo surgery were allocated to the combination surgery and radiotherapy group, and those not willing to undergo surgery were allocated to the radiotherapy alone group. In both groups, chemotherapy was performed if indicated. The modified Tokuhashi and Tomita scores were used to evaluate the prognosis of spinal metastasis. Each patient’s general condition was assessed using Frankel classification grading.
3. Surgical Procedures
The patients who undergo surgery were a posterior approach. Debulking tumor from the posterolateral aspect after laminectomy was performed, and the posterior stabilization was achieved using a pedicle screw-rod system. The range of stabilization was decided based on bone quality, the number of affected vertebrae, and deformity. After surgery, postoperative rehabilitation was adjusted case by case depending on patients’ status with immobilization support devices.
4. Sample Size Calculation
The sample size was based on a mean of health state values between the radiotherapy alone and the combined surgery and radiotherapy at 12 months (0.019 ± 0.027, 0.448 ± 0.451, respectively) from the study of Miyazaki et al. [2]. Two-sided, 2-independent means sample size calculation was used at the 0.05 significance level for the difference. The power of the test was 0.2. Thus, the number of each group was 12 patients.
5. Statistical Analysis
Demographic and clinical characteristics of study participants were analyzed descriptively. Categorical data were reported as frequency and percentage, and normally distributed continuous data were reported as mean ± standard deviation. Fisher exact test and Student t-test were used to comparing categorical data and normally distributed continuous data, respectively. The Kaplan-Meier method was used to estimate the survival function from lifetime data. All statistical analyses were performed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA), and a 2-tailed p-value that less than 0.05 was considered statistically significant.
6. Economic Evaluation
A CUA was performed to compare the cost and health status between the combined use of surgery and radiotherapy and radiotherapy alone in patients with spinal metastasis. The intervention of interest was palliative surgery that provides better quality of life, but the cost of treatment is higher. We performed the analysis using a societal perspective and lifetime time horizon as recommended by the Thailand health technology assessment (HTA) guideline [11]. Our findings are presented as an incremental cost-effectiveness ratio (ICER) in United States dollar (USD) per quality-adjusted life years (QALYs) gained. The interpretation of the cost-effectiveness of the surgery was based on an official willingness-to-pay (WTP) threshold of 160,000 Thai Baht (THB)/QALY (5,113 USD/QALY), as reported by the Thai Health Economic Working Group [12]. An annual discount rate of 3% was used for both costs and health outcomes.
7. Economic Model
According to expert opinion and our review of the literature, the health status outcome should include both ambulatory status and pain improvement after the treatment. A decision tree was constructed to divide patients into 2 groups–those who received surgical treatment and those who did not. After treatment, the patients in each group were classified into 1 of the 4 following outcomes of treatment: ambulatory with less pain, nonambulatory with less pain, ambulatory without pain improvement, and nonambulatory without pain improvement (Fig. 1A). After that, a Markov model with 3-month cycle durations was adopted to capture the lifetime costs and health outcomes of the treatment. In the Markov model, patients could remain either in the same state or in transition to a poorer state due to the progression of the disease. In this model, we assumed that patients underwent surgery only one time and that no patients would transition to an improved health state, as shown in Fig. 1B.

Decision tree (A) and Markov model (B). A decision tree was constructed to divide patients into the 4 following groups according to the health status outcome of each treatment: ambulatory with less pain, nonambulatory with less pain, ambulatory with pain, and nonambulatory with pain. In the Markov model, patients could remain in the same health state or transition to worse health states. Sx+RT, combined surgery, and radiotherapy; RT, radiotherapy alone.
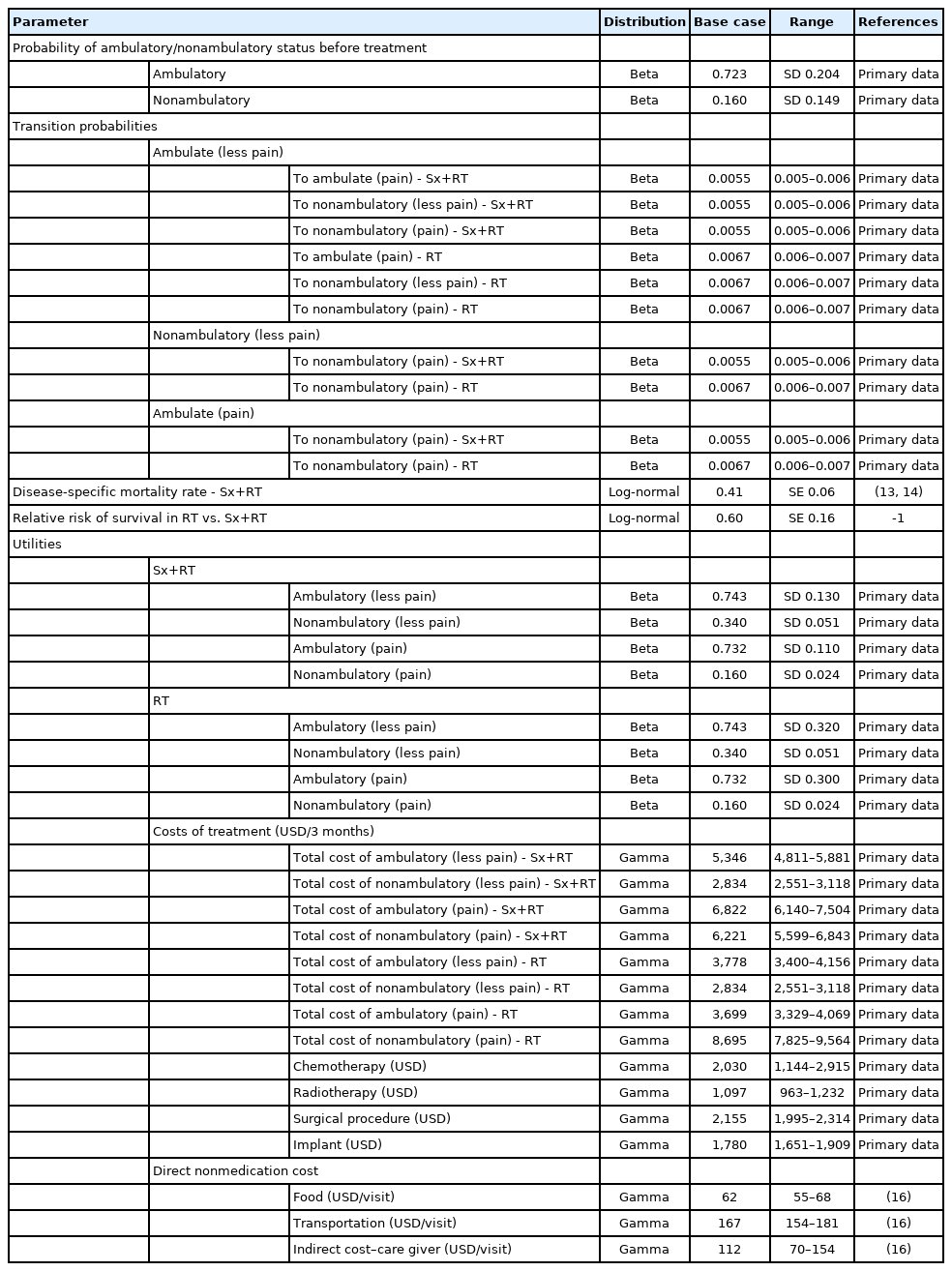
8. Input Parameters
The transition probabilities and utility of each group were obtained from the result of the cohort included in this study. The disease-specific mortality rate was based on the overall local control rate of the disease from studies conducted by Bishop et al. [13] and Pessina et al. [14]. The probability of ambulatory and nonambulatory status before treatment was obtained from the studied cohort. Regarding the adoption of societal perspective, in this study, we included both direct medical costs (e.g., nursing service, medication, diagnostic imaging) and direct nonmedical costs (e.g., food, transportation). Indirect costs of patients were not included due to our assumption that lost or impaired ability to work or engage in leisure activities due to morbidity would be captured in the disutility of QALY [15]. Direct costs of treatment, hospital visit rates (both outpatient and inpatient), and utility data were obtained from the studied cohort. Direct nonmedical costs were obtained from a standard cost list in the Thailand HTA guideline [16]. All costs were converted to 2020 USD using an exchange rate of 1 USD = 31.3 THB and the consumer price index [17]. Detail and sources of the model input parameters used in this study are shown in Table 1.
9. Cost-Utility Analysis
The primary outcome of the base case analysis was the ICER obtained from a comparison between the combined surgery and radiotherapy treatment strategy versus radiotherapy alone.
One-way sensitivity analyses were performed to study the effects of altering uncertainty parameters within the 95% confidence interval (CI) ranges, including all clinical effects, transitional probabilities, costs, and utilities, on the ICER from the model. In cases where the 95% CI range was unavailable, a range of mean ± 15% was applied. The results of 1-way sensitivity analysis are presented using a tornado diagram. A probabilistic sensitivity analysis (PSA) was performed using Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, USA) to simultaneously examine the effects of all parameter uncertainties [18]. The distributions of each probability were assigned the following [19]: transitional probability, and utility parameters were specified to beta-distribution. Costs were assigned a gamma distribution. Relative risk of mortality parameters was given a log-normal distribution. A Monte Carlo simulation was run to obtain 1,000 different simulations reflecting a range of values for the total cost, outcomes, and ICER. The results of the PSA are presented as a cost-effectiveness plane and a cost-effectiveness acceptability curve.
RESULTS
1. Clinical Results
Twenty-four patients were prospectively enrolled and followed for 6 months after treatment. There were 6 men and 18 women. Twelve patients underwent surgical treatment and radiotherapy. The others underwent only radiotherapy. Patient demographic and clinical characteristics are shown in Table 2. No statistically significant difference was observed for any of the parameters shown in Table 2 between the surgery group and the nonsurgery group. Patients with various types of cancer were included (Table 2). All patients in both groups underwent radiotherapy. The average back pain score at the 3-month follow-up in the surgery group was significantly lower than the average score in the radiotherapy alone group (31.67 ± 30.92 vs. 55.45 ± 20.67, respectively; p = 0.024), but there was no significant difference between groups for pain at the 6-month follow-up. Ambulatory status and the survival rate were also not significantly different between the 2 treatment groups (Table 3).
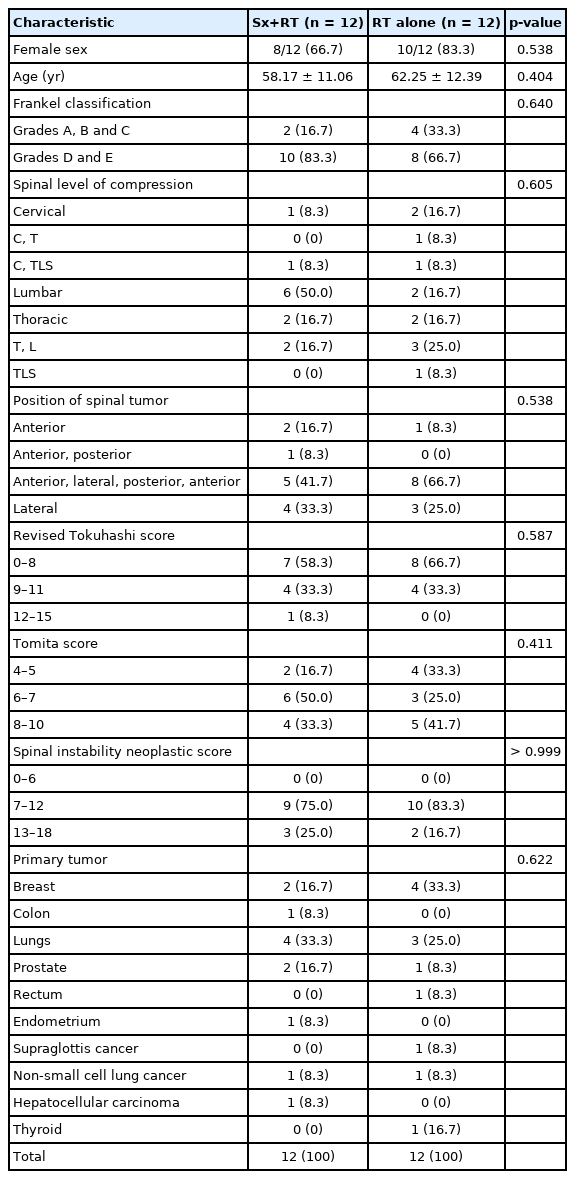
Patient demographic and clinical characteristics compared between the combined surgery and radiotherapy (Sx+RT) group and the radiotherapy alone (RT) group
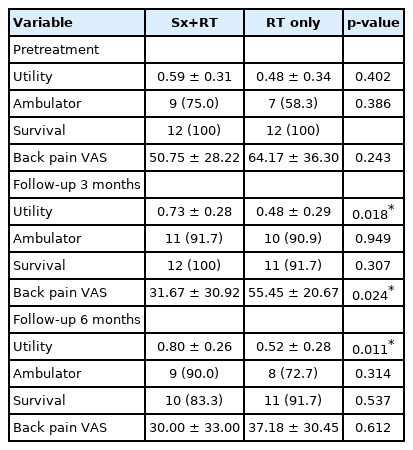
Utility, ambulator, survival, and back pain visual analogue scale (VAS) score at different time points compared between the combined surgery and radiotherapy (Sx+RT) group and the radiotherapy alone (RT) group
The mean preoperative utility value was 0.592 ± 0.314 in the surgery group, and 0.479 ± 0.345 in the radiotherapy alone group (p = 0.402). At both the 3- and 6-month follow-up, the mean utility value in the surgery group was significantly higher than that in the radiotherapy alone group (3 months: 0.701 ± 0.328 vs. 0.433 ± 0.297, respectively; p = 0.018; and, 6 months: 0.804 ± 0.264 vs. 0.506 ± 0.270, respectively; p = 0.011) (Table 3).
2. Cost-Utility Analysis
1) Base case analysis
The estimated total lifetime cost per patient for surgery and radiotherapy versus radiotherapy alone was 59,863.14 USD versus 24,526.97 USD, respectively. The number of QALYs was 1.54 and 0.92 for the combination treatment group and the radiotherapy alone group, respectively. The ICER for the combination surgery and radiotherapy treatment was 57,074.01 USD per QALY gained compared to radiotherapy alone (Table 4). This finding demonstrates the combination treatment option to be non-cost-effective when judged according to the official WTP threshold in Thailand.
2) One-way sensitivity analysis
Fig. 2 shows the most influential variables in our model to be the utility of ambulatory status with less pain after combination surgery and radiotherapy, the utility of ambulatory status with less pain after radiotherapy alone, and relative risk of survival in radiotherapy alone versus combination surgery and radiotherapy. However, within the range of each parameter, none yielded a cost-effective result.
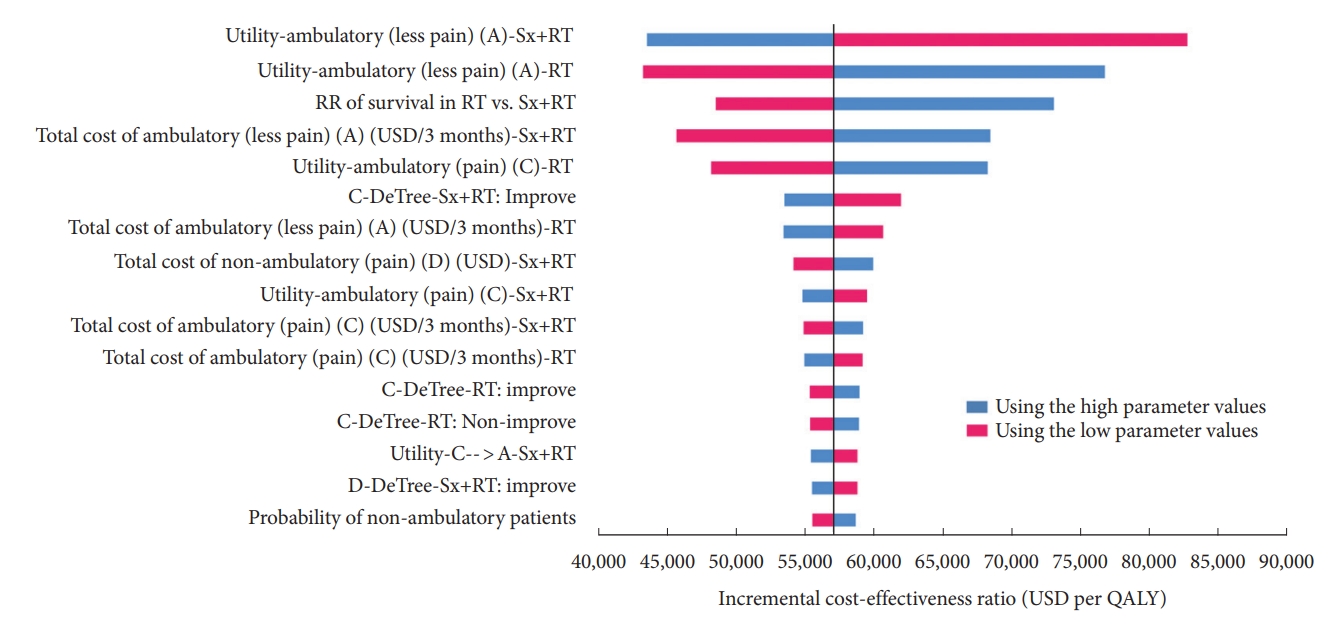
Tornado diagram. This model illustrates the result of 1-way sensitivity analysis that was performed to study the effects of altering uncertainty parameters within the 95% confidence interval ranges, including all clinical effects, costs, utilities, and the discount rate on the ICER calculated from the model. ICER, incremental cost-effectiveness ratio; Sx+RT, combined surgery and radiotherapy; RT, radiotherapy alone; USD, United States dollar; QALY, quality-adjusted life-year.
3) Probabilistic sensitivity analysis
Results of the PSA based on 1,000 Monte Carlo simulations are presented in a cost-effectiveness plane (Fig. 3A). Despite the variation in base case parameter inputs, most of the plots were in the upper-right quadrant, which suggests the combination surgery and radiotherapy treatment strategy to be more effective, but more expensive than radiotherapy alone. All simulations were plotted above the WTP threshold line, which means that none of the scenarios could be considered cost-effective in Thailand’s healthcare setting. The results of the PSA are also presented as a cost-effectiveness acceptability curve, as shown in Fig. 3B. At the Thailand WTP threshold, the probability of the combination therapy strategy being cost-effective is zero. If the Thailand WTP was increased to 56,000 USD per QALY gained, the probability of surgery combined with radiotherapy being cost-effective was 50% compared to radiotherapy alone.
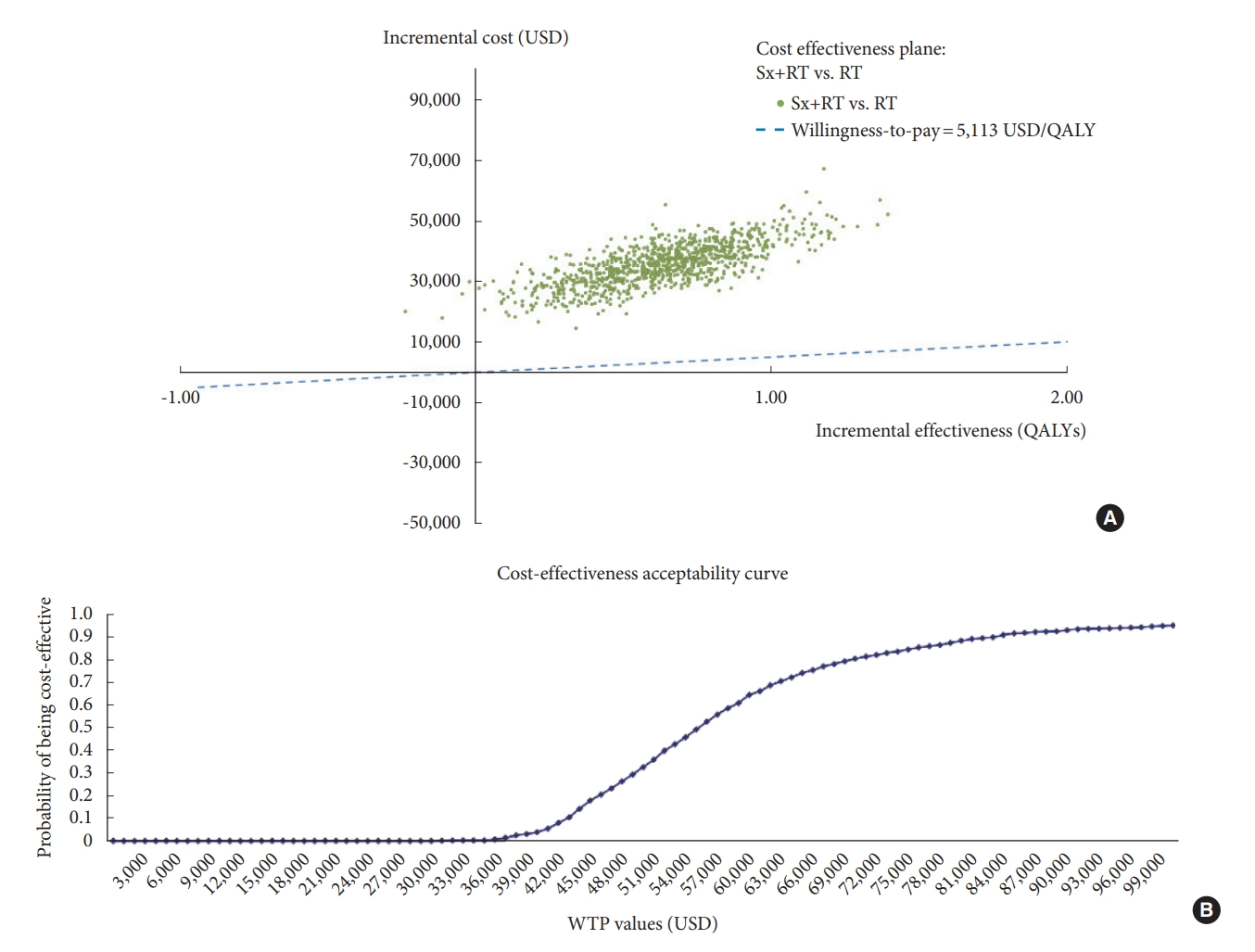
Multivariate probabilistic sensitivity analysis. The result was based on 1,000 Monte Carlo simulations. The results are shown as a cost-effectiveness plane (A), and a cost-effectiveness acceptability curve (B). Sx+RT, combined surgery and radiotherapy; RT, radiotherapy alone; USD, United States dollar; QALY, quality-adjusted life-year.
DISCUSSION
Many studies have investigated the cost-utility of the surgical treatment among spinal metastasis patients. Furlan et al. [8] reported an ICER of 250,307 USD per QALY when surgery plus radiotherapy was compared to radiotherapy alone. They adopted a Markov model approach and analyzed the results based on the data from the study of Patchell et al. [3] combined with Ontario-based physician fee and hospital cost data in Canada. They found and reported surgery plus radiotherapy to be cost-effective at a WTP threshold of 50,000 USD per QALY [8]. In Japan, Miyazaki et al. [2] also found surgical treatment to be cost-effective with an ICER of 42,003 USD per QALY gained at a WTP of 50,000 USD per QALY gained. Finally–in Belgium, Depreitere et al. [7]. Reported an ICER for surgical management of spinal metastasis of 13,635 EUR per QALY compared to radiotherapy alone. Taken together, these reported findings indicate that palliative surgery is cost-effective for spinal metastasis patients in developed countries.
During the 6-month follow-up after treatment, our findings showed significant improvement in the quality of life of patients in the combined surgery and radiotherapy group compared to the quality of life of patients in the radiotherapy alone group. In this study, there was no significant difference in ambulation between the 2 groups. However, the radiotherapy alone group had less ambulatory status after 6 months. Therefore, the effectiveness of radiotherapy alone in terms of maintaining or restoring ambulatory status may be limited. A prior study also reported that surgical intervention significantly improved ambulation, pain relief, and quality of life in spinal metastasis patients [20].
For the CUA, the ICER of the combined surgery and radiotherapy group relative to the radiotherapy alone group was 57,074.01 USD per QALY gained, which indicated that the surgery group is not cost-effective compared to the radiotherapy alone group. Moreover, the results of our sensitivity analyses showed no cost-effectiveness of the combination therapy regardless of the parameter values used. We conducted a literature review for studies that also compared combination treatment with radiotherapy alone in spinal metastasis (Table 5), and some previously reported results conflict with the results of our study. The observed differences between and among studies may be due to differences in the WTP threshold and cost of treatment of each country. However, the survival rate was not only affected by the choice of treatment but also other factors were included, so the highly selection of spinal metastatic patients who seemed to have better prognosis and outcome after the treatment was required. One of the significant prognostic factors in spinal metastasis patients was reported to be ambulatory status [21]. Schoenfeld et al. [9] reported a QALY of 0.800 among patients who received nonoperative treatment, and a QALY of 0.823 in patients with independent ambulatory status at presentation. In patients with nonambulatory status at presentation, they reported a QALY of 0.089 in patients who received nonoperative treatment and a QALY of 0.813 in patients who received operative treatment. The ICER for a surgical procedure was 899,700 USD per QALY and 48,600 USD per QALY in patients with independent ambulatory and nonambulatory status at presentation, respectively. Similar to the result reported by Schoenfeld et al. [9] our results showed significant improvement in utility among surgical patients with nonambulatory status to be a factor that positively influences cost-effectiveness, so the observed improvement in utility and survival in the surgery group strongly influenced cost-effectiveness in this economic model.
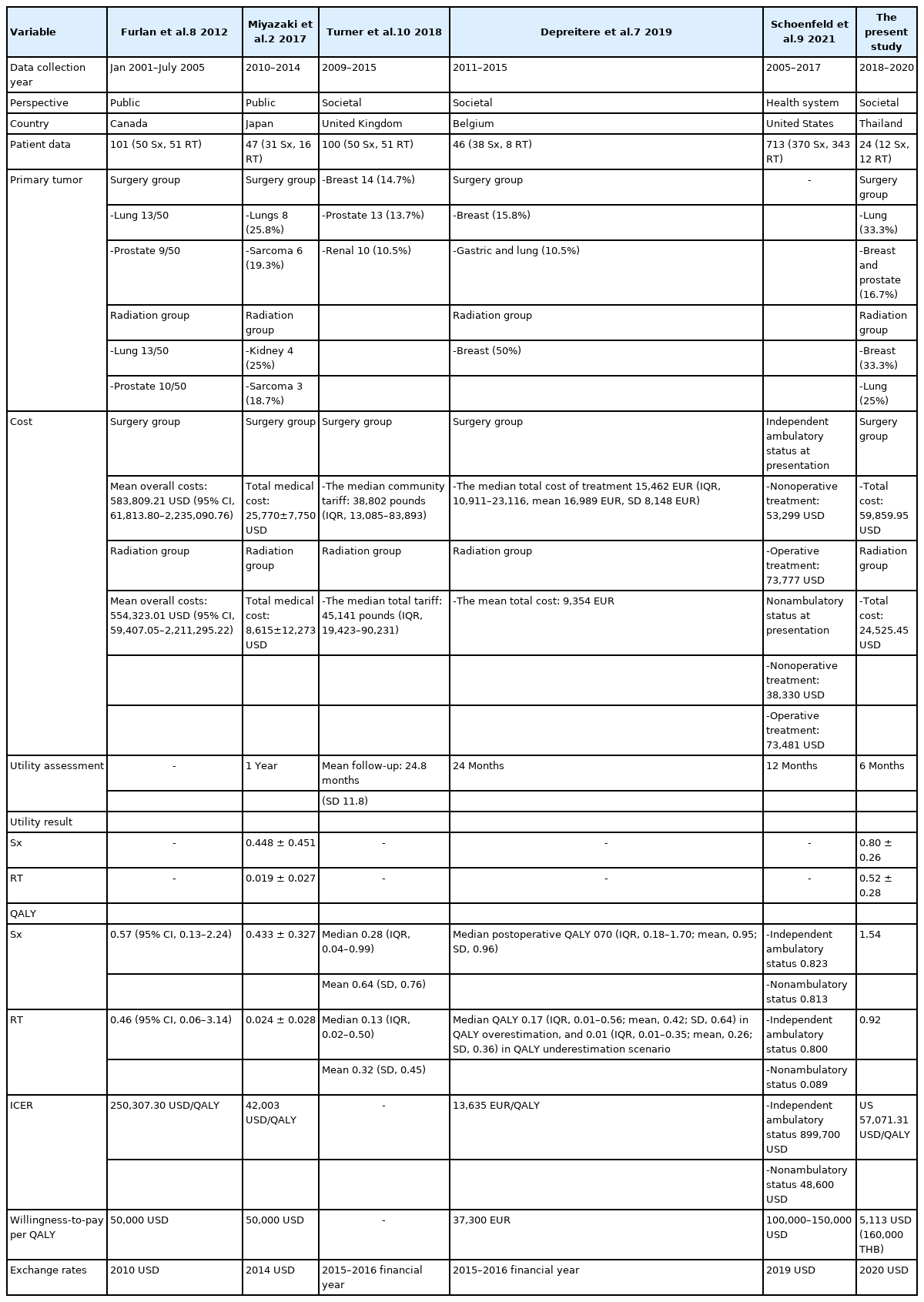
Literature review for previous studies that compared cost-utility between radiotherapy alone and combined surgery (Sx) and radiotherapy (RT) for spinal metastasis
There were many methods of surgery that may have affected the outcomes. Lee et al. [22] compared the postoperative result between palliative, debulking and en bloc surgery in spinal metastases. The result showed the debulking surgery group had the highest postoperative complications than the others but no difference in the improvement of neurological deficit after surgery. The proper surgical option on each patient may improve outcomes [22]. Additionally, stereotactic body radiation therapy has become a fundamental tool for the treatment of spine metastasis that provided good local control, especially in radioresistant tumor [23,24]. The separation surgery followed by stereotactic radiation therapy was effective in decompression and long-term local control [25]. Compared with conventional radiotherapy, stereotactic body radiotherapy at a dose of 24 Gy in 2 daily fractions was superior to conventional external beam radiotherapy at a dose of 20 Gy in 5 daily fractions in improving the complete response rate for pain [26]. However, the receipt of stereotactic body radiotherapy is limited because of a lack of medical resources. So, most patients with spine metastases were treated with conventional radiotherapy usually with 10 fractions [27] same as in Thailand.
To our knowledge, our study is the first prospective cohort study to compare utility outcomes after treatment in spinal metastasis patients in a developing country. This study has several strengths. First, our study data were prospectively collected. Second, we adjusted the mortality rates of these patients by incorporating the Thai age-standardized mortality rate to reflect baseline health of Thai population. Third, all cost data were retrieved from reliable local sources. Fourth and last, we conducted a comprehensive literature review to determine the overall mortality rate and the progression of disease after treatment in both groups for use as model input parameters.
This study has some mentionable limitations. From the reference literature, a study reported by Miyazaki et al. [2] showed a more significant difference in the health state of the surgery group versus radiotherapy alone, and the follow-up time was longer than the 6-month follow-up in our study. Second, there was a limited sample size. Third, our center is a university hospital so the costs of care are higher than those charged by rural general and provincial hospitals in Thailand. The further multicenter study may be needed that includes all healthcare settings in Thailand.
CONCLUSION
Surgical treatment for spinal metastasis significantly improved the quality of life of spinal metastasis patients compared with radiotherapy alone over the evaluated 6-month posttreatment follow-up period. However, the surgical treatment strategy was not found to be cost-effective compared to radiotherapy alone at the current WTP threshold in Thailand. A highly selective strategy for identifying spinal metastasis patients before surgical treatment is suggested to optimize all modifiable measurement parameters for all stakeholders.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: PL; Data curation: SW, PP, AT; Formal analysis: PP; Methodology: PT, CK, AT, PL; Project administration: PT, SW; Visualization: CK; Writing - original draft: PT; Writing - review & editing: PL.
Acknowledgements
The authors gratefully acknowledge the patients that generously agreed to participate in this study, and Mr. Pongsathorn Samphaotong of the Orthopaedic Research Unit of the Department of Orthopaedic Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University for assistance with statistical analysis.