 |
 |
- Search
| Neurospine > Volume 19(2); 2022 > Article |
|
|
Abstract
Objective
Endoscopic transforaminal lumbar interbody fusion (Endo-TLIF) has gained increasing popularity among spine surgeons. However, with the use of fluoroscopy, intraoperative radiation exposure remains a major concern. Here, we aim to introduce Endo-TLIF assisted by O-arm-based navigation and compare the results between O-arm navigation and fluoroscopy groups.
Methods
Sixty-four patients were retrospectively analyzed from May 2019 to September 2020; the nonnavigation group comprised 34 patients, and the navigation group comprised 30 patients. Data on radiation dose, blood loss, postoperative drains, surgery time, complications, and length of hospital stay (LOS) were collected. Clinical outcomes were evaluated from postoperative data such as fusion rate, Oswestry Disability Index (ODI), and visual analogue scale (VAS). Radiation dose and surgery time were selected as primary outcomes; the others were second outcomes.
Results
All patients were followed up for at least 12 months. No significant differences were detected in intraoperative hemorrhage, postoperative drains, hospital LOS, or complications between the 2 groups. The radiation dose was significantly lower in the navigation group compared with the nonnavigation group. The time of cannula placement and pedicle screw fixation was significantly reduced in the navigation group. No significant differences were detected between the clinical outcomes in the 2 groups (VAS and ODI scores).
Conclusion
The present study demonstrates that O-arm-assisted Endo-TLIF is efficient and safe. Compared with fluoroscopy, O-arm navigation could reduce the radiation exposure and surgical time in Endo-TLIF surgery, with similar clinical outcomes. However, the higher doses exposed to patients remains a negative effect of this technology.
As the elderly population continues to grow, an increasing number of people suffer from lumbar degenerative disease (LDD), which causes pain and disability. Spinal fusion is considered an effective technique for treating LDD, and this technique is continuously developing to achieve the goal of maximizing outcomes and minimizing morbidity. Minimally invasive spinal (MIS) surgery has gained popularity among spinal surgeons because of advances that reduce intraoperative trauma, require smaller incisions, require less recovery time, and result in fewer perioperative complications [1,2]. Spinal endoscopy techniques have developed rapidly and are widely used in treating LDD. A newly emerging endoscopic spinal surgery, endoscopic transforaminal lumbar interbody fusion (Endo-TLIF), is manipulated via the transforaminal corridor with little bone removal and maximum preservation of the surrounding structures [3,4]. Many previous studies have suggested that Endo-TLIF is an effective and safe procedure for LDD [5-7].
However, as in other MIS surgeries, fluoroscopic assistance is essential for Endo-TLIF because surgeons must reach the proper target and place the pedicle screws percutaneously. In fact, fluoroscopy is used both in the first step and throughout the procedure because it is difficult to identify the operation direction through the percutaneous pathway. In addition, further fluoroscopic checks are required for the insertion of the polyetheretherketone (PEEK) cage and fixation of the pedicle screws. Therefore, intraoperative radiation exposure for both patients and surgeons is of significant concern.
In recent years, navigation systems have been successfully applied in various surgical fields [8] including neurosurgery, endoscopy, bronchoscopy, and arthroscopy. They are also used in spinal surgery, and many studies have suggested that they can effectively reduce radiation exposure and surgical time [9-11].
There have been very few studies on the navigation systems used in Endo-TLIF. Therefore, we aimed to introduce Endo-TLIF using the O-arm-based navigation system and compare the results between the navigation and fluoroscopy groups.
We retrospectively analyzed 64 patients who underwent Endo-TLIF assisted by O-arm navigation or conventional 2-dimensional (2D) fluoroscopy in our center between May 2019 and September 2020. The Ethics Committee of the Second Affiliated Hospital of Army Medical University approved this study, and written informed consents were obtained from all patients. Patients who met all of the following criteria were included: (1) age Ōēź 18 and Ōēż 80 years, (2) diagnosis of lumbar spondylolisthesis (below Meyerding grade II), lumbar instability, or lumbar spinal nerve canal stenosis, and (3) conservative therapy for Ōēź 3 months prior. The exclusion criteria included inoperable physical ailments or mental disease, history of lumbar spinal surgery, spinal infection or tumor, and traumatic lesions. One experienced surgeon performed all the surgeries.
Perioperative data such as radiation dose, blood loss, postoperative drains, surgery time, complications, and length of hospital stay (LOS) were collected. In addition, the time required for specified steps in the surgery was recorded, including the navigation set-up time, cannula placement time, and percutaneous pedicle screw fixation time. Clinical outcomes were evaluated from postoperative data such as Oswestry Disability Index (ODI), visual analogue scale (VAS), and modified MacNab criteria. Surgical complications were assessed, including severe nerve root injury, vascular damage, hematoma, and cauda equina injury. Additionally, patient spine fusion was assessed using computed tomography (CT) images at 12 months postoperatively. The bridging trabecular bone formation between the vertebral body was regarded as solid fusion in the CT images. Radiation dose and surgery time were selected as primary outcomes, and the others were secondary outcomes. The radiation dose was collected from the radiation generator, and the duration of radiation exposure was also collected.
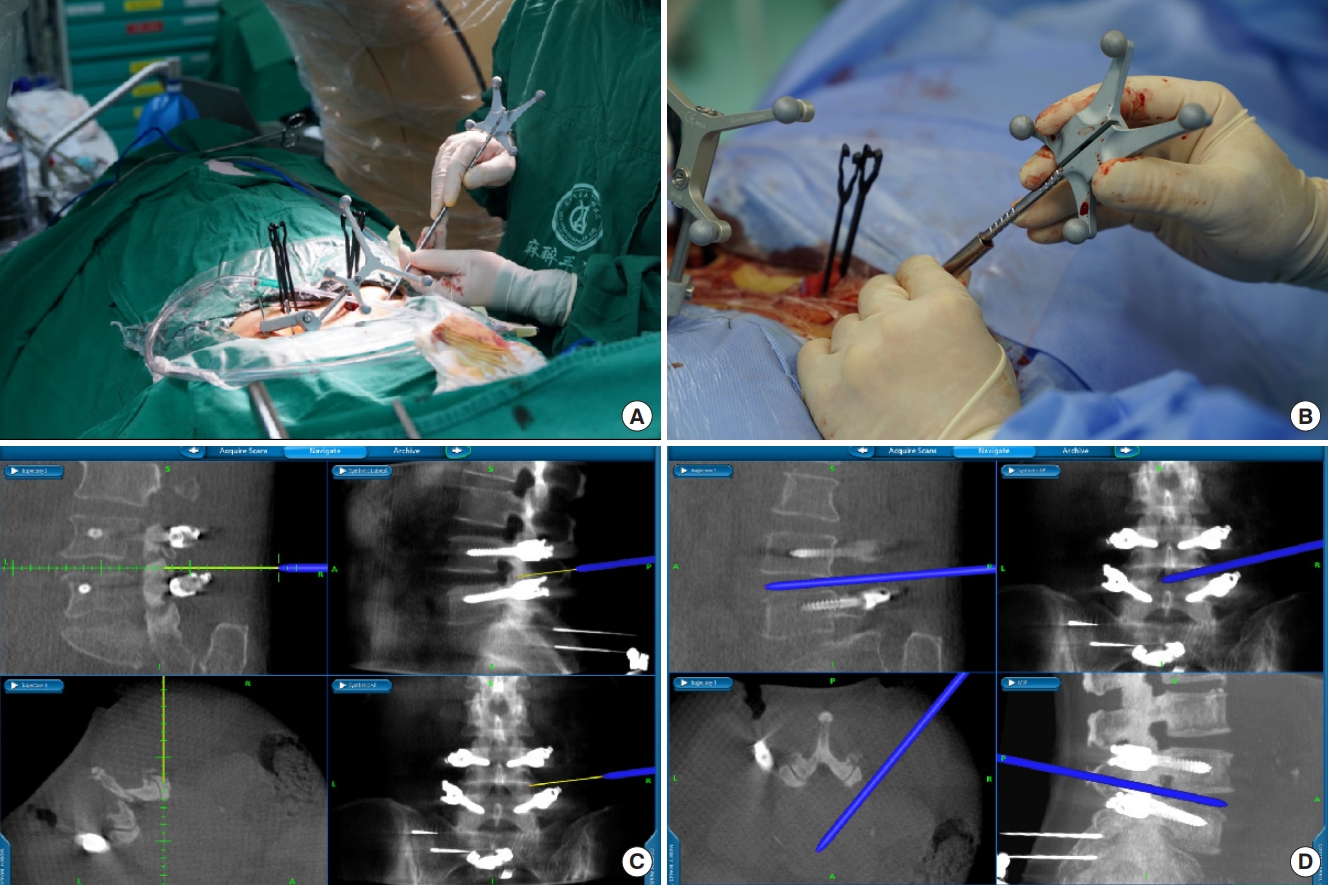
Patients with general anesthesia were placed in the prone position. A nerve monitoring system monitored somatosensory-evoked potentials and free-running electromyography throughout the operation. Two K-wires (2.0-mm diameter) were used to anchor the reference frame to the iliac crest (Fig. 1A). Next, the O-arm (O-arm Surgical Imaging System and Stealth-Station; Medtronic, Minneapolis, MN, USA) was used to obtain intraoperative 3-dimensional (3D) images (Fig. 1B). Then acquired CT pictures were instantly transmitted to the computer, and multiplanar images of the lumbar spine were reconstructed using the navigation system. Subsequently, surgical instruments were registered to be traced intraoperatively in real time. In general, navigation preparation time, including reference frame fixation, O-arm scan, picture transmit, and instrument registration, is less than 10 minutes.
The entry point of pedicle screws was determined using 3D-image guidance to optimize screw length and avoid neurovascular structures. The pedicle screw was placed at a suitable depth using a navigated screwdriver (Fig. 2A, B). The image of screw trajectory and position was displayed on the monitor in real time, and the surgeon could make appropriate adjustments according to the image (Fig. 2C, D). After the screws were in place, C-arm was used to confirm the final position of the screws. Next, we use a spinal needle to reach the target point via the navigation system. Sequential dilation was performed to expand the soft tissue, and a double-cannula device was docked on the lateral aspect of the facet joint to perform foraminoplasty under navigation guidance. The navigation system showed the depth and pathway of the reamer or bone drill on a computer screen in real time until foraminoplasty was completed (Fig. 3). After the working cannula was advanced through the dilator and its position was confirmed using C-arm, reamers of different diameters were used to remove the degenerative disc tissue. Thereafter, the intervertebral disc was filled with allografts and recombinant human bone morphogenetic protein, and PEEK cages were implanted via an expandable tube (ZELIF, Sanyou, China). The final position of PEEK cages was identified using C-arm. Finally, a standard percutaneous endoscopic lumbar discectomy procedure was performed.
The operation was performed with the assistance of traditional fluoroscopy, as previously reported [12]. After surgery, analgesic and anti-inflammatory treatments were administered.
The IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA) was used to analyze data, and Statistical significance was defined as p-values less than 0.05. Statistics are expressed as mean ┬▒ standard deviation or frequency. The Independent-sample t-test, chi-square test, and Mann-Whitney U-test were used to examine differences between the 2 groups, as appropriate.
Thirty-four patients were included in the nonnavigation group (14 men and 20 women), and 30 patients in the navigation group (13 men and 17 women). The follow-up time of all patients was at least a year. No significant differences in patient demographics were detected between the 2 groups (Table 1).
The dose of radiation administered was 7.58 ┬▒ 0.84 mGy in the navigation group; this was significantly lower than in the nonnavigation group (59.08 ┬▒ 9.77 mGy). The duration of radiation exposure was 59 seconds in the nonnavigation group and 9 seconds in the navigation group (p < 0.001). Intraoperative blood loss, postoperative drainage, hospital LOS, and complications were not significantly different between the 2 groups (Table 1). The navigation set-up time was 5.9 ┬▒ 0.84 minutes. Both cannula placement time (22.6 ┬▒ 2.7 minutes) and pedicle screw fixation time (37.0 ┬▒ 2.8 minutes) were significantly shorter in the navigation group. The total operation time was also reduced in the navigation group (p < 0.001) (Table 2). Compared with preoperative scores, both VAS and ODI scores significantly improved after surgery at different times in both groups (Table 3). Nevertheless, there were no significant differences between the 2 groups (Table 3). The excellent and good rates were 91.2% in the nonnavigation group and 93.3% in the navigation group. No significant difference was observed between the excellent and good rates of the 2 groups (p = 0.682). No major complications occurred during the surgery. Only 2 occurrences of transient ipsilateral dysesthesia were recorded, and the clinical symptoms disappeared with conservative treatment. The spine fusion was 94.1% (32 cases) and 93.3% (28 cases) respectively in the nonnavigation group and navigation group at 12 months postoperatively, and no significant difference was observed between the groups. However, all patients in the 2 groups had achieved solid spine fusion at the final follow-up, and there was no subsidence occurrence in both groups.
As a minimally invasive procedure, Endo-TLIF has been successfully manipulated to treat LDD and achieve positive clinical outcomes [7,13]. Jin et al. [13] presented a consecutive case series of Endo-TLIF, demonstrating satisfactory clinical and radiological results. It indicated that Endo-TLIF is a promising surgical alternative for treating LDD. In 2020, Wu et al. [14] compared Endo-TLIF with open-TLIF in the treatment of LDD, supporting the hypothesis that Endo-TLIF is a viable option for treating single-segment LDD with little trauma, rapid recovery, and inexpensive cost. These both suggest that Endo-TLIF is an effective technique with less trauma and faster recovery. Intraoperative ionization-based imaging techniques are essential for MIS surgery to expose the spine visually. Compared with open procedures, x-rays are more frequently used during the operation, which increases surgery time and causes harm to both patients and medical staff [15,16]. Therefore, the associated radiation exposure remains a major concern, especially for surgeons who are frequently exposed [17].
Compared with open surgery, MIS techniques such as MIS-TLIF are highly dependent on fluoroscopy as the limited exposure fields and constrained working tube, which results in higher radiation exposure to both patients and the surgeon [18]. In a meta-analysis, the results indicated that mean fluoroscopy in MIS-TLIF was 94 seconds which was 2-fold of open surgery [19]. In addition, Godzik et al. [20] also reported that radiation exposure to the surgeon in the MIS-TLIF group was 408.3 ┬▒ 192.3 ╬╝Sv which was significantly higher than lateral transpsoas lumbar interbody fusion (208.6 ┬▒ 146.9 ╬╝Sv). A previous prospective cohort study [21] showed that Endo-TLIF had less intraoperative blood loss, less patient postoperative pain, and shorter hospital stay with similar surgical outcomes when compared with MIS-TLIF. These outcomes prove that Endo-TLIF is better than MIS-TLIF in certain diseases. However, as a less invasive surgery than MIS-TLIF, there are many other percutaneous procedures in Endo-TLIF besides percutaneous screw placement, leading to more radiation exposure. The advent and development of navigation technology have had a profound impact on spinal surgery [22,23]. Computer-assisted 3D navigation can provide high-resolution images and a more detailed view of the pedicles, improving the precision of spinal screw placement. As reported in a previous study, the nerve injury risk and clinical complications could be decreased through this technique [24]. Zhao et al. [25] compared the occurrence of postoperative hydrothorax between O-arm navigation and free-hand in spinal deformity surgery. They found that the volume of postoperative hydrothorax could be significantly reduced using the O-arm navigation, and this was ascribed to the improvement in screw implantation accuracy. Besides this, the O-arm navigation system can significantly reduce the radiation exposure of surgeons. Images can be obtained using navigation systems, with the surgeons outside the operating theater, with no additional intraoperative CT scan or fluoroscopy required to continue with the procedures. A prospective randomized study compared radiation exposure between 2D and 3D fluoroscopic techniques. The results suggested that the surgeon radiation exposure in the 2D fluoroscopy group was 9.96 times higher than that in the navigation group [26]. In the present study, the mean radiation dose in the navigation group was 7.58 ┬▒ 0.84 mGy, much lower than that in the nonnavigation group. Our results are consistent with those of a previous study [27].
In our study, although there is an additional mean 5.9-minute navigation set-up time before surgery, the total duration of surgery in the navigation group was significantly shorter than that in the nonnavigation group (119.8 ┬▒ 10.5 minutes vs. 134.2 ┬▒ 10.2 minutes). This may be ascribed to the reduced time of cannula placement (22.6 ┬▒ 2.7 minutes vs. 34.6 ┬▒ 3.7 minutes) and pedicle screw placement (37.0 ┬▒ 2.8 minutes vs. 47.1 ┬▒ 2.8 minutes) in the navigation group. The results showed that the efficiency of Endo-TLIF was improved by navigation. In another retrospective study, the effect of navigation on surgical efficiency was explored [28]. The total operative time decreased significantly in the O-arm navigation group compared with the free-hand group. In the present study, clinical outcomes such as VAS and ODI scores improved significantly in the 2 groups postoperatively. Differences between the nonnavigation and navigation groups were not detected significantly in the VAS and ODI scores. Also, hospital LOS and complications were not significantly different in the 2 groups. Several previous studies have assessed the impact of O-arm navigation on clinical outcomes (nerve injury and reoperation rate). These studies support the hypothesis that navigation-assisted spinal surgery could improve clinical outcomes by reducing nerve injury and reoperation rates for mispositioned screws [29-31]. All surgeries in our study were performed by senior doctors; hence, it was not difficult for them to place screws accurately in the lumbar pedicle. Therefore, we did not study the screw placement. No patients underwent reoperation in either of the 2 groups; only 2 occurrences of transient ipsilateral dysesthesia were recorded in the nonnavigation group, and the clinical symptoms disappeared with conservative treatment.
Endo-TLIF surgery assisted by O-arm navigation offers several advantages. First, the surgical efficiency could be improved, especially in some percutaneous procedures, including cannula placement and pedicle screw fixation. Second, the radiation exposure to operation staff can be reduced, having a positive effect on protecting their health. In addition, surgeons can determine the desired screw sizes and rod lengths and assess the extent of the discectomy. However, some disadvantages have also been reported. First, O-arm for intraoperative CT navigation resulted in higher radiation doses to patients compared with C-arm [32-34]. In a multicenter study, the results indicated that the mean doses for patients in the O-arm group were 4 times higher than those in the C-arm group [35]. Radiation exposure shows a positive dose effect in breast cancer mortality [36] and has been linked to various cancers [37]. Although some minimized-dose O-arm Protocols could be used to reduce negative effects for patients [38], the impact of exposure for patients in O-arm navigation remains a problem. Moreover, O-arm-assisted Endo-TLIF surgery is comparatively expensive and may lead to an additional financial burden on patients. Although the cost of new technologies is reducing steadily over time, more research on cost-effectiveness is needed to justify the navigation technique financially [39]. There are some limitations to our study. This was a retrospective study that compared the intraoperative data and clinical outcomes between the 2 groups. The sample size was relatively small, and inherent selected bias could not be ignored. Randomized controlled trials with large sample sizes and long-term follow-up are needed in future.
We have shown that Endo-TLIF assisted by O-arm navigation is efficient and can reduce radiation exposure. O-arm navigation could reduce radiation exposure and surgical time in Endo-TLIF surgery with clinical outcomes similar to those with fluoroscopy. Navigation is a promising alternative for patients undergoing Endo-TLIF surgery. However, the higher doses exposed to patients remain a negative effect of this technology.
NOTES
Funding/Support
This study was supported by the technological innovation project of military clinical medicine of the second affiliated hospital of Army Medical University (Grant No.2018JSLC0014).
Author Contribution
Conceptualization: JG, XH, HL, CL, YT, YZ; Data curation: JG, XH, LL, HL; Formal analysis: JG, XH, HW, YT; Methodology: J JG, XH, LL, YT; Project administration: C Li, YT, YZ; Visualization: LL, HL, HW, YT, YT; Writing - original draft: JG, XH; Writing - review & editing: JG, XH, C Li, YT, YZ.
Fig.┬Ā1.
(A) The percutaneous iliac pin with attached reference array is fixed in place. (B) The O-arm device is in place and prepared for image capture.

Fig.┬Ā2.
(A) Image of a navigated screwdriver with an attached tracking array, and (B) it was registered intraoperatively. (C, D) The track of the Access Tracker was visible in real time and the surgeon could make appropriate adjustments.

Fig.┬Ā3.
(A, B) The navigated trocar-like puncture probe was used during foraminoplasty. (C) The entire puncture trajectory was designed and accurately assisted by navigation. (D) The depth of the processed intervertebral space was evaluated by the Access Tracker.
Table┬Ā1.
Patient demographics and perioperative data
Table┬Ā2.
Comparison of surgery time between the 2 groups
Table┬Ā3.
Comparison of clinic outcomes between the 2 groups
REFERENCES
1. Banczerowski P, Czigl├®czki G, Papp Z, et al. Minimally invasive spine surgery: systematic review. Neurosurg Rev 2015;38:11-26. discussion 26.



2. Altshuler M, Mueller K, MacConnell A, et al. Does minimally invasive spine surgery reduce the rate of perioperative medical complications? A retrospective single-center experience of 1435 degenerative lumbar spine surgeries. Eur Spine J 2021;30:122-7.



3. Lee SH, Erken HY, Bae J. Percutaneous transforaminal endoscopic lumbar interbody fusion: clinical and radiological results of mean 46-month follow-up. Biomed Res Int 2017;2017:3731983.




4. Ahn Y, Youn MS, Heo DH. Endoscopic transforaminal lumbar interbody fusion: a comprehensive review. Expert Rev Med Devices 2019;16:373-80.


5. Wagner R, Haefner M. Uniportal endoscopic lumbar interbody fusion. Neurospine 2020;17(Suppl 1):S120-8.




6. Heo DH, Hong YH, Lee DC, et al. Technique of biportal endoscopic transforaminal lumbar interbody fusion. Neurospine 2020;17(Suppl 1):S129-37.




7. Kolcun JPG, Brusko GD, Basil GW, et al. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: operative and clinical outcomes in 100 consecutive patients with a minimum 1-year follow-up. Neurosurg Focus 2019;46:E14.

8. Luo X, Mori K, Peters TM. Advanced endoscopic navigation: surgical big data, methodology, and applications. Annu Rev Biomed Eng 2018;20:221-51.


9. Kim TT, Johnson JP, Pashman R, et al. Minimally invasive spinal surgery with intraoperative image-guided navigation. Biomed Res Int 2016;2016:5716235.




10. Klingler JH, Scholz C, Kr├╝ger MT, et al. Radiation exposure in minimally invasive lumbar fusion surgery: a randomized controlled trial comparing conventional fluoroscopy and 3D fluoroscopy-based navigation. Spine (Phila Pa 1976) 2021;46:1-8.

11. Vaishnav AS, Merrill RK, Sandhu H, et al. A review of techniques, time demand, radiation exposure, and outcomes of skin-anchored intraoperative 3D navigation in minimally invasive lumbar spinal surgery. Spine (Phila Pa 1976) 2020;45:E465-76.


12. Gong J, Huang Z, Liu H, et al. A modified endoscopic transforaminal lumbar interbody fusion technique: preliminary clinical results of 96 cases. Front Surg 2021;8:676847.



13. Jin M, Zhang J, Shao H, et al. Percutaneous transforaminal endoscopic lumbar interbody fusion for degenerative lumbar diseases: a consecutive case series with mean 2-year follow-up. Pain Physician 2020;23:165-74.

14. Wu W, Yang S, Diao W, et al. Analysis of clinical efficacy of endo-LIF in the treatment of single-segment lumbar degenerative diseases. J Clin Neurosci 2020;71:51-7.


15. Bronsard N, Boli T, Challali M, et al. Comparison between percutaneous and traditional fixation of lumbar spine fracture: intraoperative radiation exposure levels and outcomes. Orthop Traumatol Surg Res 2013;99:162-8.


16. Mariscalco MW, Yamashita T, Steinmetz MP, et al. Radiation exposure to the surgeon during open lumbar microdiscectomy and minimally invasive microdiscectomy: a prospective, controlled trial. Spine (Phila Pa 1976) 2011;36:255-60.

17. Bindal RK, Glaze S, Ognoskie M, et al. Surgeon and patient radiation exposure in minimally invasive transforaminal lumbar interbody fusion. J Neurosurg Spine 2008;9:570-3.


18. Khan NR, Clark AJ, Lee SL, et al. Surgical outcomes for minimally invasive vs open transforaminal lumbar interbody fusion: an updated systematic review and meta-analysis. Neurosurgery 2015;77:847-74. discussion 874.

19. Kim CH, Lee CH, Kim KP. How High Are Radiation-related risks in minimally invasive transforaminal lumbar interbody fusion compared with traditional open surgery? A meta-analysis and dose estimates of ionizing radiation. Clin Spine Surg 2016;29:52-9.

20. Godzik J, Mastorakos GM, Nayar G, et al. Surgeon and staff radiation exposure in minimally invasive spinal surgery: prospective series using a personal dosimeter. J Neurosurg Spine 2020 Feb 7:1-7. https://doi.org/10.3171/2019.11.SPINE19448. [Epub].


21. Ao S, Zheng W, Wu J, et al. Comparison of Preliminary clinical outcomes between percutaneous endoscopic and minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative diseases in a tertiary hospital: is percutaneous endoscopic procedure superior to MIS-TLIF? A prospective cohort study. Int J Surg 2020;76:136-43.


22. Xi Z, Chou D, Mummaneni PV, et al. The navigated oblique lumbar interbody fusion: accuracy rate, effect on surgical time, and complications. Neurospine 2020;17:260-7.




23. Park P. Impact of spinal navigation on the oblique lumbar interbody fusion. Neurospine 2020;17:268-9.




24. Houten JK, Nasser R, Baxi N. Clinical assessment of percutaneous lumbar pedicle screw placement using theO-arm multidimensional surgical imaging system. Neurosurgery 2012;70:990-5.



25. Zhao Z, Liu Z, Hu Z, et al. Improved accuracy of screw implantation could decrease the incidence of post-operative hydrothorax? O-arm navigation vs. free-hand in thoracic spinal deformity correction surgery. Int Orthop 2018;42:2141-6.



26. Villard J, Ryang YM, Demetriades AK, et al. Radiation exposure to the surgeon and the patient during posterior lumbar spinal instrumentation: a prospective randomized comparison of navigated versus non-navigated freehand techniques. Spine (Phila Pa 1976) 2014;39:1004-9.

27. Chang CC, Chang HK, Wu JC, et al. Comparison of radiation exposure between O-arm navigated and C-arm guided screw placement in minimally invasive transforaminal lumbar interbody fusion. World Neurosurg 2020;139:e489-95.


28. Khanna AR, Yanamadala V, Coumans JV. Effect of intraoperative navigation on operative time in 1-level lumbar fusion surgery. J Clin Neurosci 2016;32:72-6.


29. Verma R, Krishan S, Haendlmayer K, et al. Functional outcome of computer-assisted spinal pedicle screw placement: a systematic review and meta-analysis of 23 studies including 5,992 pedicle screws. Eur Spine J 2010;19:370-5.




30. Xiao R, Miller JA, Sabharwal NC, et al. Clinical outcomes following spinal fusion using an intraoperative computed tomographic 3D imaging system. J Neurosurg Spine 2017;26:628-37.


31. Watkins RG, Gupta A, Watkins RG. Cost-effectiveness of image-guided spine surgery. Open Orthop J 2010;4:228-33.




32. Costa F, Tosi G, Attuati L, et al. Radiation exposure in spine surgery using an image-guided system based on intraoperative cone-beam computed tomography: analysis of 107 consecutive cases. J Neurosurg Spine 2016;25:654-9.


33. Mendelsohn D, Strelzow J, Dea N, et al. Patient and surgeon radiation exposure during spinal instrumentation using intraoperative computed tomography-based navigation. Spine J 2016;16:343-54.


34. Tabaraee E, Gibson AG, Karahalios DG, et al. Intraoperative cone beam-computed tomography with navigation (O-ARM) versus conventional fluoroscopy (C-ARM): a cadaveric study comparing accuracy, efficiency, and safety for spinal instrumentation. Spine (Phila Pa 1976) 2013;38:1953-8.

35. Su AW, McIntosh AL, Schueler BA, et al. How does patient radiation exposure compare with low-dose o-arm versus fluoroscopy for pedicle screw placement in idiopathic scoliosis? J Pediatr Orthop 2017;37:171-7.


36. Hoffman DA, Lonstein JE, Morin MM, et al. Breast cancer in women with scoliosis exposed to multiple diagnostic x rays. J Natl Cancer Inst 1989;81:1307-12.


37. Boice JD Jr, Morin MM, Glass AG, et al. Diagnostic x-ray procedures and risk of leukemia, lymphoma, and multiple myeloma. JAMA 1991;265:1290-4.



- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























