 |
 |
- Search
|
|
||
Abstract
NOTES
Fig. 1.

Fig. 2.

Fig. 3.
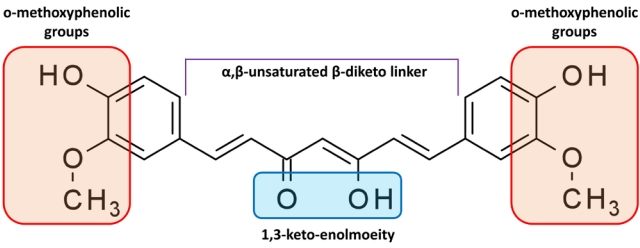
Fig. 4.
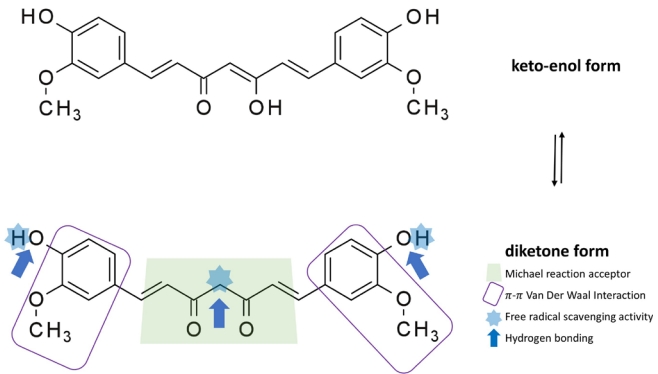
Table 1.
| Study | Specimen/sample size/SCI method | Study design (experimental groups) | Curcumin treatment method | Summary of results |
|---|---|---|---|---|
| Zu et al., [24] 2014 | Male Sprague-Dawleyrats/N = 64/Striking bar falling (diameter: 3 mm) 5-cm height (150 gcf) on T8 level | Sham/DMSO (n = 16) | 40 mg/kg | Curcumin increased gray–white matter interface, tissue edema/AQP-4 expression, and GFAP/pJAK-STAT expression |
| Sham/Curcumin (n = 16) | Single IP injection | |||
| SCI/DMSO (n = 16) | 30 min after SCI | Moderately improved BBB scores | ||
| SCI/Curcumin (n = 16) | ||||
| Wang et al., [59] 2014 | Female BALB/c mice/N = No description/10-g force clip for 3 sec, extradural on T9 level | Sham | 50 mg/kg | Curcumin decreased tissue expression of GFAP and Iba-1 and increased NF-200 |
| SCI/DMSO | Single IP injection | Decreased levels of IL-1β, NO, and NF-κB | ||
| SCI/Curcumin | Immediately after SCI | Increased neuromotor scores (Basso mouse scale) | ||
| Lin et al., [60] 2015 | Wild-type C57BL/6JNarl mice/ N = 18/Weight dropped | Sham control (n = 6) | 40 mg/kg | Curcumin attenuated the downregulation of CISD2 in SCI and LPS-treated astrocytes. |
| Guide was lifted up to 4 mm to perform a hemitransection | SCI (n = 6) | Single IP injection | (CISD2 exerts antiapoptotic and anti-inflammatory effects on neural cells) | |
| SCI+Curcumin (n = 6) | 30 min after SCI | |||
| Yuan et al., [23] 2015 | Female Sprague-Dawley rats/N = No description/Aneurysm clip (fixed force of 50 g) for 60 secon T9 level | Sham | Various dose of curcumin (300, 100, and 30 mg/kg) | Curcumin inhibited the expression of proinflammatory cytokines (TNF-α, IL-1β, and NF-κb) |
| SCI | ||||
| SCI+Curcumin 30 | IP injection once per day for 7 days | Reduced the expression of the intracellular components and GFAP through its anti-inflammatory effects. | ||
| SCI+Curcumin 100 | Suppressed reactive gliosis. | |||
| SCI+Curcumin 300 | Inhibited the generation of TGF-β1, TGF-β2, and SOX-9 | |||
| SCI+Methylprednisolone | Improved BBB scores | |||
| Ni et al., [61] 2015 | Male Sprague-Dawley rats/N = 48/30-g force extradural com- pression with a clip for 30 sec on T8–9 level | Sham (n = 16) | 100 mg/kg | Curcumin modulated the TLR4/NF-κB inflammatory signaling pathway and significantly ameliorated SCI-induced spinal cord edema and apoptosis. |
| SCI (n = 16) | IP injections at 15 min after SCI | |||
| SCI+Curcumin (n = 16) | BBB scores significantly increased | |||
| Yuan et al., [9] 2017 | Female Sprague-Dawley rats/N = 280/50-g force clip compres- sion for 60 sec on T9 level | Sham (n = 70) | 100 mg/kg | Curcumin regulated both the NF-κB and SOX-9 signaling pathways. |
| SCI (n = 70) | IP injections | Downregulated the expression of chemokines, MCP-1, RANTES, and CXCL10, released by astrocytes. | ||
| SCI+Curcumin (n = 70) | Immediately after surgery and once every 24 hr for 7 days | |||
| SCI+DMSO (n = 70) | Decreased macrophage and T-cell infiltration | |||
| Ruzicka et al., [40] 2018 | Wistar rats/N = 135/Balloon-in- duced compression using Fogarty catheter on T8 level | Saline (n = 34) | High dose once a week (60 mg/kg diluted in olive oil) intrathecally 4 times (immediately after SCI followed up for 3subsequent weeks), low-dose IP injection daily (6 mg/kg diluted in olive oil) (immediately after SCI and on the 28th day) | The combined therapy facilitated axonal sprouting and modulated the expression of proregenerative factors and production of inflammatory responses. |
| Curcumin (n = 27) | ||||
| MSC (n = 28) | Both curcumin and curcumin combined with MSC therapy improved BBB score and the combined treatment group showed additional improvement in advanced locomotor performance. | |||
| Curcumin+MSC (n = 26) | ||||
| Ruzicka et al., [62] 2018 | Wistar rats/N=131/Ballooninduced compression using | Behavioral group study: | Curcumin 6 mg/kg, | Curcumin and EGCG alone or in combination increased axonal sprouting, decreased glial scar formation, and altered the levels of macrophage inflammatory protein 1-alpha, interleukin-1β, interleukin-4, and interleukin-6. |
| Saline (n=10) | EGCG 17 mg/kg | |||
| Fogarty catheter for 5 min | Curcumin (n=13) | IP daily | ||
| EGCG (n=19) | Curcumin 60 mg/kg | |||
| Curcumin+EGCG (n=9) | EGCG 17 mg/kg | All treatments displayed significant behavioral recovery (BBB score) with no obvious synergistic effect after the administration of the combined therapy of curcumin and ECGC | ||
| Cytokine group study: | IM weekly for 28 days | |||
| Saline (n=20) | ||||
| Curcumin (n=20) | ||||
| EGCG (n=20) | ||||
| Curcumin+EGCG (n=20) | ||||
| Lee et al., [4] 2019 | Sprague-Dawley rats/N=35/Clip with 30-g force for 2 min | Sham (n=32) | 200 mg/kg/day for 8 weeks, IP | SCI+hyperglycemia+curcumin group: SOD activity increased, malondialdehyde and ED-1 macrophage marker levels decreased, IL-6, IL-8, TNF-α, phosphorylated extracellular signal-regulated kinase, phosphorylated JNK, and phosphorylated p38 levels decreased, |
| SCI only (n=32) | ||||
| SCI+Hyperglycemia (n=32) | ||||
| SCI+Hyperglycemia+Curcumin (n=32) | Better BBB score | |||
| Yardım et al., [63] 2021 | Male Sprague-Dawley rats/N=35/PTX-induced SCI | Control (n=7) | 100 mg/kg or 200 mg/kg | Curcumin reduced mRNA expression levels of NF-κB, TNF-α, IL-6, iNOS, and GFAP and increased the levels of Nrf2, HO-1, and NQO1. Curcumin suppressed the activation of apoptotic and autophagic pathways by increasing Bcl-2 and Bcl-xL and decreasing p53, caspase-3, Apaf-1, LC3A, LC3B, and beclin-1 mRNA expression levels |
| Curcumin (n=7) | Oral daily for 10 days | |||
| PTX (n=7) | ||||
| PTX+Curcumin100 (n=7) | ||||
| PTX+Curcumin200 (n=7) |
SCI, spinal cord injury; DMSO, dimethyl sulfoxide; AQP-4, aquaporin 4; GFAP, glial fibrillary acidic protein; pJAK-STAT, phosphorylated Janus kinase-signal transducer and activator of transcription; BBB, Basso, Beattie, Bresnahan; BALB, Bagg albino; IP, intraperitoneal; NF-200, neurofilament-200; IL, interleukin; NO, nitric oxide; NF-κB, nuclear factor kappa B; LPS, lipopolysaccharide; TNF, tumor necrosis factor; TGF, transforming growth factor; SOX-9, sex-determining region Y-box transcription factor 9; MCP-1, monocyte chemoattractant protein-1; RANTES, regulated upon activation, normal T cell expressed and presumably secreted; CXCL10, C-X-C motif chemokine ligand 10; MSC, mesenchymal stem cells; EGCG, epigallocatechin gallate; IM, intramuscular; iNOS, inducible nitric oxide synthase; Nrf2, nuclear erythroid 2-related factor 2; HO-1, hemeoxygenase 1; NQO1, NAD(P)H:quinone oxidoreductase 1; PTX, paclitaxel; LC3A, light chain 3 A; LC3B, light chain 3 B.
Table 2.
| Study | Specimen/sample size/SCI method | Study design (experimental groups) | Curcumin treatment method | Summary of results |
|---|---|---|---|---|
| Akar et al., [64] 2017 | Wistar rats/N=40/Spinal cord ischemia induced by clamping the aorta | Sham (n=10) | 100 mg/kg, IP at 30 min before ischemia | Decreased MDA levels in the spinal cord |
| Ischemia–reperfusion (n=10) | Increased SOD and GPx levels caused by curcumin | |||
| Curcumin (n=10) | Dissolved in 5 N NaOH | Neurological outcome scores were significantly better when compared with those of the IR group. | ||
| Solvent (n=10) | ||||
| Xi et al., [65] 2019 | Sprague-Dawley rats/N=24/A hammer was dropped on T8 level | Sham/control (n=8) | 80 mg/kg/day, IP | Oxidative stress and apoptosis (caspase-3 activity and B cell lymphoma 2-associated X protein levels) were suppressed |
| SCI (n=8) | Tetrahydrocurcumin for 2 weeks | Tetrahydrocurcumin inhibits oxidative stress response by regulating FOXO4 in SCI model rats. | ||
| Tetrahydrocurcumin treatment (n=8) | Tetrahydrocurcumin increased the BBB scores | |||
| Daverey et al., [10] 2020 | Male Wistar rats/N=18/30-mm spinal cord section/Hypoxia | Sham | One hour incubation with 50 μM curcuminin 95% N2 and 5% CO2 | Curcumin inhibited hypoxia-induced HIF1-α expression and tissue damage by improving the morphology of astrocytes and remarkably reducting vacuolation. |
| Hypoxia | ||||
| Hypoxia+Curcumin | ||||
| Sham | It inhibited the hypoxia-induced upregulation of GFAP and neurofilament-H (NF-H) after hypoxia and downregulated the expression of proinflammatory cytokines such as TNF-α and IL-1. | |||
| Hypoxia | ||||
| Hypoxia+Curcumin | ||||
| Hypoxia+BAY11-7082 | Curcumin exerted its neuroprotective effect through cross-talk between the NF-κB and Nrf2 signaling pathways. | |||
| Daverey and Agraw-al, [66] 2020 | Human astrocytes | Human astrocytes | Human astrocytes: | Riluzole protects white matter injury by the activation of Nrf2/HO-1 and caspase 9. |
| Male Wistar rats/N=21/30-mm spinal cord section/Hypoxia | Sham | Riluzole (1 μM) | ||
| Hypoxia | Curcumin (1 μM) | Curcumin’s neuroprotective effect is mediated through the inhibition of HIF-1α, GFAP, NF-H, and caspase 9. | ||
| Hypoxia+Curcumin | Rat SCI model (white | |||
| Hypoxia+Riluzole | matter injury) | |||
| Hypoxia+Riluzole+Curcumin | Riluzole (10 μM) | Curcumin is more effective than riluzole in reducing GFAP and NF-H injury. | ||
| Curcumin (50 μM) |
SCI, spinal cord injury; IP, intraperitoneal; MDA, Malondialdehyde; SOD, serum superoxide dismutase; IR, ischemia–reperfusion; FOXO4, forkhead box protein O4; BBB, Basso, Beattie, Bresnahan; HIF1-α, Hypoxia inducible factor 1-α; GFAP, glial fibrillary acidic protein; TNF, tumor necrosis factor; IL, interleukin; Nrf2, nuclear erythroid 2-related factor 2; HO-1, hemeoxygenase 1; NF-H, neurofilament protein-H.
Table 3.
| Study | Stem cell types and specimen/SCI method | Study design (experimental groups) | Curcumin treatment method | Summary of results |
|---|---|---|---|---|
| Son et al., [36] 2014 | Neural progenitor cell (NPC) from the spinal cord of Sprague-Dawley rats | Examine cellular proliferation (MTS assay) in control (no curcumin) and curcumin groups at 6 different dose levels | In culture medium at 0.1, 0.5, 1, 10, 20, and 50 μM | Lower dosage (0.1, 0.5, 1 μM) of curcumin increased SC-NPC proliferation. |
| However, higher dosage decreased SC-NPC proliferation. | ||||
| Curcumin stimulates the proliferation of SC-NPCs via the MAP kinase signaling pathway, especially involving the p-ERK and p-38 proteins. | ||||
| Requejo- Aguilar et al., [39] 2017 | Ependymal stem/progenitor cells of the spinal cord (EpSP- Ci) of Sprague-Dawley rats | PA (as vehicle) (n=12) | Intrathecal administration | PA–C enhances neuroprotection, increases axonal growth |
| PA–curcumin–Cy5.5 (n=15) | PA–curcumin–Cy5.5 (10 μM) (combination treatment: ep-SPCs and a pH-responsive polymer–curcumin conjugate) | PA–C can improve functional recovery in acute SCI | ||
| Contusion 250 kdyn | Also enhances functional recovery in a rodent model of chronic SCI. | |||
| Infinite Horizon Impactor | *PA (polyacetal): enhances blood bioavailability and stability and provides a means for highly localized delivery. | |||
| Bang et al., [37] 2018 | Neural stem/progenitor cells de- rived from Sprague-Dawley rats N = 60/Clip with a closing force of 30 g & a 2-min com- pression | Sham (n=20) | Implanting indwelling intrathecal catheters/A concentration of 1 μmol/L for curcumin | SCI-Curcumin group: |
| SCI-Curcumin (n=20) | The co-immunoreactivity of nestin/BrdU was higher | |||
| SCI-Vehicle (n=20) | The GFAP immunoreactivity and lesion cavity was lower | |||
| The BBB score was better (up to 14 days) | ||||
| Wanjiang et al., [41] 2020 | hUC-MSC/Female Sprague- Dawley rats/N = 180/50-g aneurysm clip compression on for 60 sec on T9 level | Sham (n=30) | IP, 100 mg of curcumin, dissolved in 1 mL of DMSO and 0.5 mL of NS | Curcumin suppressed hUC-MSC apoptosis through the ERK1/2 signaling pathway |
| SCI+Veh (n=30) | ||||
| SCI+cur (n=30) | 1st injection: 30 min after the operation | The combination of curcumin and hUC-MSC therapies improved motor function after SCI in rats. | ||
| SCI+hUC-MSC (n=30) | ||||
| SCI+cur+hUC-MSC (n=30) | Once/day for 14 days. | |||
| SCI+cur+hUC-MSC+U0126 (n=30) | ||||
| Bonilla et al., [42] 2021 | Induced pluripotent stem cells (iPSC-NSC) & Human MSC Female Sprague-Dawley rats/200 kdyne contusion on T8 level iPCS-NSC (n = 8) | Control (n=16) | A pH-responsive polyacetal–curcumin nanoconjugate (PA–C) delivery into the intrathecal space in contusive SCI with stem cell transplantation. | PA–C-treated or PA–C and iPSC-NSC + MSC-treated groups: Smaller scars, whereas PA–C and iPSC-NSC + MSC therapy induced the preservation of β-III tubulin-positive axons. |
| MSC (n=11) | ||||
| iPCS-NSC+MSC (n=11) | ||||
| PA-C (n=6) | iPSC-NSC + MSC transplantation fostered the preservation of motoneurons and myelinated tracts, whereas PA–C therapy polarized microglia into an anti-inflammatory phenotype. | |||
| iPSC-NSC+MSC+PA-C (n=7) | ||||
| Elkhenany et al., [67] 2021 | Human induced neural progeni- tor cells (iNPC) Female Sprague-Dawley rats/200 kdyn Infinite Horizon Impactor on T8 level | HA_PM_iNPC (non SCI) (n=3) | PM-embedded curcumin | PM-embedded iNPCs and CURC with PPY fibers supported a significant increase in neuropreservation (as measured by higher βIII tubulin staining of neuronal fibers) and decrease in the injured area (as measured by the lack of GFAP staining). |
| HA_PPY_PM_iNPC (non SCI) (n=3) | ||||
| HA_PM_CURC (n=3) | ||||
| HA_PPY_PM_CURC (n=3)HA_PM_iNPC (n=3) | ||||
| HA_PPY_PM_iNPC (n=3) | *HA: hyaluronic acid | |||
| HA_PM_CURC_iNPC (n=3) | *PM: Corning® PuraMatrixTM peptide hydrogel | |||
| HA_PPY_PM_CURC_iNPC (n=3) | *PPY: polypyrrole-coated fibers |
SCI, spinal cord injury; SC-NPC, spinal cord neural progenitor cell; MAP, mitogen-activated protein; p-ERK, phospho-extracellular signal-regulated kinase; PA, polyacetal; PA-C polyacetal-curcumin; epSPC, ependymal stem/progenitor cells of the spinal cord; GFAP, glial fibrillary acidic protein; BBB, Basso, Beattie, Bresnahan; hUC-MSC, umbilical cord mesenchymal stem cell; IP, intraperitoneal; DMSO, Dimethyl sulfoxide; ERK, extracellular signal-regulated kinase; CURC, curcumin.
Table 4.
| Study | Specimen/sample size/SCI method | Study design (experimental groups) | Curcumin treatment method | Summary of results |
|---|---|---|---|---|
| Kim et al., [3] 2014 | Male Sprague-Dawley rats/N = 36/clipping 30-g force for 2 min on T9 level | Sham (n=12) | 200 mg/kg IP daily for 7 days | Curcumin group: Higher BBB scores 7–14 days after surgery (by antiinflammatory and antioxidant action/ED-1, MDA, and SOD were measured) |
| SCI/vehicle (n=12) | ||||
| SCI/curcumin (n=12) | ||||
| Machova Urdzikova et al., [43] 2015 | Male Wistar rats/N = 60/or balloon compression using Fogarty catheter (2 Fr) on T8 level | Control (n=30) | 60 mg/kg Epidural locally | Curcumin group: Improved behavioral recovery (BBB scores and plantar sensory performance scores) within the first week following SCI (by anti-inflammatory action/NF-κB, MIP1a,IL4, IL1b, IL2, IL6, IL12p70, TNF-α, and RANTES were measured) |
| Curcumin (n=30) | Immediately after injury and 6 mg/kg in olive oil IP daily for 1–28 days | |||
| Liu et al., [44] 2018 | Male Sprague-Dawley rats/N = 60/10-g rod dropped, from 25-mm height on T9–10 level | SCI-Curcumin (n=27) | 200 mg/kg IP daily for 56 days | SCI-Curcumin group: Improvement in the BBB score |
| SCI-MP (n=27) | MP-treated group better within the first 14 days | |||
| Sham group (n=6) | Cur-treated group better from 21–49 days after SCI | |||
| Paralleled BBB scores of the 2 treatment groups on 56 days after SCI (by anti-inflammatory action/Bax, Bcl-2, Caspase-3, and GFAP were measured) | ||||
| Luo et al., [68] 2021 | Female Sprague-Dawley rats/N = 24/2-mm segment of the spinal cord removed at T9 level | Control (n=6). | FC/FI-Cur hydrogel was implanted into the lesion area. | FC/FI-Cur hydrogel group: Significantly promoted BBB walking score (by anti-inflammatory action/immunofluorescence staining of antibodies CD68, S100, neurofilament 200, GFAP, myelin basic protein, etc. were measured) |
| FC hydrogel (n=6) | ||||
| FC/FI hydrogel (n=6) | FC: Fmoc-grafted chitosan | |||
| FC/FI-Cur hydrogel (n=6) | FI: Fmoc peptide |
BBB, Basso, Beattie, Bresnahan; SCI, spinal cord injury; ED-1, CD68/SR-D1 antibody (marker for activated macrophages); MDA, malondialdehyde; SOD, superoxide dismutase; NF-κB, nuclear factor kappa B; MIP1a, macrophage inflammatory protein-1 alpha; IL, interleukin; TNF-α, tumor necrosis factor-alpha; RANTES, regulated upon activation, normal T cell expressed and presumably secreted; MP, methylprednisolone; IP, intraperitoneal; GFAP, glial fibrillary acidic protein.
REFERENCES

- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























