Successful Criteria for Indirect Decompression With Lateral Lumbar Interbody Fusion
Article information
Abstract
Objective
No consensus criteria have been established regarding ideal candidates for indirect decompression with lateral lumbar interbody fusion (LLIF), and contributing factors of indirect decompression failure were rarely reported. We aim to investigate the success rate of indirect decompression by LLIF with proposed selection criteria and identify risk factors associated with indirect decompression failure, defined as persistent pain requiring revision with direct decompression.
Methods
Data from 191 patients undergoing LLIF were retrospectively reviewed. All the following criteria must be fulfilled: (1) dynamic clinical symptoms (pain relief in supine position), (2) presence of reducible disc height (recovered disc height in supine position), (3) no profound weakness, and (4) no static stenosis. The success rate of indirect decompression with LLIF and results after at least 1 year of follow-up were collected. Preoperative, procedure-related, and postoperative factors were assessed for their relationship with failure.
Results
Of 191 patients,13 patients (6.8%) required additional direct decompression due to persistent pain, giving a criteria success rate of 93.2%. Factors associated with indirect decompression failure included low bone mineral density (T-score < 2.1), low reducible disc height (<13%), low postoperative disc height (< 10 mm), high-grade cage subsidence, and use of plate fixation.
Conclusion
We proposed patient selection criteria for indirect decompression with LLIF which had a satisfactory success rate and identified factors associated with the need for additional direct decompression. Our proposed criteria may assist selection of patients likely to achieve good results following indirect decompression with LLIF, and optimize selection based on risk factors of failure.
INTRODUCTION
Degenerative disease of the lumbar spine is one of the most common causes of morbidity in aging societies. Many patients with degenerative lumbar disease, who failed conservative treatment, ultimately required surgery [1,2]. In cases with neural compression and segmental instability, decompression and fusion procedures are usually performed. In recent years, lateral lumbar interbody fusion (LLIF), either the prepsoas (oblique lumbar interbody fusion, OLIF) or transpsoas approach (extreme lateral lumbar interbody fusion, XLIF), has become a popular surgical procedure. Decompression of neural elements is achieved indirectly by restoring disc and foraminal height, unbuckling of the ligamentum flavum, and correction of sagittal and coronal deformities. Many reports have shown favorable results following indirect decompression with LLIF for lumbar degenerative diseases [3-6]. LLIF has many advantages, especially in elderly patients with multiple comorbidities who cannot tolerate a long operative time or significant blood loss, which can occur with traditional open direct decompressive surgery. The LLIF procedure avoids aggressive muscle dissection and preserves the posterior spinal ligament and musculature. However, in some circumstances, the indirect decompression effect is not sufficient to relieve neural compression, and thus necessitates a subsequent revision surgery for direct decompression. Most studies have defined the need for revision with direct decompression as an indirect decompression failure [7-10]. To date, only a few studies with small subjects have used an algorithmic approach to select cases for this procedure [11]. A systematic review by Kirnaz et al. [9] estimated the pooled incidence of indirect decompression failure following LLIF as 9%, but limited studies in the review had clear patient selection criteria, and most did not investigate the causes of failure.
Despite the benefits and clinical success of indirect decompression and fusion with LLIF, no consensus criteria have been established regarding ideal candidates for this procedure, and factors that might predict candidates in whom the procedure is likely to fail. We developed a set of clinical and radiographic criteria for selecting surgical candidates. The objectives of the present study were to (1) determine the success and failure rates in patients who underwent indirect decompression by LLIF using our proposed criteria and (2) evaluate risk factors resulting in failure (persistent symptoms requiring subsequent direct decompression at the index level that occurred within 6 months following the LLIF procedure).
MATERIALS AND METHODS
1. Study Design, Patient Sample, and Selection
We conducted a retrospective analysis of consecutive patients who underwent indirect decompression with LLIF (XLIF or OLIF) at our hospital between April 2014 and June 2020. Patients with lumbar degenerative diseases undergoing 1–2 levels of indirect decompression with LLIF were eligible for inclusion if they met all of the following criteria: (1) dynamic clinical symptoms, defined as pain developing when standing or walking, but subsiding by > 50% when resting in a supine position; (2) no profound weakness, defined as having motor power less than grade IV; (3) reducible disc height, defined as the presence of a recovered disc height at least 1 mm when changing from upright to supine positions; and (4) no static stenosis such as facet cysts or bony lateral recess (evaluated with magnetic resonance imaging [MRI] and computed tomography [CT], respectively). Patients who were preoperatively planned for combined direct and indirect decompression and patients with incomplete 1-year follow-up data were excluded. The participants were classified into 2 groups based on the outcome of symptom resolution postoperatively: success, and failure group which was defined by persistent pain requiring reoperation for direct decompression within 6 months after LLIF procedure. This study was approved by the Institutional Review Board of Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital (approval #729/63) and written informed consent was obtained from all patients. The research was conducted according to the World Medical Association Declaration of Helsinki.
2. Surgical Technique
All surgeries were performed at a single institution by 1 of 3 senior spine surgeons. The LLIF procedure was performed in the right lateral decubitus or prone position. Intraoperative neurophysiological monitoring was performed in all patients with XLIF. The skin incision directed to the operated level was determined using O-arm navigation or fluoroscopy. Muscle dissection was performed layer by layer to identify the corridor for LLIF using transpsoas for XLIF or the prepsoas approach for OLIF. The dilator and tubular retractor were used to access the intervertebral disc index. Annulotomy, discectomy, and cartilaginous endplate removal were performed. The trial cage for XLIF (NuVasive Inc., San Diego, CA, USA) or OLIF (Clydesdale Spinal System, Medtronic, Minneapolis, MN, USA) was inserted using an orthogonal maneuver. After the appropriate size was checked under the fluoroscope, a cage filled with bone graft or bone substitute was implanted. The instrumentation was performed in most cases using selected fixation, such as percutaneous posterior pedicular screw-rod fixation or lateral fixation with a plate or screw-rod system. Otherwise, standalone LLIF was selected in some cases. The implant position was confirmed using O-arm navigation or fluoroscopy before wound closure. All patients were treated according to the same pain control protocol and ambulated the day after the operation. If no complications occurred, they were discharged approximately 3 days postoperatively.
3. Data Collection and Outcome Measurement
Postoperative data were retrospectively collected from the electronic medical records and radiographs of consecutive patients treated with XLIF (NuVasive Inc.) or OLIF (Medtronic OLIF25) according to the study inclusion and exclusion criteria.
Patients were scheduled for follow-up examinations at 1, 3, 6, 12, and 24 months postoperatively for clinical and radiographic assessments. Demographic clinical data retrieved included age, sex, bone mineral density (BMD), body mass index, diagnosis, number of operated levels, smoking status, history of previous lumbar surgery, comorbidities, length of follow-up period, preoperative Oswestry Disability Index (ODI), visual analogue score of leg (VASL), and back pain (VASB). The operative data collected included LLIF type, cage size, lordotic angle, cage position, type of fixation, and use of biologics. The preoperative and postoperative radiographic parameters were recorded, including reducible disc height (percentage of disc height discrepancy between supine and standing position) (Fig. 1), disc and foraminal height, lordotic angle, spinal canal diameter, and cage subsidence. Disc height was calculated as the average of the sum of the anterior and posterior disc heights. The lordotic angle was measured as the angle between the upper endplate of the upper vertebra and the lower endplate of the lower vertebrae of the fusion level. Bony fusion was evaluated with CT scan and defined as presence of continuous bony bridge connecting 2 vertebrae [12,13]. Cage subsidence grading was measured by the percentage of endplate collapse as described by Marchi et al. [14] which was classified as low-grade (grade 0: 0%–24% and grade I: 25%–50%) and high-grade subsidence (grade II: 51%–74% and grade III: 75%–100%).
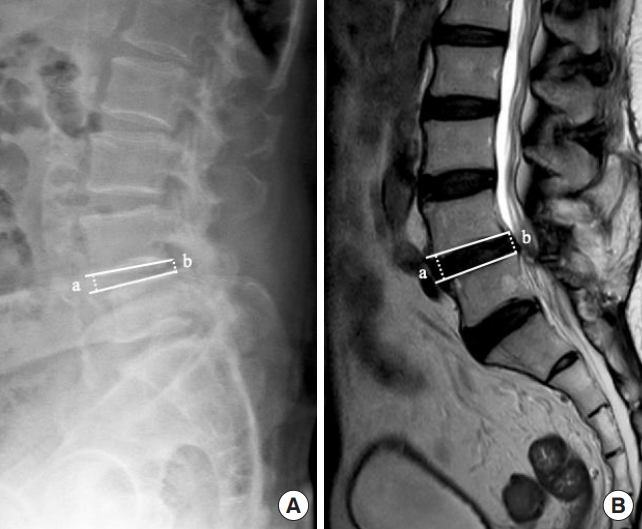
Reducible disc height, defined as presence of disc height discrepancy between standing (A) and supine position (B) in a case with spondylolisthesis L4–5. The narrow L4–5 intervertebral disc space in a standing plain radiograph was restored in the supine position, as evidenced in the magnetic resonance imaging. The disc height is calculated as the mean of anterior and posterior disc height = (a+b)/2.
Functional outcomes were assessed postoperatively in the form of the ODI, VAS of back and leg pain, and the satisfaction score at each follow-up. The radiographic outcome measures, including disc height, spinal canal parameters, and segmental lordosis, were evaluated. Bony fusion and postoperative complications, such as cage subsidence, migration, infection, or pseudarthrosis, were also evaluated by CT scan and MRI. Examples of success and failure cases of indirect decompression were shown in Figs. 2, 3.
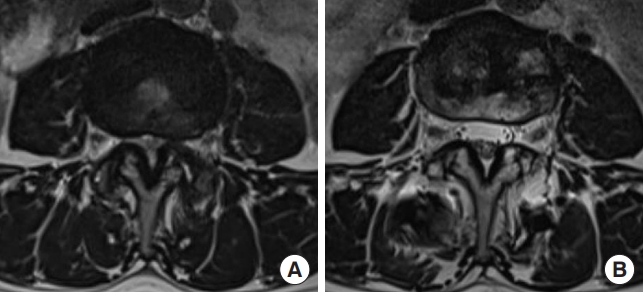
Preoperative (A) and postoperative (B) T2-axial magnetic resonance images showing an indirect decompression effect in a case with successful indirect decompression with lateral lumbar interbody fusion.
4. Statistical Analysis
Demographic and disease-related characteristics were described in the patients overall and in the failure group. Formal comparisons between continuous parameters fixed within patients were made using the Wilcoxon rank-sum test for continuous variables and Fisher exact test for categorical variables. Comparisons of variables that differed by level in patients with procedures at 2 levels were assessed using generalized estimating equations (GEE). Receiver-operating curve (ROC) analysis was used to identify thresholds for preoperative and postoperative radiographic parameters with an increased risk of failure, and cutoff values were determined using the point that maximized the sensitivity and specificity (Youden index) [15]. GEE with a binomial family, logit link, exchangeable correlation matrix, and robust variance estimates were used to account for the within-patient correlation when assessing associations between demographic, operative, and pre- and postoperative radiographic characteristics, and the risk of failure. Factors with p<0.1, in univariate models, were adjusted for in a multivariable model. The probabilities predicted by this model were calculated assuming equal numbers of observations for each categorical term in the model. Decisions regarding statistical and clinical significance were made on the basis of p-values and 95% CI [16]. Statistical analysis was conducted using Stata 17.0 (Statacorp, College Station, TX, USA).
RESULTS
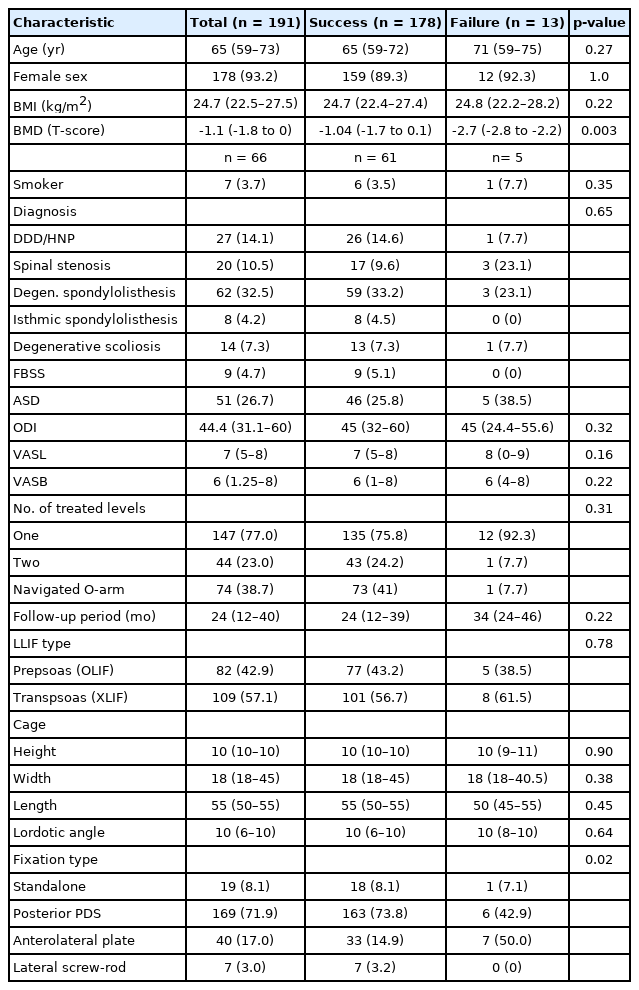
Patient demographic data and clinical and procedure-related characteristics are summarized in Table 1. Of the 217 eligible patients who underwent LLIF procedures, a total of 191 (235 fusion levels) had all required clinical and radiographic data and returned for scheduled follow-up visits in the first 12 months with a median (IQR) follow-up of 24 months (12–40 months). Of the 191 patients, 147 underwent fusion at one level and 44 underwent fusion at 2 contiguous levels. Thirteen patients (6.8%) experienced unsuccessful indirect decompression at any level, giving an overall success rate of 93.2% (178 of 191). Demographic and baseline characteristics were similar between the groups. The overall median (IQR) age was 65 years (59–73 years), with a female predominance (93%, 178 of 191). The most common diagnoses were degenerative spondylolisthesis, followed by adjacent segment disease, degenerative disc disease, or herniated nucleus pulposus in 32.5%, 26.7%, and 14.1%, respectively. The most commonly operated level was L4–5 (56%). Patients in the failure group had a fixation type of anterolateral plate more than those in the success group, whereas other procedure-related characteristics did not differ between the groups, including the number of treated levels, different fusion segments, different approach directions (either OLIF or XLIF), cage profile, use of O-arm navigator, and follow-up period. In 67 patients who had a BMD available, the median (IQR) BMD was significantly lower in the failure group: -2.7 (-2.8 to -2.2) versus -1.0 (-1.7 to 0.1) respectively; p<0.001.
All patient-reported outcomes, including ODI, VASL, and VASB score following surgery in the success group showed statistically significant improvements compared to the failure group at all follow-up assessments. The average ODI changes in the success group and the failure group were -29.3±17.2 and 1.4±9.6, respectively. The VASL changes were -4.6± 3.5 and -1.7± 2 while the VASB changes were -4.6± 3.5 and -1.5± 2.3, respectively. The median time to reoperation with additional direct decompression in the failure group was 4.5 months (range, 2.5–6.0 months).
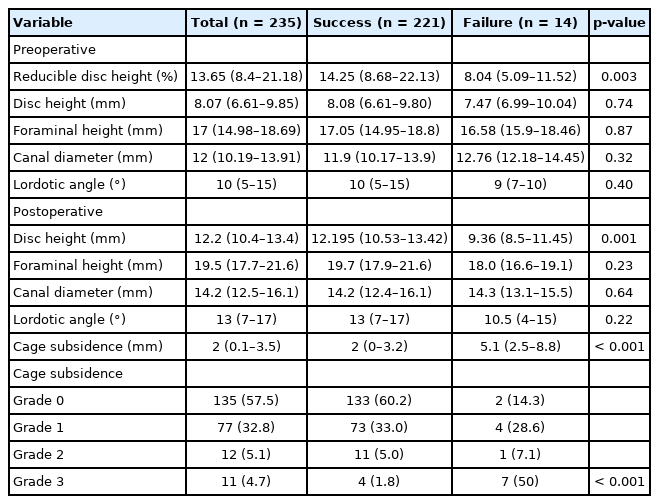
The preoperative and postoperative radiographic factors are shown in Table 2. Reducible disc height was the only preoperative parameter that differed significantly between the success and failure groups, with a median (IQR) of 14.3% (8.7%–22.1%) and 8.0% (5.1%–11.6%), respectively (p<0.001). Postoperative radiographic factors that differed significantly between the outcome groups were postoperative disc height and cage subsidence. Postoperative disc height was higher in the success group than the failure group at a median (IQR) of 12.2 mm (10.5–13.4 mm) and 9.4 mm (8.5–11.5 mm), respectively. Likewise, cage subsidence grading was also higher in the success group at median (IQR) of 2.0 (0–3.2) and 5.1 (2.5–8.8), respectively. Overall mean fusion rate of LLIF in this study was 93.2%, which was not significantly different between both groups. The fusion rate also did not differ by the number of fused levels.
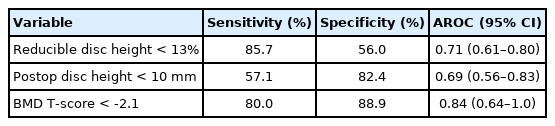
In our ROC curve analysis, we assessed cutoff thresholds that maximized the ability of the continuous parameters to discriminate patients with failure. The cutoffs were BMD T-score < -2.1 (AROC= 0.84; 95% CI, 0.64–1.0), reducible disc height < 13% 0.71 (0.61–0.80), and postoperative disc height ≤ 10 mm 0.69 (95% CI, 0.56–0.83). The sensitivity, specificity, and AROC of these parameters at their cutoff points are shown in Table 3. Five factors showed an association with failure in the univariate analysis (Table 4). Of these, BMD assessments were only conducted in 35% of the study participants, therefore we were unable to adjust for this variable in our multivariable model. In the model that adjusted for the remaining factors, 3 factors were independently associated with a significantly increased risk of failure. These were high-grade subsidence (OR, 13.9; 95% CI, 3.4–56.0; p<0.001), reducible disc height < 13% (OR, 18.9; 95% CI, 3.8–93.9; p<0.001), and postoperative disc height ≤ 10 mm (OR, 3.6; 95% CI, 0.98–12.9; p=0.054). In those with none of these risk factors, the predicted failure probability (95% CI) was 0.3 (0%–0.8%). The combination of high-grade subsidence, postoperative disc height ≤ 10 mm, and reducible disc height < 13% resulted in a predicted failure probability of 71.7% (44.9%–98.5%).
DISCUSSION
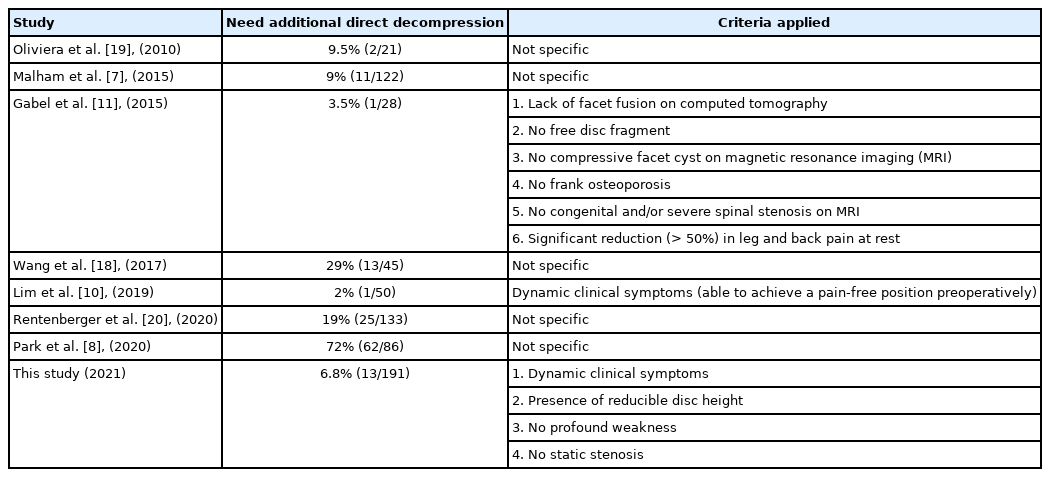
In this study, we reported the rate of indirect decompression failure after applying our selection criteria to be as low as 6.8% which was relatively lower than those reported in previous studies [8,9,17-19]. All criteria were made of patient characteristics which all of them were nonmodifiable patient factors, so we believed that these factors would comprise a practical prerequisite for the procedure. While the analyzed risk factors leading to failure were modifiable factors either preoperatively or intraoperatively. These factors should be adjusted to optimize the outcomes. The identified risk factors leading to indirect decompression failure in this study included low BMD, low preoperative reducible disc height, low postoperative disc height, fixation with anterolateral plate and high-grade subsidence. The reported rate of failure in a study by Oliveira et al. [19] showed that 9.5% of patients had insufficient relief of nerve compression symptoms and required additional direct posterior decompression. The causes of failure were identified to be cage subsidence, loss of sagittal alignment correction, and persistent central and foraminal stenosis. Rentenberger et al. [20] reported an 18.8% surgical revision rate due to neurological symptoms, pain, or radiculopathy, while other studies have reported significantly higher failure rates. The rate of additional posterior decompression after XLIF was 60% in a study reported by Kim et al. [21], and Park et al. [8] reported that the need for direct decompression and instrumentation in a second operation was as high as 72.1% in patients with leg pain that improved ≤ 50% after the index procedure.
Although many published studies have reported indirect decompression failure following LLIF, only a few have provided clear guidance for selecting appropriate candidates to undergo indirect decompression with LLIF. Lim et al. [10] proposed the prerequisite of preoperative postural pain status to guide patient selection for indirect decompression with XLIF. The ability to achieve a pain-free position, such as sitting or lying, was a clinical predictor of successful XLIF for patients with lumbar spinal stenosis. An algorithmic approach to predict success of indirect decompression with LLIF was suggested by Gabel et al. [11] in a case series of 28 patients. Patients who achieved pain relief at rest and lacked facet fusion, free disc fragments, facet cysts, osteoporosis, and severe spinal stenosis were unlikely to require revision surgery for direct decompression.
In this study, we proposed criteria for selecting surgical candidates for indirect decompression with LLIF. Each criterion had a significant impact on the results of the procedure; therefore, all criteria must be met for patients to be eligible for the surgery. First, we defined dynamic clinical symptoms as the ability to achieve pain relief of more than 50% when resting in a supine position compared with standing or walking. This definition was referenced in the study by Gabel et al. [11] who also proposed the same criterion. As mentioned earlier, the reduction of preoperative pain when resting in a supine position is one of the clinical predictors of successful LLIF without direct decompression in patients with lumbar spinal stenosis [10]. Significant pain relief could imply that the dynamic disc distraction and ligamentotaxis effects from LLIF resulted in an increased interlaminar space and that unbuckling of the ligamentum flavum was effective and adequate. Conversely, persistent pain despite resting in a supine position suggests the presence of severe spinal canal stenosis with significant static nerve compression that would only be sufficiently relieved with a direct decompression [10]. Moreover, even the direct decompressive laminectomy was also reported to result in a significantly better pain relief in patients with dynamic clinical symptoms versus those with constant pain not improved with posture [22]. Second, patients must have no significant weakness, which is defined as having motor strength less than grade IV; the greater severity of weakness correlates with a higher degree of neural compression that may not be sufficiently relieved by indirect decompression alone. The third criterion was the presence of a disc height discrepancy ≥ 1 mm between supine and upright positions. The ability to restore the disc height when lying in a supine position indicates the flexibility of the affected segment. Thus, we hypothesized that patients with intervertebral disc structures with less stiffness are more likely to obtain more disc height from the LLIF interbody cage, leading to an increased indirect decompression effect. Similarly, Choi et al. [23] described the discrepancy of the disc height on postural change. This phenomenon may suggest the advanced desiccation and vertical instability of the segment, which results in poor outcomes following surgical decompression alone. This sign was also described as an effective screening method for discogenic back pain in patients with lumbar disc degeneration [24]. The last criterion was that spinal canal stenosis must not be caused by facet cyst or bony lateral recess, evaluated with MRI and CT [2,25], as these static lesions significantly decrease the indirect decompression effect from the ligamentotaxis mechanism and have been proven to contribute to failure of indirect decompression alone [2,18,25].
With all the criteria applied, the success rate of indirect decompression with LLIF was 93.2%. To the best of our knowledge, when compared with previous literature, this study included more subjects and also demonstrated a relatively high success rate with significant improvement of clinical and radiographic outcomes (Table 5). Our proposed criteria were developed because of their theoretical advantages that would contribute to satisfactory results, and each was supported by evidence from previous studies. The suggested criteria consisted of a combination of clinical and radiographic considerations. Moreover, each criterion had a clear and strict definition that was simple to apply in clinical practice.
All of the patients in the failure group had revision surgery with posterior direct decompression. Intraoperative findings revealed significant remaining neural compression especially from the buckling ligamentum flavum, that were correlated with the postoperative MRI. Following the direct decompression, the pain was significantly relieved in all patients. Thus, we concluded that poor outcomes among these patients may contribute from the failed indirect decompression. To avoid revision surgery, many studies have identified factors contributing to the failure of indirect decompression with LLIF. Wang et al. [18] studied the preoperative radiographic factors of failed indirect decompression via XLIF and concluded that bony lateral recess stenosis is a significant factor resulting in failure to achieve adequate decompression via XLIF. Oliveira et al. [19] reported multiple factors including the presence of congenital stenosis or short pedicles, uncontained disc fragments, locked facets with calcified discs, the presence of posterior endplate osteophytes compromising the lateral recess, and synovial cysts and radiculopathy unimproved with flexion posture.
We also analyzed risk factors of indirect decompression failure, leading to reoperation for direct decompression. A low postoperative disc height, especially less than 10 mm, was found to be associated with failure. This finding is in accordance with the study by Park et al. [8] which found that the subjects who needed direct decompression following LLIF had mean postoperative disc height of 9.4 mm. The degree of postoperative disc height had positive effects on the increased foraminal height and ligamentotaxis that indirectly decompressed the neural elements.
Although OLIF and XLIF had different approach directions that made the cage position slightly different, our study did not find any significant effect on postoperative clinical outcomes following both approaches. The number of operated levels, different fusion segments, and cage characteristics were also not the contributing factors to failure. These findings were consistent with previous literature [8,9,26].
The degree of reducible disc height, defined as the preoperative disc height discrepancy between the standing and supine positions, also affected the surgical outcome. A higher disc height obtained in the supine position was associated with successful results. This could be explained by the lower segmental stiffness, resulting in a greater postoperative disc height and more indirect decompression effect. Moreover, if the segment is too rigid to be restored, the risk of subsidence increases. Our results showed that when the restored disc height in the supine position was less than 13%, the risk of failure increased significantly.
Although not an independent predictor of failure, our study found an increased risk of failure when supplementary fixation was achieved using anterolateral plates after adjusting for other covariates. Previous biomechanical studies also showed that this type of fixation may not be strong enough, thus leading to an increased risk of subsidence as a consequence of high cage and endplate stress. The standalone LLIF construct has also been reported to be at a higher risk of subsidence and failure; however, the number of patients with this type of construct in our study was low. Therefore, the failure rate of this construct may be underrecognized [27-29]. According to our results and previously published data, we note that both standalone LLIF and anterolateral plate fixation are at high risk of failure and recommend their use with caution, especially in patients with osteoporosis or significant instability.
Our study’s overall fusion rate of LLIF was as high as 93.2%, regardless of the number of fused levels, and did not significantly differ between both groups. Despite using bone substitutes, the fusion rate of LLIF in our study was comparable to those reported in previous studies and also not different from posterior fusion using autologous bone graft [12,30,31]. The benefits of LLIF in terms of bony fusion included the larger cage containing more bone grafts, and the ability to be placed to cover dense apophyseal rings bilaterally for structural support [30].
Cage subsidence is generally known as a major cause of indirect decompression failure as it directly lessens the decompression effect after LLIF, resulting in revision surgery [32-34]. This is supported by our results that high-grade subsidence (grades II–III) was associated with failure. Therefore, intraoperative endplate injury should be avoided, especially in patients with other known risk factors of subsidence, such as advanced age, osteoporosis, and specific endplate morphology [14,35].
There are some limitations to our study. The retrospective design was subject to selection bias as well as incomplete data; however, we attempted to minimize these factors by enrolling all eligible patients and using electronic medical records. The BMD as an important risk factor for failure was only available in approximately one-third of patients, so assessing how other factors might be influenced after adjustment for BMD was not possible. Additionally, because of the small number of revision surgeries, the power to ascertain a potential association of factors that might be associated with a small to moderate increased risk of failure was reduced. As etiologies of indirect decompression failure were multifactorial, there were other factors that potentially contributed to failure in LLIF. For example, the preoperative shape of spinal canal stenosis could potentially affect postoperative clinical and radiological results following indirect decompression with LLIF. However, previous studies failed to reach conclusion on the correlation between shape of spinal canal and outcomes after decompression [36,37]. Moreover, the shape of the spinal canal was difficult to determined and classified into groups to be analyzed. Thus, a further study focusing on types of spinal canal morphology and indirect decompression effect was required. The strengths of our study include the large number of patients, all operated on at a single center, and identical standard operating procedures and similar standards of care to all patients. Nevertheless, further studies with larger sample sizes are necessary to confirm our findings and identify additional significant risk factors for failure. Moreover, applying our proposed criteria for patient selection in posterior fusion with direct decompression surgery would be an interesting further study.
CONCLUSION
Our study presents the patient selection criteria for indirect decompression with LLIF, which resulted in a satisfactory success rate. Risk factors for reoperation with direct decompression included low BMD, low preoperative disc height discrepancy between standing and supine position, use of supplementary fixation with anterolateral plate, low postoperative disc height, and high-grade cage subsidence. Our proposed criteria and reported risk factors may provide guidance for spine surgeons to select appropriate patients who could achieve good results following indirect decompression with LLIF and optimize patient selection based on modifiable risk factors resulting in failure.
Notes
Conflict of Interest
WL and WS have received speaker and consultant honoraria from Medtronic company. Other authors declare they have no financial interests. The other authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: WY, KJ, WL, WS; Data curation: VK; Formal analysis: SK; Methodology: WY, WL; Project administration: WS; Visualization: WY, KJ; Writing - original draft: KJ; Writing - review & editing: WY, VK, WS.