Learning Curve and Complications of Unilateral Biportal Endoscopy: Cumulative Sum and Risk-Adjusted Cumulative Sum Analysis
Article information
Abstract
Objective
The purpose of this study was to investigate the learning curve and complications of unilateral biportal endoscopy (UBE) in the treatment of lumbar disc herniation (LDH) and lumbar spinal stenosis (LSS).
Methods
This was a retrospective cohort analysis of 197 consecutive patients who received UBE unilateral laminotomy bilateral decompression (UBE-ULBD) or lumbar discectomy (UBE-LD) surgery, including 107 males and 90 females with an average age of 64.83±14.29 years. Cumulative sum (CUSUM) and risk-adjusted cumulative sum analysis (RA-CUSUM) were used to evaluate the learning curve, with the occurrence of complications defined as surgical failure, and variables of different phase of the learning curve were compared.
Results
The cutoff point of learning curve of UBE surgery was 54 cases according to CUSUM analysis. The learning curve of UBE-ULBD and UBE-LD were divided into 3 phases. The first cutoff points were 31 and 12 cases, and the second cutoff point were 67 and 32 cases respectively. With the progress of the learning curve, the operation time and postoperative hospital stays decreased. The visual analogue scale and Oswestry Disability Index at the last follow-up were significantly lower than that before surgery. The incidence of surgical failure was 6.11% and began to decrease after the 89th case based on RA-CUSUM analysis. The surgical failure rate decreased from 10.11% to 2.78 after the 89th case with significant different.
Conclusion
UBE surgery is effective in the treatment of LDH and LSS with low incidence of complications. But a learning curve of at least 54 cases still required for mastering UBE surgery.
INTRODUCTION
With increasing age, the incidence of lumbar disc herniation (LDH) and lumbar spinal stenosis (LSS) gradually increases. The herniated intervertebral disc, hyperplastic facet joint, thickened ligamentum flavum (LF) and lamina lead to a reduction in spinal canal volume and compression of the central spinal canal, lateral recess or foramen, causing symptoms such as intermittent claudication, low back pain and lower limb pain. LDH and LSS are the most common indications for lumbar surgery [1,2]. Although traditional open surgery has been proven to be effective, it still has some disadvantages, such as large tissue injury, poor postoperative spinal stability, and many complications [3]. Microscopy and full-endoscopy have the advantages of less trauma and quick recovery after surgery, but due to the influence of the working cannula, the range of movement of the instrument is limited, so excessive facet joint resection may be needed for sufficient decompression, which may affect the stability of the spine after operation [4,5].
Recently, the unilateral biportal endoscopy (UBE) technique was used for the treatment of LDH and LSS, and several articles have reported satisfactory effect of UBE surgery [5-8]. As an emerging technology, the safety and learning curve of UBE have also received widespread attention. Surgeons hope to master the skills urgently and require advices and references, but there are still few studies on the learning curve of UBE surgery so far.
The purpose of this study was to analyze the learning curve of UBE surgery through cumulative sum (CUSUM) analysis based on operation time and risk-adjusted cumulative sum (RA-CUSUM) analysis based on surgical failure rate [9,10]. To the best of our knowledge, there has been no research on using CUSUM and RA-CUSUM analysis to determine the learning curve of UBE until now.
MATERIALS AND METHODS
1. Patient Selection
Consecutive patients who underwent UBE surgery in the Department of Orthopedics, Hangzhou Hospital of Traditional Chinese Medicine from December 2019 to December 2020 were analyzed retrospectively. The operation methods included unilateral laminotomy bilateral decompression (UBE-ULBD) and lumbar discectomy (UBE-LD). All operations were performed by the same surgeon who had extensive experience in percutaneous endoscopic lumbar discectomy (PELD) but had never performed arthroscopic surgery.
The institutional review committee of Hangzhou Hospital of Traditional Chinese Medicine (No. 2022KY087) approved the study. This study was not considered to require informed consent. There was no treatment other than that routinely implemented during hospitalization, as well as no additional risk for the patients involved.
Inclusion criteria: (1) Patients presented with low back pain (visual analogue scale [VAS]≥ 6), with or without lower limb radiation pain or intermittent claudication (walking distance≤ 100 m). (2) Magnetic resonance imaging (MRI) showed stenosis of the central spinal canal, lateral recess or nerve root canal. (3) Systematic conservative treatment for more than 3 months was unsuccessful. (4) Patients undergoing UBE-ULBD or UBE-LD surgery performed by the same surgeon.
Exclusion criteria: (1) More than 2 surgical levels. (2) Lumbar spondylolisthesis greater than grade Ⅰ (Meyerding grade). (3) Lumbar scoliosis (Cobb angle> 20°). (4) Patients with a history of lumbar spinal canal decompression surgery or lumbar interbody fusion surgery at the same level. (5) Patients with spinal infection, tumor, tuberculosis.
2. Surgical Procedure
The patient was placed in a prone position under general anesthesia with the abdomen suspended. The midline, horizontal line of the intervertebral space and surface projection of pedicles were identified on the anteroposterior (AP) view of the fluoroscope. By adjusting the operating table, the horizontal line of the intervertebral space of the targeted level was ensured to be perpendicular to the ground on lateral view of the fluoroscope.
Taking the left approach as an example, the viewing portal was located on the cranial side and the working portal on the caudal side. The left-side approach was easier to perform given that most surgeons had the dominant hand on the right side. Two 1-cm incisions were made 1.5 cm above and below the horizontal line of the intervertebral space of the ipsilateral pedicular medial line (Fig. 1A). Deep fascia was incised perpendicular to the skin incision. The saline was suspended at a height of approximately 70–100 cm from the incision and connected to a 30° arthroscope.
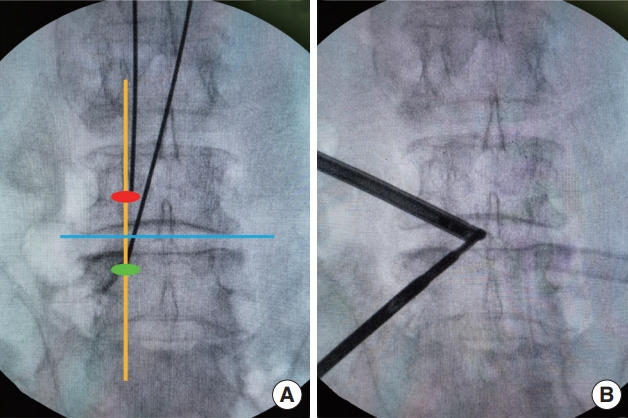
Schematic of the incision design (A) and intersection of the 2 portals (B). Blue line: horizontal line of the intervertebral space. Yellow line: ipsilateral pedicular medial line. Red oval: viewing portal. Green oval: working portal.
Multifidus muscles were dissected from the spinous process (SP) and the lamina space to form a primary workspace. The tubular dilators were inserted to expand the portals until the tips met at the junction between the base of the SP and the lamina on AP view, and were replaced by the endoscope and instrument subsequently (Fig. 1B). The paraspinal soft tissues were cleared with a radiofrequency probe (BONSS, Jiangsu, China), and the medial inferior articular process, superior margin of inferior lamina and SP were exposed.
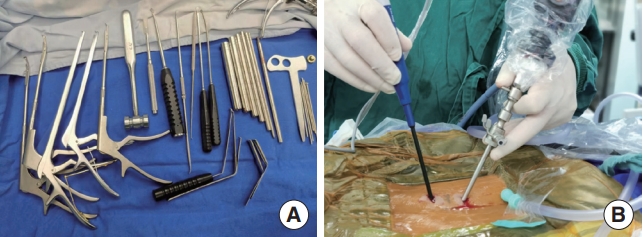
Laminectomy was performed with a burr until reaching the insertion of the LF. The LF was carefully dissected and completely resected in pieces. In UBE-LD surgery, the axillary and shoulder areas of the traversing nerve root were explored to confirm the position of the herniated disc. The annulus fibrosus was incised by a radiofrequency probe or scalpel, and the nucleus pulposus was removed by pituitary forceps. In UBE-ULBD surgery, the lateral recess was decompressed with a straight Kerrison punch, and the range of decompression reached the inner wall of the pedicle. Then, the base of the SP was partly removed with the osteotome. Decompression of the contralateral spinal canal and lateral recess was performed using the curved Kerrison punch, with the inner wall of the contralateral pedicle as a reference. Finally, the contralateral LF was removed. The radiofrequency probe was used for hemostasis after confirming complete decompression. Then, the incision was closed, and a drain was placed. The instruments and schematic of UBE surgery was showed in Fig. 2.
3. Data Collection and Analysis
1) Data collection
Basic information from all patients was collected, including age, sex, type of stenosis, surgical segment, body mass index (BMI), and hypertension. The operation time, estimated blood loss (EBL), complications and postoperative hospital stays were recorded after the operation. The operation time was calculated from the beginning of anesthesia to the closure of the incision. Since UBE surgery was performed under continuous fluid irrigation, and saline permeates into the soft tissue, the amount of bleeding was estimated by the surgeon. The VAS was used to evaluate the degree of low back pain and leg pain, and the Oswestry Disability Index (ODI) was used to evaluate limb function. Records were made before the operation, 1 month after the operation and at the last follow-up.
2) CUSUM
The learning curve based on operation time was calculated by CUSUM analysis. The formula was defined as: CUSUM=
3) RA-CUSUM
In this study, surgical failure was defined as occurrence of complications, including nucleus pulposus residue, dural tear, epidural hematoma, nerve root injury, and infection. Univariate bivariate logistic regression was used to analyze potential risk factors, such as sex, age, surgical segment, EBL, BMI, hypertension and operation time. The variables with p<0.05 were included in the multivariate logistic regression model to predict the probability of surgical failure in each case. The scatter diagram of RA-CUSUM was drawn according to the following formula:
4. Statistical Analysis
Continuous variables with a normal distribution are expressed as the mean±standard deviation. A t-test was used to compare the 2 groups of variables. One-way analysis of variance (ANOVA) and repeated measures ANOVA followed by the least significant difference test was used to compare multiple groups of variables. Continuous variables with a skewed distribution are expressed as the median (interquartile range, IQR), and the rank sum test was used for comparisons between groups. The classified variables were expressed as percentages, and comparisons between groups were performed by the chi-square test followed by Bonferroni correction. A p-value of < 0.05 was considered statistically significant.
RESULTS
1. Characteristics of Patients
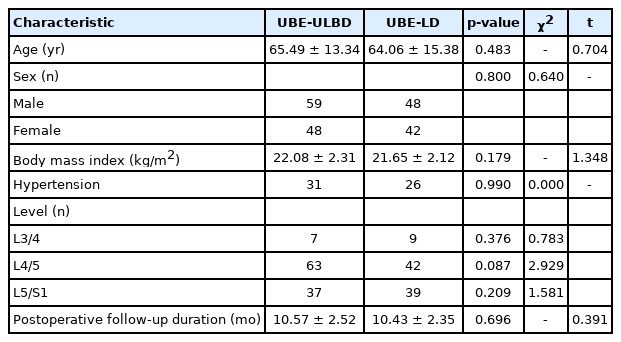
Table 1 shows the detailed baseline characteristics of all patients. A total of 197 consecutive patients who underwent single-segment UBE surgery were enrolled in this study, including 90 cases of UBE-LD and 107 cases of UBE-ULBD. The average follow-up time was 10.51±2.44 months (8–20 months). There were 107 males and 90 females, including 57 patients with hypertension, with an average age of 64.83±14.29 years (34–91 years) and an average BMI of 21.89±2.23 kg/m2. The operative segments included 16 cases of L3/4, 115 cases of L4/5 and 66 cases of L5/S1. The statistic differences of baseline characteristics between UBE-LD and UBE-ULBD groups were not noticed.
2. Surgical Outcomes
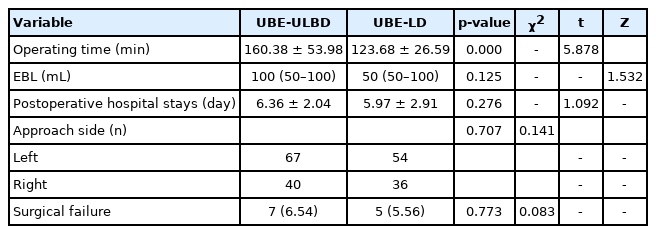
The average operation time was 143.61±47.25 minutes (Table 2), and the operation time of UBE-ULBD was longer than that of UBE-LD (160.38±53.98 minutes vs. 123.68±26.59 minutes, p<0.05). The median EBL was 100 mL with an IQR of 50. The average postoperative hospital stays were 6.18±2.47 days (2–17 days). The VAS and ODI scores at the last follow-up were significantly improved compared with those before the operation (p<0.05) (Table 3, Fig. 3).

The visual analogue scale (VAS) and Oswestry Disability Index (ODI) at each point in time. UBE-ULBD, unilateral biportal endoscopy unilateral laminotomy bilateral decompression; UBE-LD, unilateral biportal endoscopy lumbar discectomy.
In our study, a total of 12 cases were regarded as surgical failure because of complications (Table 4), including residual nucleus pulposus (3 cases, 1.52%), dural tear (4 cases, 2.03%), epidural hematoma (2 cases, 1.02%), and nerve root injury (3 cases, 1.52%). UBE-ULBD was considered failed in 7 cases (6.54%) and UBE-LD in 5 cases (5.56%), and the difference was not statistically significant (p=0.773).
Residual nucleus pulposus was found in 3 patients with highly migrated LDH in early phase (3rd, 13th, 68th), all of which were reoperated by PELD surgery and satisfactory results were obtained. Four patients with dural tears were fixed with gelatin sponges during the operation, and no cerebrospinal fluid leakage, lumbar pseudomeningoceles or meningitis was observed after the operation. Among them, 1 patient developed irritability, increased heart rate, hyperextension of both lower limbs and hypertonia in the recovery of general anesthesia, which relieved spontaneously after 2 hours. A case of epidural hematoma suddenly developed radiation pain in the right lower limb on the third day after the operation, and the symptoms were relieved immediately after the hematoma clearance operation (Fig. 4). The other patient did not present any clinical symptoms, and epidural hematoma was found only on MRI after the operation. Among the patients with nerve root injury, 2 presented abnormal skin sensation in the nerve control area of the lower extremities, which recovered after conservative treatment, and 1 presented a transient decrease in extensor muscle strength of the dorsalis pedis, which gradually recovered to normal during the follow-up.
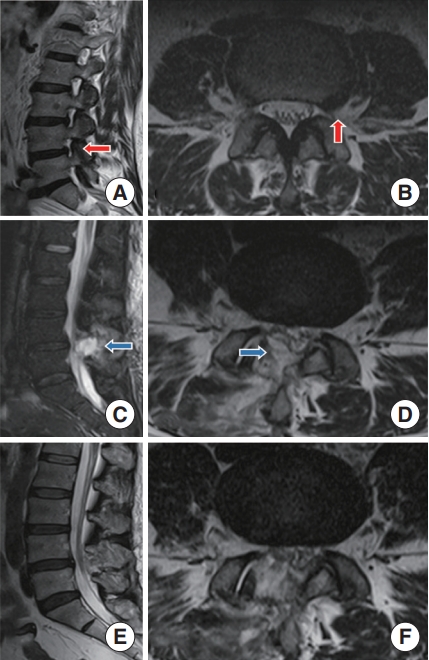
(A, B) A case of epidural hematoma after UBE-LD surgery: a 52-year-old woman underwent UBE-LD surgery for lumbar disc herniation in the left foramen area (red arrows). (C, D) Radiation pain of the right lower limb with a visual analogue scale of 9 suddenly appeared on the third day after operation, the magnetic resonance imaging (MRI) showed the epidural hematoma (blue arrows). (E, F) The symptoms were relieved immediately after hematoma clearance operation, and MRI indicated that the epidural hematoma had been removed. UBE-LD, unilateral biportal endoscopy lumbar discectomy.
3. Learning Curve of CUSUM Analysis
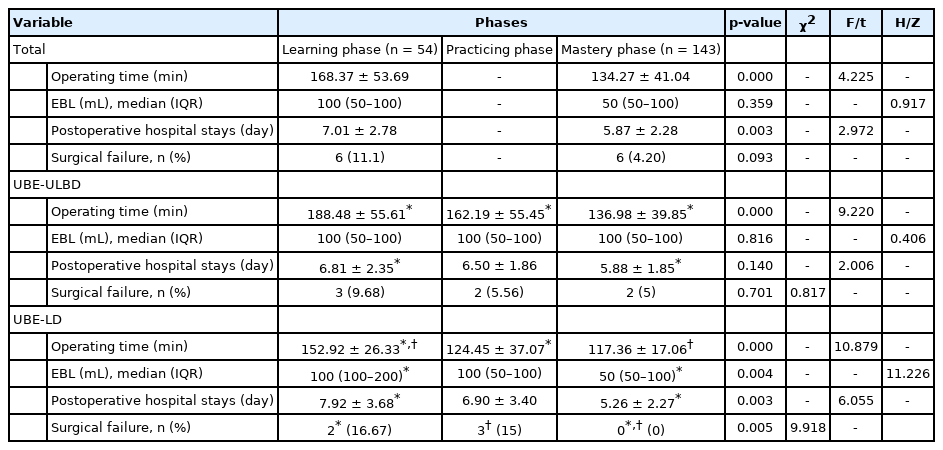
Curve fitting was performed for the scatter diagram drawn according to the CUSUM value (Fig. 5), and the fitting function formula was as follows: CUSUM=118.28991+4.8622×n+2.124 85 ×n2-0.05692 ×n3+5.21264e-4×n4-9.49534e-7×n5-1.33934e-8×n6+8.28645e-11×n7-1.42398e-13×n8 (R2= 0.9711, p = 0.000). When the number of surgical cases accumulated to 54 of the 197 cases, the slope of the curve changed from positive to negative. Therefore, the cutoff point required to achieve technical proficiency of UBE surgery was considered as 54 cases. We further divided the learning curve of 197 cases of UBE surgery into 2 phases for data comparison: the learning phase (case 1–54) and the mastery phase (case 55–197).

Cumulative sum (CUSUM) graph of the cohort. UBE-ULBD, unilateral biportal endoscopy unilateral laminotomy bilateral decompression; UBE-LD, unilateral biportal endoscopy lumbar discectomy.
CUSUM=118.28991+4.8622 ×n+2.12485 ×n2-0.05692 ×n3+5.21264e-4×n4-9.49534e-7×n5-1.33934e-8×n6+8.28645e-11× n7-1.42398e-13×n8
CUSUM analysis of different surgical methods showed that the first cutoff points of UBE-ULBD and UBE-LD were 31 cases and 12 cases, and the second cutoff point were 67 and 32 cases respectively. Therefore, the learning curve of UBE-ULBD and UBE-LD was divided into 3 phases respectively: the learning phase, practicing phase and mastery phase (Figs. 6, 7).

Cumulative sum (CUSUM) graph of unilateral biportal endoscopy unilateral laminotomy bilateral decompression.
CUSUM= 163.19249+28.77753 ×n+1.33211 ×n2-0.07247 ×n3+1.17327e-4×n4+4.12196e-5×n5-8.26555e-7×n6+6.36981e-9×n7-1.77889e-11×n8

Cumulative sum (CUSUM) graph of unilateral biportal endoscopy lumbar discectomy.
CUSUM=-126.94879+112.59308 ×n-11.3327 ×n2+0.56427 ×n3-0.01506 ×n4+2.17584e-4×n5-1.60262e-6×n6+4.71398e-9×n7
The comparison of patient characteristics and perioperative data in different phases are listed in Table 5. The operation time and postoperative hospital stays decreased with the improvement of mastery (p<0.05). The incidence of complications in the learning phase and the mastery phase were 11.1% and 4.20% respectively. There was no significant difference the surgical failure rate and EBL of different phases (p>0.05), with the exception of the learning phase and the mastery phase of UBE-LD group.
4. Learning Curve of RA-CUSUM Analysis
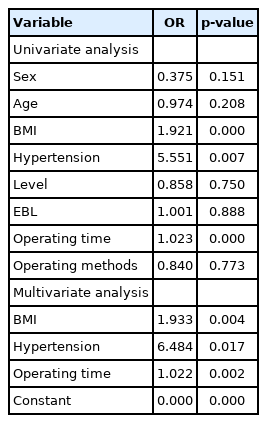
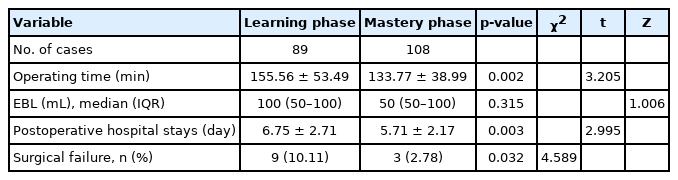
Univariate binary logistic regression showed that BMI, hypertension and operation time were risk factors for surgical failure (p<0.05; odds ratio [OR], 1.921, 5.551, 1.023) (Table 6). The fitting curve generated according to the results of RA-CUSUM began to decrease after the 89th operation, which meant that the probability of surgical failure began to decrease (Fig. 8). It indicated that 89 cases were needed to overcome the learning curve of UBE surgery in RA-CUSUM analysis. Therefore, the learning curve was divided into a learning phase (case 1–89) and a mastery phase (90–197) according to the cutoff point.
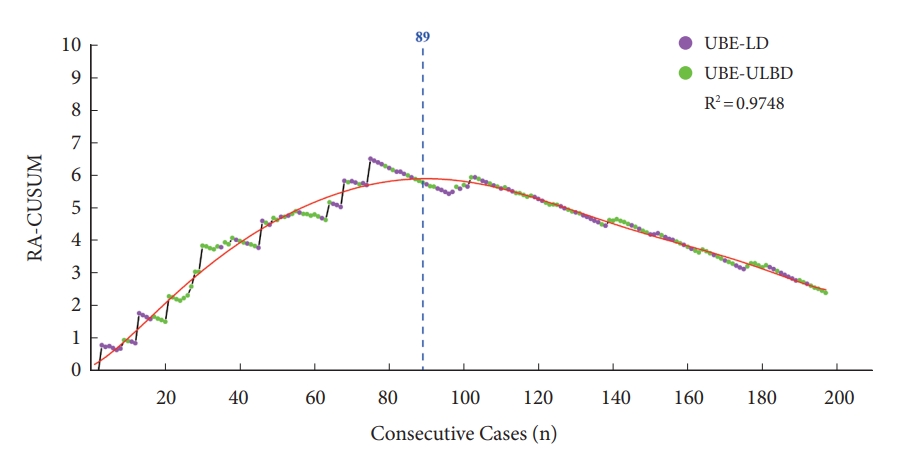
Risk-adjusted cumulative sum analysis (RA-CUSUM) graph of the cohort. UBE-ULBD, unilateral biportal endoscopy unilateral laminotomy bilateral decompression; UBE-LD, unilateral biportal endoscopy lumbar discectomy.
RA-CUSUM=0.12342+0.05918 ×n+0.00397 ×n2-1.45566e-4×n3+2.55111e-6×n4-2.61582e-8×n5+1.53628e-10×n6-4.74322e-13×n7+5.95452e-16×n8
As listed in Table 7, the surgical failure rate of the mastery phase (2.78%) was significantly lower than that of the learning phase (10.11%). In mastery phase, the operation time and the postoperative hospital stays were significantly lower than that in the learning phase (p<0.05). However, there was no significant difference in EBL between the 2 phases.
DISCUSSION
To reduce the trauma and complications caused by surgery, spinal surgeons have been committed to the combination of endoscopic technology and minimally invasive concepts. The effectiveness of lumbar discectomy and decompressive laminotomy by posterior approach using the microendoscopy or full-endoscopy have been reported [11-14]. However, due to the working cannula of the microscopic or full-endoscopic system, it is sometimes difficult for the instrument to tilt to the opposite side, especially in ULBD surgery. For the purpose of complete contralateral decompression, it is necessary to tilt the operating table or even remove the contralateral facet joint needlessly in most cases [4,5,15]. Excessive resection of the facet joint leads to the decrease of spinal stability after surgery. Ito et al. [4] indicated that the preservation rates for facet joints of UBE-ULBD and Micro-ULBD were 78% and 86% on the ipsilateral side, respectively, while those on the contralateral side were 85% and 94%. UBE technology established portals through the skin without a cannula, so the range of movement of the instruments was large, the decompression was complete and no excessive bony removal was required. UBE has the characteristics of less trauma, less bleeding and rapid recovery, and good effectiveness in the treatment of LDH and LSS according to previous studies [15-17]. In our study, UBE-ULBD and UBE-LD also showed good clinical efficacy through the decrease of VAS and ODI (Table 3, Fig. 3).
As an emerging technique, in the early proficiency phase of UBE surgery, surgeons must go through the process of learning and practicing. The learning curve reflects the rate of skills acquired within a certain period of time, which is usually determined by the number of surgical cases required for beginners' surgical techniques to achieve relative stability [18,19]. Although the reference value of the learning curve is limited by subjective factors, it can be used to summarize objective and replicable experiences, provide technical references and reduce unnecessary learning costs. To date, there are few articles on the learning curve of UBE. Study of Choi et al. [20] indicated that the operation time of UBE surgery was close to the average and remained stable after the 36th cases. Kim et al. [21] considered that at least 34 cases were required to achieve sufficient mastery of lumbar interbody fusion by UBE. A surgeon with no experience with endoscopic surgery was considered to achieve adequate UBE surgical ability in the 58th cases according to the study of Park et al. [22]. Comparation of the learning curve of UBE and other endoscopic surgery has reference significance for surgeons who have engaged in other spinal endoscopic techniques in the past. The cutoff point of full-endoscopic surgery ranged from 10 to 43 cases, with an average of about 22 cases [23-25], and which of microscopic surgery was reported to be between 20 and 30 cases [26-29]. The cutoff point of full-endoscopy and microendoscopy seems slightly earlier than for UBE surgery. In UBE surgery, the same trigonometric imaging principle as arthroscopy was applied, and excellent hand-eye coordination was required for surgeons. In the early phase of learning, the instruments may be loss under the endoscopic view, and even enter the wrong intervertebral space for the reason of the wide range of activities of endoscope and instruments. Besides, the workspace of UBE surgery was man-made, not a natural joint space like arthroscopic surgery. Therefore, lack of experience in creation of workspace will lead to the prolongation of operation time, even the blockage of saline and blurred field of vision.
CUSUM is an average-based test method that was originally mainly used to monitor the continuous change trend of the industrial sector. Because this statistical method meets the requirements of clinical technical learning and quality control, it has been used to analyze learning curves in medicine since the 1970s [9,30]. In this study, CUSUM analysis of operation time indicated that the cutoff point required to overcome the learning curve of UBE surgery was 54 cases. The average operation time in the mastery phase was about half an hour shorter than in the learning phase (168.37 minutes vs. 134.27 minutes). However, in the results of CUSUM analysis in this study, there was no significant difference in surgical failure rates among different phases of UBE surgery. Moreover, in previous studies using operation time as an evaluation index [20-22], there was no difference in the complication rate among different learning phases. Although the operation time is the key factor in determining whether the surgeon overcomes the learning curve, the evaluation of the learning curve should theoretically include the quality and safety of medical care and the substantial health benefits of patients, not just surgical proficiency. Therefore, not only the operation time but also the occurrence of complications and the failure of the operation should be considered when determining the learning curve [31].
Most of the evaluation indices used by CUSUM analysis were operation times, while the RA-CUSUM was used to evaluate other parameters that affect the outcome of the operation [32]. RA-CUSUM analysis in this study used the rate of surgical failure as reference index, and indicated that at least 89 cases were required to achieve a stable success rate. The overall failure rate of UBE surgery was 6.11%. Besides, the failure rate was 10.11% in the learning phase and 2.78% in the mastery phase, with a significant decrease after the 89th case. Therefore, after reaching a sufficiently fast operation speed, surgeons still need a period of learning and experience accumulation to control the complication rate at a low level.
Research by Lin et al. [33] indicated that the average complication rate in LDH patients who received UBE surgery (4 studies, 134 cases) was 8.3%, and 6.3% in LSS patients (6 studies, 333 patients). Kim et al. [34] reported that the surgical failure rate of UBE surgery was 10.29%, which could drop to 5.60% after the early learning stage. In our study, the complication rate of UBE surgery was similar to that previously reported. But it is worth noting that, in our study, all 3 complications occurred in the mastery phase according to RA-CUSUM were UBE-ULBD surgery (98th, 139th, 164th), and the CUSUM analysis of the operation time also showed that the cutoff point of UBE-LD surgery was earlier. Since there was no literature showing the difference between UBE-LD and UBE-ULBD in this respect, we consider that the surgeon's PELD experience may be the cause.
The incidence of dural tears during surgery in LSS patients (3.7%) was significantly higher than that in LDH patients (2.1%), and the risk of dural tears in ULBD surgery was higher [35]. In our study, dural tears also mainly occurred during UBE-ULBD surgery. The space between the dural sac and lamina became narrower in LSS patients, and adhesion between the dural sac and LF appeared, resulting in a blind area during the ULBD operation that would cause dural tears. In addition, the ligament structure between the dural sac and the surrounding spinal canal wall, the meningovertebral ligaments, is tightly connected with the LF. Therefore, the LF could be torn off together with part of the dorsal dural sac and small vessels due to pulling the LF sharply [36]. Under the combined action of meningovertebral ligaments and pressure of saline, small folds may be formed on the surface of the dural sac. It is possible to tear along with the dural sac when the lamina is removed using a Kerrison punch [37]. In this study, one patient with dural tear presented with increased intracranial pressure during resuscitation. Saline would be infused into the subdural space from the high-pressure workspace after a dural tear, which directly leads to an increase in intracranial pressure, presenting with neck pain, headache and even seizure after surgery [38,39].
Symptoms of epidural hematoma were usually observed within 24 hours after surgery, but approximately 43% of cases did not develop symptoms until 4 days or later after operation [40]. One patient with epidural hematoma in our study developed symptoms on the third day after the surgery. Therefore, close observation should be carried out within 1 week after the surgery. In this study, regression analysis indicated that hypertension was one of the risk factors for surgical failure (OR, 6.484), which may be related to the effect of intraoperative bleeding on the visual field of surgery. Hypertension was also one of the risk factors for epidural hematoma [41]. In hypertensive patients with poor blood pressure management, the increase in blood pressure was more obvious after recovery from anesthesia, and unpredictable bleeding may occur [41].
This research was a single-center study and the surgery was performed by the same surgeon, so our experience does not apply to other surgeons. For the reason that the study of the learning curve should first be based on the subjective factor of the surgeon's experience. Moreover, objective factors such as the volume of patients, medical insurance policies, different equipment and devices all play important roles in the learning process. Therefore, other surgeons may have cutoff point earlier or later than ours.
SUGGESTIONS
1. How to Avoid Complications
We recommend that the deep layer of the contralateral LF be preserved during the operation and resected at the end. If the LF is removed first, the operation should be performed on the outside of the epidural fat as far as possible [37]. The meningovertebral ligaments need to be explored and severed with nerve dissectors or curettes. Timely reduction of water pressure and repair by patch after the tear happened may reduce the probability of intracranial hypertension. Complete hemostasis during the surgery and blood pressure management during the perioperative period were important for the prevention of epidural hematoma. The position of the migrated LDH is changeable, and local adhesion is serious most of the time, so the conventional approach may be blocked by the bony structure and result in poor outcomes [42]. The position of the incisions can be adjusted according to the position of the herniation, whole spinal canal exploration needs to be performed before the end of the operation, and the working portal and viewing portal can be exchanged if necessary to expand the exploration range.
2. How to Shorten the Learning Curve
In the early phase of learning, we did not avoid some difficult cases deliberately, which leads to the prolongation of the operation time and the rapid rise of the learning curve in CUSUM analysis. The resection of LF is a relatively time-consuming and tricky step in the operation. Therefore, we suggest beginners to choose simple LD surgery by the left-side approach in early phase, which may reduce the difficulty of practicing. Apply of 0° endoscope in the early phase can make beginners adapt to UBE surgery more quickly. Additionally, standardized training and practicing on models or cadavers are of great help to shorten the learning curve.
CONCLUSIONS
In this work, the learning curve of UBE surgery was evaluated by CUSUM and RA-CUSUM analysis based on operation time and incidence of complications, and satisfactory clinical outcomes were achieved with low incidence of complications. Our data indicated that the number of cases for overcoming the learning curve of UBE surgery was 54 cases, and increased to 89 cases when the incidence of complications was taken into account. The appropriate early cases selection and standardized training are helpful to shorten the learning curve.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
(1) National Key R&D program of China (project number:2019YFC0121400). (2) Medical Health Science and Technology project of Zhejiang Province (project number: 2022KY997).
Author Contribution
Conceptualization: JX, DW; Data curation: JB, WG; Formal analysis: JX, JB, WG; Methodology: JX, DW; Project administration: HP; Writing - original draft: JX, DW, JL; Writing - review & editing: WZ, HP.