|
 |
- Search
|
|
||
Abstract
Objective
This study aimed to investigate the reliability and diagnostic accuracy of typical dermatomes and myotomes for determining the pathologic level in surgically verified patients with cervical radiculopathy.
Methods
Patients who underwent single-level surgery due to cervical radiculopathy with at least a 60% reduction in preoperative symptoms or recovery of muscle power after surgery were included. The observed clinical symptoms (pain, paresthesia, motor weakness) were compared to those of typical cervical dermatomes and myotomes.
Results
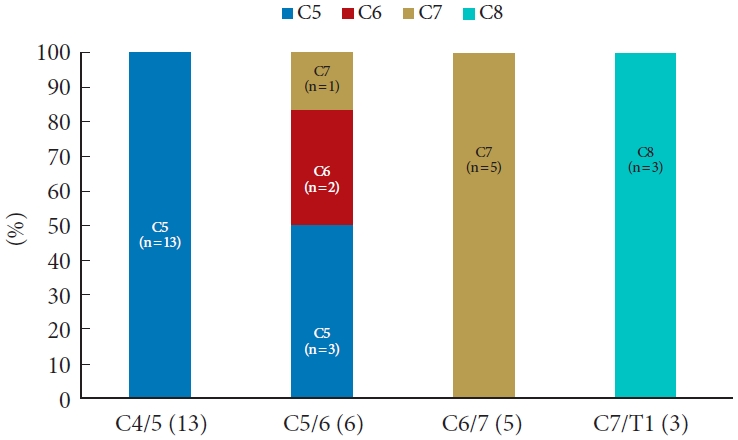
Among the 227 patients reviewed, 142 (62.6%) had a standard dermatomal pattern, and 74 of 110 (67.3%) had a standard myotomal pattern. The myotome of C5/6 radiculopathy showed much more variance than those of other cervical segments. Among the patients with severe motor weakness (muscle strength Ōēż grade 3 or obvious muscle atrophy), all those with involvement of root C5, C7, and C8 showed a typical pattern (C4/5: 13 of 13 patients, C6/7: 5 of 5 patients, C7/T1: 3 of 3 patients), while only 2 of the 6 patients (33.3%) with severe motor weakness caused by C5/6 radiculopathy fit the typical pattern.
Cervical radiculopathy is a common condition that usually results from compression of the cervical nerve roots, which is frequently caused by cervical disc herniation or cervical spondylosis [1]. Neurologic signs and symptoms of cervical radiculopathy vary depending on the pathologic level of the cervical segment, but specific diagnosis might become easier if the root(s) compression lesion shown in advanced imaging, such as magnetic resonance imaging (MRI), matches the clinically expected level [1-4]. Therefore, an effort has been made to evaluate the reliability of various signs and symptoms in determining the pathologic level(s). Cervical radiculopathy is generally considered to present in a reproducible pattern of dermatome and myotome attributable to the involved cervical root [4,5].
Unfortunately, most neurologic manifestations used to determine cervical pathologic level do not have high diagnostic accuracy in the real world [5,6]. Additionally, in clinical practice, patients who have severe nerve root compression on cervical spine MRI frequently do not complain of any symptoms. Therefore, the imaging findings should be carefully correlated with the neurological examination. Spine surgeons are required to differentiate the pathologic level of cervical radiculopathy with asymptomatic radiographic cervical nerve root compression [6].
Riew stated that only about half of cervical radiculopathy patients had a ŌĆ£typicalŌĆØ pattern of clinical symptoms [4,5]. This is well known to experienced cervical spine surgeons, and similar findings have been reported in the past. However, data on the variability with which cervical radiculopathy presents in real clinical practice and how often the actual presentation might deviate from the typical human dermatomes and myotomes remains limited. Such information would be useful to surgeons making diagnoses as to the causative root level. Therefore, the aim of this study was to determine how often patients present with typical myotome and dermatome patterns in a surgically verified population undergoing single-level cervical surgery for radiculopathy.
After Institutional Review Board approval of Kangwon National University Hospital (A-2019-08-002-004), a retrospective review was performed on the records of all patients with single-level cervical radiculopathy who underwent surgery. Patients with single-level radiculopathy were selected to correlate the presenting symptoms with a specific root level. Surgical treatment methods included anterior cervical discectomy and fusion (ACDF), anterior disc replacement (ADR), and posterior foraminotomy (PF); all the surgical procedures were performed by the same surgeon between March 2011 and March 2018. ACDF was the most common surgical method and ADR was performed in relatively young patients without dynamic instability (> 2.0 mm translation on flexion-extension lateral radiographs) and without severe spondylosis. Patients with a high amount of neck pain due to facet arthropathy were not indicated for ADR surgery. The electronic medical records were reviewed to obtain data consistent with the studyŌĆÖs inclusion criteria, which included (1) MRI imaging demonstrating evidence of single-level nerve root compression at the level thought to be causing symptoms; (2) relief of symptoms, especially radiating pain and/or motor weakness, after decompression surgery; and (3) at least a 60% reduction in preoperative symptoms or recovery of G1 or more of muscle power by the 6-month postoperative follow-up. Patients with myelopathic symptoms and identifiable cervical spinal cord compression and cord signal change in MRI imaging and those in which bilateral root compression was present were excluded. All patients included in this study underwent a nonsurgical treatment, such as physical therapy, medications, or epidural steroid injection, for at least 3 months before surgery, or were assessed to have a progressive or clinically significant motor weakness in the muscle strength test. The single nerve roots involved in this study were limited to the fifth, sixth, seventh, and eighth cervical roots.
We analyzed patient demographics, level of root lesion, clinical symptoms (pain, sensory change, and motor weakness), duration of symptoms, and the degree of pre- and postoperative pain. To evaluate pre- and postoperative pain, the Neck Disability Index (NDI) and visual analogue scale (VAS) for neck/arm pain scores were also analyzed [7].
All manual motor grade scores were evaluated by a single skilled examiner. The most commonly accepted method of assessing muscle strength is the Medical Research Council (MRC) scale [8]. This method involves testing key muscles against the examinerŌĆÖs resistance and grading the patientŌĆÖs strength on a 0 to 5 scale. (grade 0, no muscle activation; grade 1, trace muscle activation, such as a twitch, without achieving full range of motion; grade 2, muscle activation with gravity eliminated, achieving full range of motion; grade 3, muscle activation against gravity; grade 4, muscle activation against some resistance; grade 5, muscle activation against examinerŌĆÖs full resistance). Testing the strength of the elbow flexors, elbow extensors, wrist extensors, finger flexors, and hand intrinsic muscles allows for a methodical evaluation of the C5 to C8 nerve roots (Table 1). Severe motor weakness was defined by an MRC score Ōēż grade 3 or by the observation of distinct muscle atrophy.
The location and characteristics of clinical symptoms (pain, paresthesia, and numbness) reported by the patient were described on pictorial maps (Fig. 1). Depending on the severity of symptoms, more marking could be done. To determine the involved level through the pain pattern, the dermatomal pattern of arm pain was considered first. If it was difficult to select just one level using the pattern of arm pain, both arm and axial neck pain were considered together. When the pain pattern was broad, the level was determined as the most painful area. The pathologic disc level using clinical symptoms was assessed by 2 independent examiners (1 staff and 1 fellow). In case of a disagreement on the disc level, the conclusion was drawn through a mutual discussion.
The data were analyzed using the IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). For surgical outcomes, the change from the baseline in each group was evaluated using paired t-tests. Differences between the 2 groups were evaluated using Student t-test for continuous variables and the chi-square test for categorical variables.
The medical records of 227 patients met the inclusion criteria of the study and were reviewed. Baseline patient characteristics and the surgical procedures performed are presented in Table 2. Overall, 227 cervical segments were included in this study (C4/5:30; C5/6:115; C6/7:69; C7/T1:13), and there was no significant difference in the demographics of the patients who underwent surgery on different cervical segments. The symptom onset varied between 0.5 and 100 months (mean, 8.1 months).
Among the 227 patients, 209 (92%) underwent anterior decompression surgery and 18 (8%) underwent posterior decompression surgery (PF). No differences in VAS scores for arm and neck pain or NDI scores in the pre- and postoperative period were noted between the anterior (ACDF and ADR) and posterior (PF) surgery groups (Table 2).
Arm pain was the most common presenting symptom, occurring in 213 patients (93.8%). The mean VAS score of preoperative arm pain was 7.26┬▒ 2.02 (range, 0ŌĆō10), while that postoperatively was 1.34┬▒ 1.79 (range, 0ŌĆō6) (p< 0.01). Pain in the axial neck (around the neck, shoulder, scapula, and interscapula) was recorded in 185 patients (81.4%). The mean preoperative axial neck pain was 6.29┬▒ 2.47 (range, 0ŌĆō10), which decreased to 1.13┬▒ 1.42 (range, 0ŌĆō6) postoperatively (p< 0.01) (Table 3).
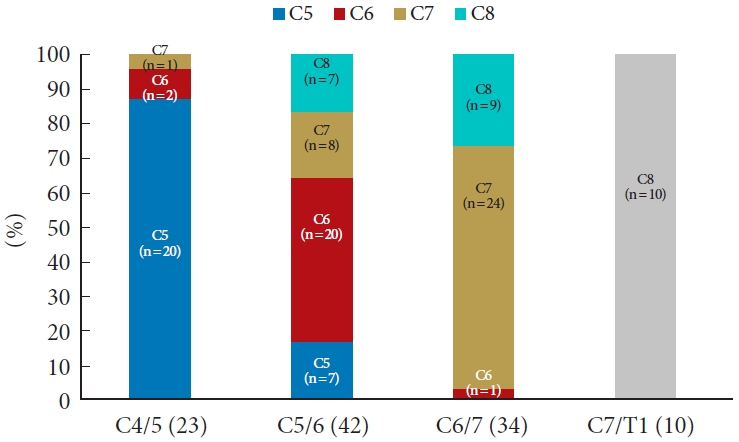
Among the 30 patients with C4/5 radiculopathy, 20 (66.6%) showed a typical dermatomal pattern in which the involved root and the location of the symptom were consistent. Meanwhile, 7 patients (23%) at this level complained of different dermatome pain (C6: 4 patients and C7: 3 patients), and in 3 patients (10%), symptoms were too broad or poorly localized to be characterized as one specific level. In patients with C5/6 radiculopathy, 69 patients (60.0%) showed a typical dermatomal pattern, and 46 patients (40.0%) showed an atypical dermatomal pattern (C5:11 patients, C7:25 patients, C8:10 patients). There were 45 patients (65.2%) with a typical pattern and 23 (33.3%) with an atypical patten (C5:4 patients, C6:12 patients, C8:7 patients) in the C6/7 radiculopathy group. There were 8 patients (61.5%) with a typical pattern, 4 (30.7%) with an atypical pattern similar to that of C7 nerve compression, and 1 patient (7.6%) with poorly described pain in the C7/T1 radiculopathy group (Fig. 2).
Objective muscle weakness was initially recorded in 110 of 227 patients (48.5%), and severe motor weakness was observed in 27 patients (11.9%). No patient experienced greater weakness after the surgical treatment. In total, 20 of 23 patients (56.9%) with C4/5 radiculopathy, 20 of 42 patients (47.6%) with C5/6 radiculopathy, 24 of 34 patients (70.5%) with C6/7 radiculopathy, and 10 of 10 patients (100%) with C7/T1 radiculopathy showed typical motor weakness (Fig. 3). All patients with involvement of root C5 (C4/5 radiculopathy) and C7 (C6/7 radiculopathy) showed typical motor weakness (C4/5: 13 of 13 patients, C6/7: 5 of 5 patients). However, among the patients with severe motor weakness, only 2 of the 6 patients (33.3%) with involvement of the C6 nerve (C5/6 radiculopathy) paradoxically showed typical weakness pattern, such as elbow flexion and wrist extension (Fig. 4).
In our series, we included patients with single-level cervical radiculopathy who underwent surgery via different surgical methods. Although there are various options available for the operative intervention of cervical radiculopathy [1], anterior cervical decompression surgeries, such as ACDF and ADR have become the most common surgical methods [9]. However, an anterior cervical exposure is more difficult at the lower level of the cervical spine such as the C7/T1 cervical segment. An alternative treatment option in this region is mini open PF alone or with concurrent discectomy [9-11].
In clinical practice, when determining the surgical level causing symptoms in patients with cervical radiculopathy, the typical dermatomal map and the function of the muscle innervated by the specific nerve are considered first. The most common symptoms associated with cervical radiculopathy are pain and/or paresthesia through the upper extremities in the dermatomal distribution of the involved nerve roots [12].
McAnany et al. [6] reported that, among 239 patients with single-level cervical radiculopathy, only 129 (54%) showed radiating pain and numbness following the standard dermatomal pattern. In present study, 62.6% (142 of 227) of the patients were identified as having the standard dermatomal pattern, which was slightly higher than that in the results reported in previous studies. Presumably, the reason for the greater prevalence of patients with a standard pattern in our study is that we determined the surgical level using the pattern of pain in the axial neck (neck, shoulder, trapezius, and periscapular pains) and in the distal part of the arm and our study was performed at a single center.
Previous studies have investigated axial pain patterns provoked by cervical discography and injections into the facet joints of the cervical spine [13]. These studies suggested that stimulation of each disc results in consistent and predictable patterns of neck pain (Fig. 1B). Although the dermatomal distribution of arm pain and sensory dysfunction is the most important clue, axial neck pain including cervicogenic headache, shoulder pain, and parascapular pain might help determine the surgical target level [3,14]. According to our result, ipsilateral axial neck pain occurred in 81.4% of patients, and some patients complained of only axial neck pain without particular arm pain or sensory dysfunction. Therefore, when determining the surgical target level, it seems advantageous to combine the 2 types of pain distribution, although the neck, shoulder, and parascapular pain distributions alone is of little value for localizing the level of cervical nerve compression [14].
Weakness of single muscles or muscle groups is of great value in the localization of a lesion to a single nerve root [15,16]. However, there are many charts in the literature, which differ from one another. Thus, some authors stated that localized weakness is not of decisive value in establishing the segmental level of a radicular lesion. Weakness of the deltoid with less severe weakness of the biceps muscle has been described in lesions of root C5. Involvement of the C6 root frequently causes marked weakness in the biceps, brachioradialis, and wrist extensor muscles. Other authors have considered that weakness in the deltoid and triceps was caused by lesions of root C6. Lesions of root C7 have resulted in weakness mainly in the triceps according to some authors, but others have included weakness in the biceps and deltoid also. Most authors agree that lesions of C8 cause most marked weakness in the intrinsic muscles of the hand [17].
Based on our results, relative to muscle weakness, the pathologic nerve could be localized correctly in 67.3% (74 of 110). However, the motor weakness of C5/6 radiculopathy (C6 nerve lesion) showed much more variance, with typical motor weakness demonstrated in 47.6% (20 of 42) of the patients. When the patient had severe motor weakness (distinct muscle atrophy and motor power grade 3 or less), a single nerve root lesion except for the lesion of C6 root could be determined much more accurately. In all cases with severe weakness involving root C5, C7, or C8, the pathologic nerve root could be correctly localized. However, in C6 radiculopathy patients with severe motor weakness, 4 of 6 patients (66.7%) had weakness that did not conform to the standard pattern.
This result in our study was similar to that of a study that identified electrodiagnostic patterns for each level of cervical radiculopathy. Levin et al. [18] compared 50 cases of surgically proven solitary-root lesions with their preoperative electrodiagnostic patterns. With C5, C7, and C8 radiculopathies, changes were relatively stereotypical, with involvement of the supra- and infraspinatus, deltoid, biceps, and brachioradialis with C5; the pronator teres, flexor carpi radialis, triceps, and anconeus with C7; and the first dorsal interosseous, abductor digiti minimi, abductor pollicis brevis, flexor pollicis longus, and extensor indicis proprius with C8. The root lesion with the most variable presentation was C6. In half the patients with C6 radiculopathy, the findings were similar to C5 radiculopathies, whereas in the other half, the findings were identical to those of patients with C7 radiculopathies.
Discrepancies from the usual clinical findings in single cervical root compression might be the result of variations in the brachial plexus and the intradural connection of rootlets [5]. In cadaveric studies of the human nervous system, more than 50% of the anatomical variations occurred in the brachial plexus. Standard textbooks describe the roots of the brachial plexus as arising from the last 4 cervical nerves and the first thoracic nerve with an occasional contribution from the fourth cervical (prefixed) and second thoracic (postfixed) nerves. These variations may lead to deviation from the expected dermatome distribution as well as differences in the motor innervation of muscles of the upper limb [19-21]. Additionally, when evaluating motor function, certain joint motions cannot separate the actions of individual muscles [8,17]. Muscle function tests for the biceps and brachioradialis are such examples. It has been proposed that the biceps and brachioradialis can be separately evaluated by changing the forearm position for pronation/supination. However, we feel that it is difficult to definitely separate the actions of these 2 muscles. In this regard, normal biceps may have masked the brachioradialis dysfunction associated with the C6 lesion. Involvement of the C6 nerve root is the second most common cause of cervical radiculopathy [12]. In patients with C6 radiculopathy, motor weakness in the wrist extensors and biceps are common but weakness of the supinator, pronator teres, and triceps muscles may also be present. The diversity of muscle units in which the C6 nerve root is involved may confuse C6 radiculopathy with C5 and C7 nerve root symptoms [17].
Our study has certain limitations inherent to retrospective studies. First, the examiner was not blinded to other information. The MRI findings were sometimes known prior to the examination. More importantly, if, for example, the examiner came to believe that the patient had a C6 lesion during the muscle tests and other neurological examinations, then the overall findings may have been biased. Second, we used a 60% or greater reduction in preoperative symptoms as an inclusion criterion for the study, with the 60% or greater improvement used as an indicator that the correct level of pathology had been addressed. However, it remains possible that radiculopathy at other levels could account for the remaining symptoms left unresolved in those who did not get 100% relief.
Identification of the exact root level(s) causing radiculopathy can be important in all patients and critical in those who elect to have surgical treatment. Determining the single nerve root involved in the basis of clinical signs and symptoms may be desirable and reasonably expected based on our findings. Of the various signs and symptoms, severe motor weakness of the upper extremity is of great value in permitting specific localization to a single root. However, it should be noted that greater variation may occur when determining the pathologic disc level by evaluating the pattern of muscle weakness in patients with C6 radiculopathy. Clinicians who attempt accurate localization of cervical root lesions on a clinical basis alone must be aware of the possible variations and frequent lack of positive findings in any given patient.
NOTES
Fig.┬Ā1.
Dermatomes diagram demonstrates the dermatomal map of radiating arm pain (A) and axial neck pain including neck, scapular, interscapular (B). The red X marks indicate the location of the patientŌĆÖs reported pain.
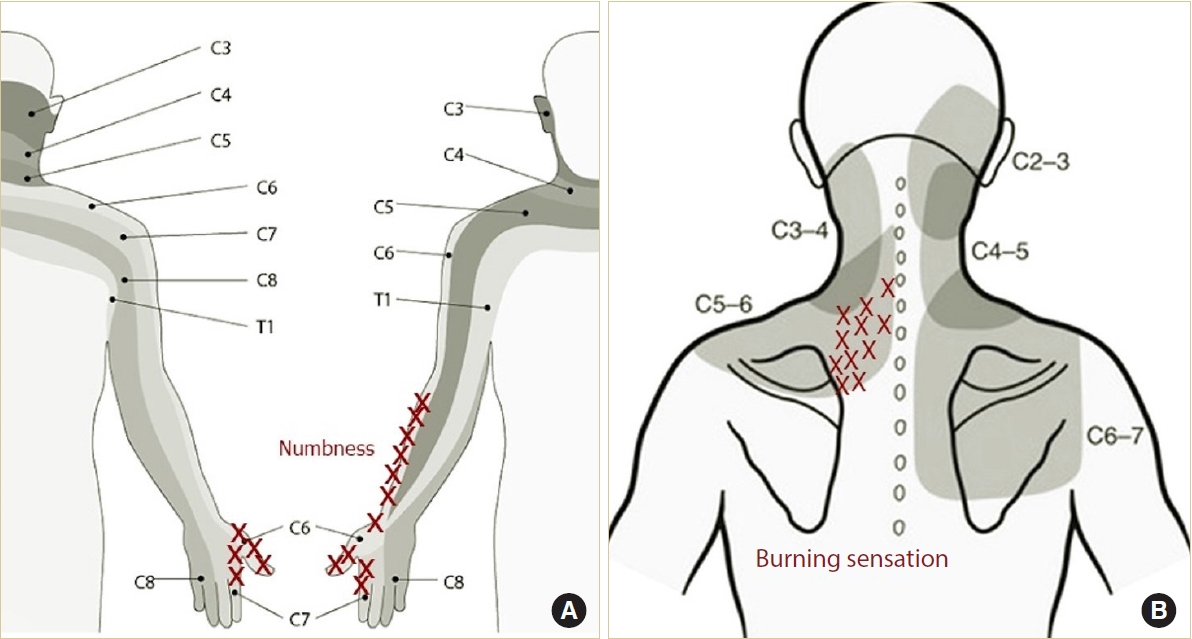
Fig.┬Ā2.
Distribution of various dermatomal patterns caused by compression of roots C5, C6, C7, and C8 in this study. NS, nonspecific.

Fig.┬Ā3.
Distribution of motor weakness patterns caused by compression of roots C5, C6, C7, and C8 in this study.
Table┬Ā1.
Compression of cervical root: summary of ŌĆ£typicalŌĆØ clinical findings
Table┬Ā2.
Demographic data
Table┬Ā3.
Surgical outcomes
REFERENCES
1. Kim HJ, Nemani VM, Piyaskulkaew C, et al. Cervical radiculopathy: incidence and treatment of 1,420 consecutive cases. Asian Spine J 2016;10:231-7.



3. Thoomes EJ, van Geest S, van der Windt DA, et al. Value of physical tests in diagnosing cervical radiculopathy: a systemat ic review. Spine J 2018;18:179-89.


4. Makhni MC, Yeung CM, Riew KD. The art of diagnosis in the cervical spine. Neurospine 2020;17:695-703.




6. McAnany SJ, Rhee JM, Baird EO, et al. Observed patterns of cervical radiculopathy: how often do they differ from a standard, ŌĆ£Netter-diagramŌĆØ distribution? Spine J 2018;19:1137-42.


7. Wainner RS, Fritz JM, Irrgang JJ, et al. Reliability and diagnostic accuracy of the clinical examination and patient self-report measures for cervical radiculopathy. Spine 2003;28:52-62.


8. James MA. Use of the Medical Research Council muscle strength grading system in the upper extremity. J Hand Surg Am 2007;32:154-6.


9. Gutman G, Rosenzweig DH, Golan JD. Surgical treatment of cervical radiculopathy. Spine 2018;43:E365-72.


10. Harrop JS, Silva MT, Sharan AD, et al. Cervicothoracic radiculopathy treated using posterior cervical foraminotomy discectomy. J Neurosurg 2003;98(2 Suppl):131-6.

11. Song KS, Lee CW. The biportal endoscopic posterior cervical inclinatory foraminotomy for cervical radiculopathy: technical report and preliminary results. Neurospine 2020;17(Suppl 1):S145-53.




12. Bono CM, Ghiselli G, Gilbert TJ, et al. An evidence-based clinical guideline for the diagnosis and treatment of cervical radiculopathy from degenerative disorders. Spine J 2011;11:64-72.


13. Lee M, McPhee R, Stringer M. An evidenceŌĆÉbased approach to human dermatomes. Clin Anat 2008;21:363-73.


14. Slipman CW, Plastaras CT, Palmitier RA, et al. Symptom provocation of fluoroscopically guided cervical nerve root stimulation: are dynatomal maps identical to dermatomal maps? Spine 1998;23:2235-42.


15. Suh BK, You KH, Park MS. Can axial pain be helpful to determine surgical level in the multilevel cervical radiculopathy? J Orthop Surg (Hong Kong) 2017;25:2309499016684091.



16. Murphy DR, Hurwitz EL, Gerrard JK, et al. Pain patterns and descriptions in patients with radicular pain: does the pain necessarily follow a specific dermatome? Chiropr Osteopat 2009;17:9.




17. Furukawa Y, Miyaji Y, Kadoya A, et al. Determining C5, C6 and C7 myotomes through comparative analyses of clinical, MRI and EMG findings in cervical radiculopathy. Clin Neurophysiol Pract 2021;6:88-92.



18. Levin KH, Maggiano HJ, Wilbourn AJ. Cervical radiculopathies: comparison of surgical and EMG localization of single-root lesions. Neurology 1996;46:1022-5.


19. Emamhadi M, Chabok SY, Samini F, et al. Anatomical variations of brachial plexus in adult cadavers; a descriptive study. Arch Bone Joint Surg 2016;4:253-8.



- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2