INTRODUCTION
Syringomyelia is characterized by chronic dilation of the central canal of the spinal cord due to the abnormal accumulation of fluid caused by cerebrospinal fluid (CSF) circulation disorder. It is a disease often associated with Chiari malformation, trauma, tumor, inflammatory disease, or idiopathic etiologies [
1-
3]. Chiari malformation type I (CM-I) is a kind of congenital dysplasia in the craniovertebral junction. Imaging often shows a small posterior fossa volume and syringomyelia located at the lower cervical and upper thoracic segments. For the pathogenesis of CM-I related syringomyelia, there has been a wide concern in the fluid dynamics, syrinx phenotypes, clinical symptoms, and imaging morphology of the posterior fossa and craniovertebral junction (CVJ) region [
4,
5].
Surgical procedures have been generally recognized for improving the neurological status of CM-I patients, such as posterior fossa decompression (PFD) or with duraplasty (PFDD), or a more thorough intradural decompression for syringomyelia, such as Foramen magnum and Magendie dredging (FMMD) [
6,
7]. By integrating clinical and radiographic characteristics of both syringomyelia and spinal biomechanics, we could better evaluate the surgical outcome of the CM-I patients after decompression surgery [
8-
10]. In particular, the relationship between CM-I related syringomyelia and scoliosis has been recognized [
11-
13]. Some studies reported that scoliosis will improve after decompression surgery for syringomyelia [
14,
15]. All of the above suggested that syringomyelia may be a relevant factor affecting the spinal alignment. However, there are few reports focused on the relationship between the changes in syringomyelia and cervical sagittal alignment parameters.
Previous researchers have proposed that the decrease of syrinx size after decompression surgery may restore cervical lordosis [
16]. But the correlation between syringomyelia and cervical sagittal alignment remains vague, especially among different kinds of syrinx configurations. Therefore, the purpose of this study was to further explore the correlation between syrinx resolution and changes in cervical sagittal alignment following FMMD in CM-I patients with syringomyelia and to further determine the respective relationship with clinical outcomes.
MATERIALS AND METHODS
1. Patient Selection
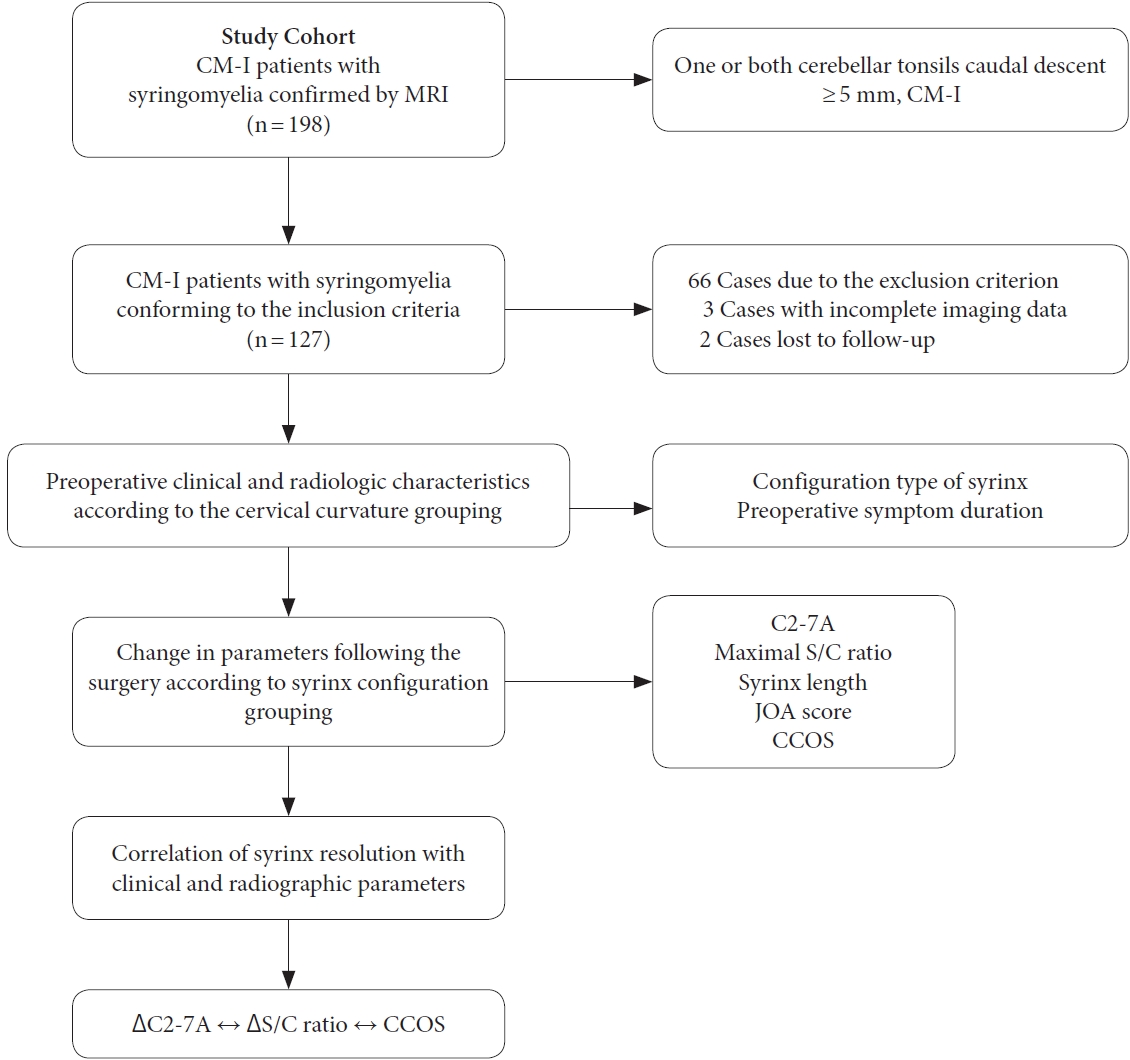
The study was approved by the institutional research ethics committee of Xuanwu Hospital (approval number: 2018030). From January 2017 to January 2020, a consecutive series of 198 CM-I patients with syringomyelia confirmed by magnetic resonance imaging (MRI) were retrospectively analyzed including their clinical records and radiologic data from a prospectively maintained database in a single center. According to screening, 66 of 198 patients were excluded from this study due to the exclusion criterion, and 5 patients had incomplete imaging data or were lost to follow-up. The exclusion criteria are as follows: (1) age < 18 years old; (2) syringomyelia associated with trauma, tumor, inflammatory disease, or idiopathic etiologies; (3) patients with atlantoaxial dislocation, degenerative cervical spondylosis, congenital vertebral anomalies, severe scoliosis, myelomeningocele, or tethered cord; (4) a history of CVJ surgery with cervical or occipital fusion and instrumentation. Finally, a total of 127 patients diagnosed with CM-I and syringomyelia who underwent intradural decompression for syringomyelia in our center met the inclusion criteria of our study. The research flow chart is shown in
Fig. 1.
2. Clinical Evaluation
The patients’ chief complaints on admission mainly include general symptoms such as headache or neck and back pain, spinal cord syndromes such as suspended sensory disorder, amyotrophy and weakness of limbs, dysfunction of urination and defecation, and other symptoms including cerebellar symptoms and cranial nerve dysfunction. Some patients had multiple clinical symptoms at the same time. The specific data were summarized in
Table 1.
3. Radiographic Evaluation
MRI and lateral radiographs of x-ray in neutral position were taken pre-and postoperatively. A routine MRI and x-ray examination was taken within one week before surgery. Follow-up MRI was conducted regularly at 1 month, 3 months (± 1 month), 6 months (± 3 months), and 1 year after surgery. After that, it was performed every 6 months until the last follow-up. All measurements were made using computer-aided software called RadiAnt DICOM Viewer (ver. 4.6.9, Medixant, Poznan, Poland). All relative radiologic measurements were repeated at least 3 times, independently by one surgeon and another radiologist.
4. X-Ray Evaluation
X-ray evaluation of the above cervical sagittal alignment parameters were shown
Supplementary Fig. 1.
Cervical lordosis angles were measured successively according to the 4-line method from the lateral radiographs in a neutral position before surgery and at the final follow-up evaluation: drawing a line parallel to the inferior endplate of the upper vertebral body, and another line parallel to the inferior endplate of the lower vertebral body. Perpendicular lines are then drawn from each of the 2 lines noted above, and the angle subtended between the crossing of the perpendicular lines is the cervical lordosis angle. A negative value of the angle meant lordosis and the positive value marked kyphosis.
Upper cervical angle (C0–2A): the included angle between the McGregor line and the line parallel to the lower endplate of the C2 body.
Lower cervical angle (C2–7A): the included angle between a line parallel to the lower endplate of the C2 body and a line parallel to that of the C7 body.
The sagittal vertical axis (C2–7 SVA): the horizontal distance between the C2 plumb line and the posterosuperior point of the C7 body.
T1 slope: The T1 slope can be used to predict the physiological curvature of the cervical spine, which was measured as the included angle between the upper endplate of T1 and the horizontal line.
5. MRI Evaluation
As proposed by Ono et al. [
17], the cerebellar tonsillar descent could be divided into 3 grades in MRI: grade 1, the cerebellar tonsil descends beyond the foramen magnum but does not reach the C1 arch; grade 2, the cerebellar tonsil reaches the C1 arch; grade 3, the cerebellar tonsil descends beyond the C1 arch.
The following indicators were measured at the T2-weighted median sagittal and transverse positions of MRI before surgery and at the final follow-up: (1) Syrinx/spinal cord ratio (S/C ratio), the ratio of syrinx diameter to the spinal cord diameter at the same level. (2) Syrinx length: the number of vertebral segments spanned by the spinal syrinx. (3) A radiographic change ratio in the maximal S/C ratio or syrinx length was calculated using the following formula: (preoperative value-postoperative value)/preoperative value. A ratio value of zero means no improvement of the syrinx size, whereas 1 means the complete disappearance of the syrinx.
6. Operation Strategy
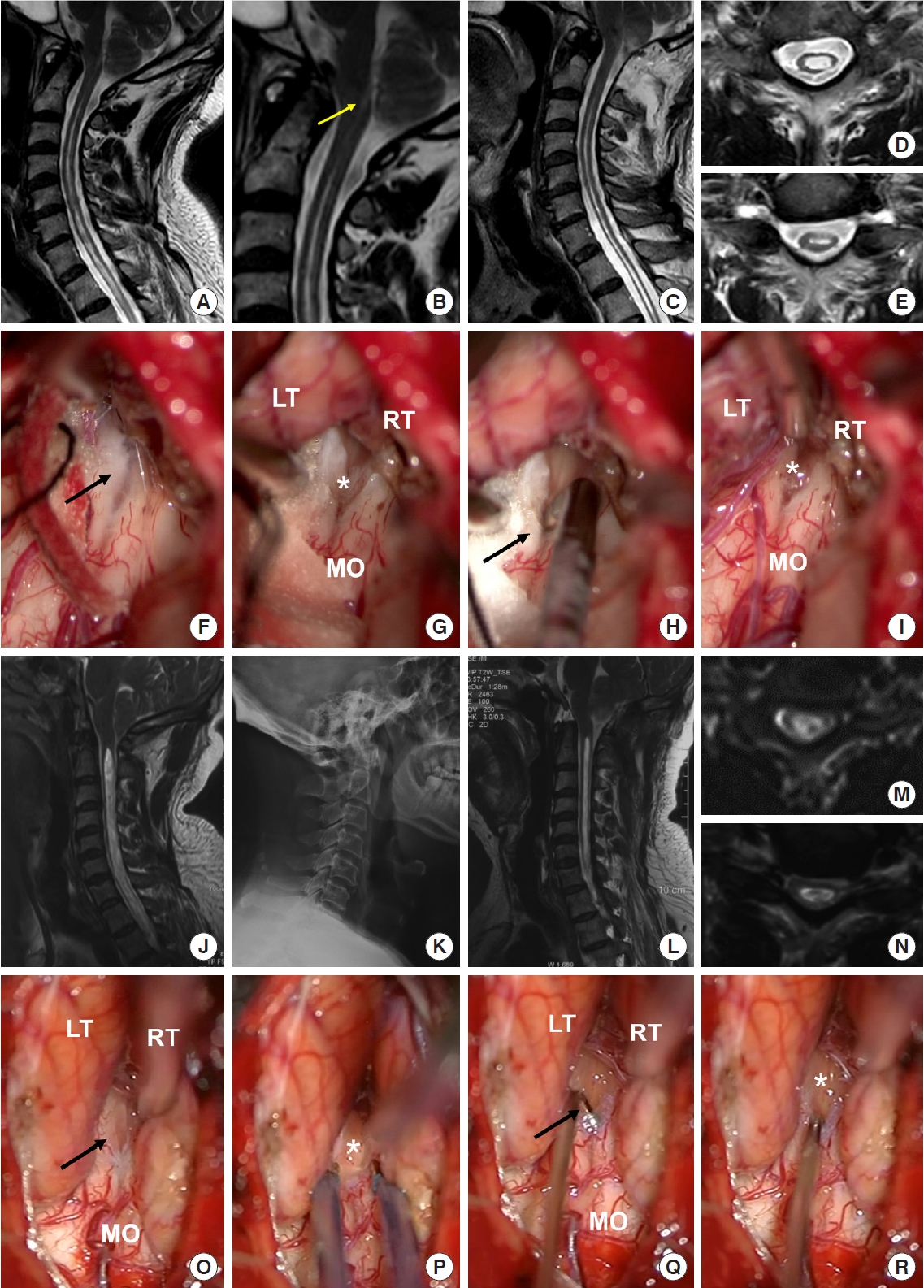
All operations in all of the 127 patients were performed via PFD with intradural exploration using FMMD to remove the factors causing CSF circulation obstruction, which was recorded via the operating microscope for all potential intradural pathology and factors (
Fig. 2). The specific surgical procedures have been described in our previous study [
6,
7].
7. Variable and Grouping Definition
The postoperative syrinx was reported to be either significantly resolved (syrinx decreased in size ≥ 20%, a more than 20% decrease in maximal S/C ratio on follow-up MRI) (
Fig. 3) or unchanged (syrinx decreased in size less than 20% or remained the same size).
The physiological curvature of the cervical spine in men is between -16˚ to -22˚ and -15˚ to -25˚ in females [
18]. The negative value less than this range (the absolute value) is defined as a straight cervical spine, the positive value is kyphosis, and the larger value than this range (the absolute value) is defined as hyperlordosis.
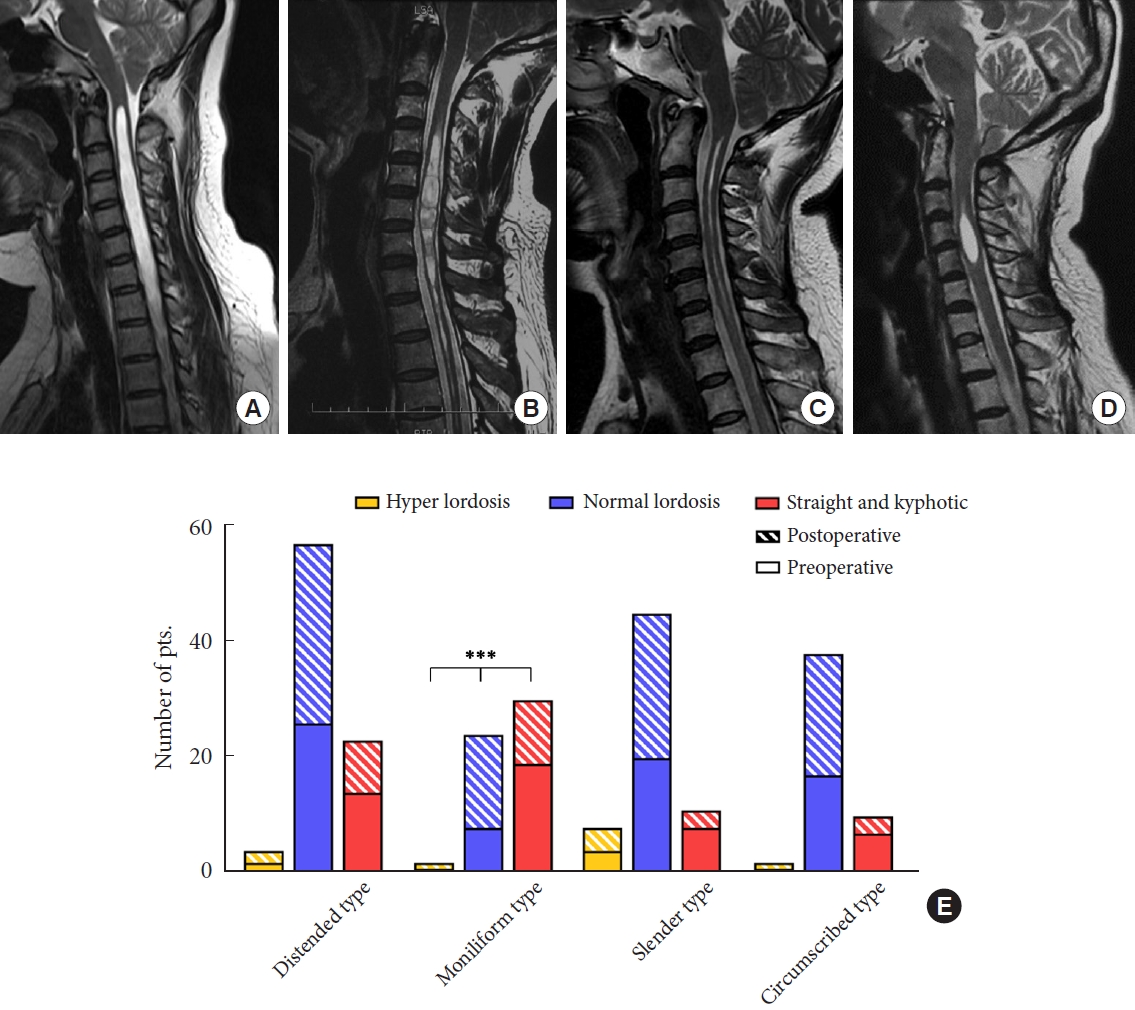
Referring to the configuration of the syringomyelia, the syrinx can be defined into 4 types: A, distended type; B, moniliform type; C, slender type; D, circumscribed type (
Fig. 4A–
D).
8. Prognostic Evaluation
All patients co mpleted the evaluation of the Japanese Orthopedic Association (JOA) scoring system to assess their neurological status before surgery and at the last follow-up. We calculated the neurologic improvement rate (ΔJOA score) according to the preoperative JOA score and the last follow-up JOA score by the following formula: (postoperative score –preoperative score)× 100%/(full score [17 points] – preoperative score).
The Chicago Chiari Outcome Scale (CCOS) was used to evaluate the surgical efficacy from 4 aspects: pain symptoms, nonpain symptoms, functionality, and complications, with scores from 1 to 4 for each item. The better the prognosis, the higher the score, with the CCOS score groupings presenting the prognosis as good between 13 and 16, no obvious improvement between 9 and 12, and poor between 4 and 8.
9. Statistical Analysis
IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis. For continuous variables, the mean values are presented with standard deviations, and the differences between the 2 groups were analyzed using the Student t-test and 1-way analysis of variance test for ≥ 3 groups. A chi-square or Fisher exact test was used for dichotomous categorical variables, and the Kruskal-Wallis H test was for multiple categorical variables. P-value < 0.05 was considered statistically significant. The relationships between clinical and radiographic parameters were assessed using Spearman rank correlation coefficients. Intraclass correlation coefficient (ICC) was used to evaluate the intra-repeatability of different observers (interobserver reliability). One independent researcher blinded to the group allocation completed the evaluations.
DISCUSSION
For syringomyelia associated with CM-I [
19-
21], patients are plagued by a series of neurological symptoms such as suspended sensory disorder, hypoesthesia, amyotrophy, or weakness of limbs. Quite a few studies have been carried out to explore the relationships between syringomyelia formation and CSF circulation obstruction in the subarachnoid space [
22,
23]. However, its specific mechanism remains controversial.
Decompression surgery with or without duraplasty is commonly used to control the development of syringomyelia, which has been reported in previous studies [
24-
27]. Although traditional surgical methods, such as PFD or PFDD, show certain curative effects in controlling the development of syrinx, they are not beneficial for all patients with syringomyelia. However, intradural pathology in various forms may cause CSF circulation obstruction, which makes great significance in the pathogenesis and outcome of syringomyelia. Therefore, the key to surgical treatment for syringomyelia likely settles in to relieve the obstruction of the foramen magnum and foramen of Magendie [
6,
7].
There is a strong association between syringomyelia and scoliosis, but there does not seem to be a significant relationship between CM-I and scoliosis in the absence of syringomyelia [
11-
13]. Syringomyelia is known to be frequently accompanied by scoliosis in some patients, possibly because of asymmetric spinal cord injury (SCI) due to chronic expansion of the central canal in the spinal cord, which means that syringomyelia may be an aggravating factor in spinal alignment. Similarly, cervical sagittal alignment reflects the cervical physiological curvature, syringomyelia as its possible aggravating factor, while there are few studies on the relationship between them.
The mean value of the C2–7A in 127 CM-I patients with syringomyelia was less lordotic than in 32 CM-I patients without syringomyelia in our center (-13.5± 7.5 vs. -20.7± 8.4, p< 0.05). We could initially infer that space occupying syrinx formation caused by the obstruction of CSF circulation might drive the loss of cervical physiological curvature as an adaptive response. Combined with the patients’ preoperative clinical and radiologic characteristics, we continued to explore the differences in the distribution of cervical curvature at baseline. The results showed that the distribution of preoperative cervical lordosis was different in various types of syringomyelia. Specifically, straight or kyphotic cervical alignment was more common in the moniliform type of syrinx. Additionally, patients with cervical alignment straight or kyphotic tend to have a short natural history. However, cervical curvature in patients with CM-I related syringomyelia was not related to syrinx length, S/C ratio, deviation, location, or other clinical characteristics. Why the straight or kyphotic cervical alignment is more concentrated in the type of moniliform syrinx has aroused our interest in further exploration.
Although the relationship between CM-I related syringomyelia and scoliosis is not fully understood. Some scholars once speculated that the syringomyelia may affect or even damage neurons in the spinal cord responsible for muscle in charge of trunk balance, thereby influencing the spinal sagittal alignment [
28]. We speculated that the development of syringomyelia could also bring certain changes to the cervical sagittal alignment as the result of chronic central SCI, which was related to the faster progression of enlargement of the central canal. Usually, the cross-fibers in front of the central canal bear the brunt, which is presented as the typical suspended sensory disorder. During the further enlargement, the anterior horn neurons and longitudinal conduction tracts were then involved in appearing manifestations such as amyotrophy, hypoesthesia, or weakness of limbs. However, whether this process is reversible depends on the degree of disturbance in the internal environment of the SCI and the degree of neuronal damage.
The decreasing of the syringomyelia size is accompanied by the stabilization or improvement of scoliosis in most patients following decompression surgery, so neurosurgical decompression is recommended for patients with CM-I, SM, and scoliosis with its potential to treat all 3 conditions at the same time [
14,
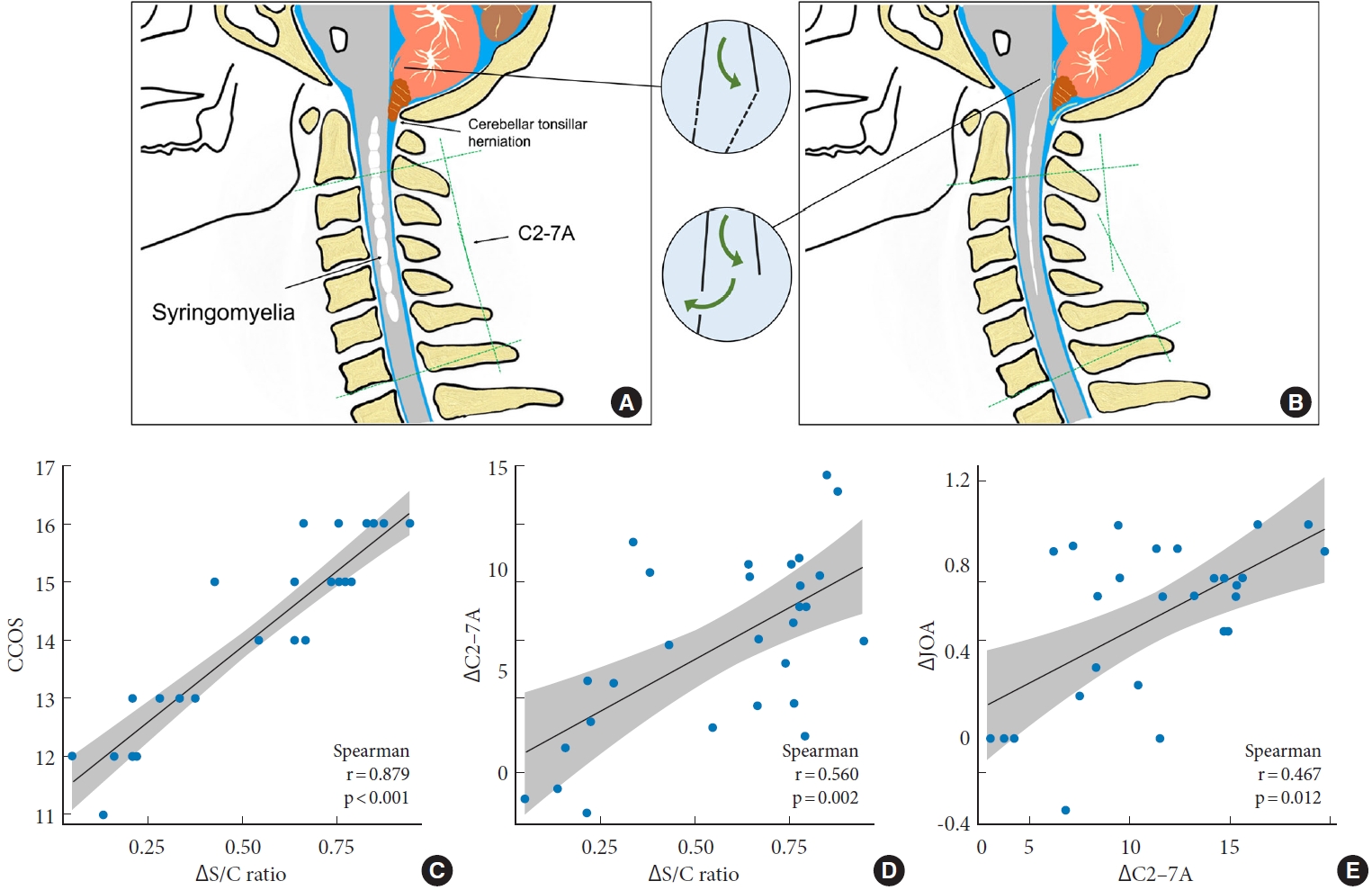
15]. Since early decompression could improve neurological status and scoliosis in patients with syringomyelia associated with CM-I, accordingly, intradural decompression for syringomyelia by FMMD may also help in restoring the cervical sagittal alignment. In our results, after FMMD, syringomyelia resolved in 108 patients (85.0%), along with significant improvements in cervical sagittal alignment and JOA score. The mean value of CCOS at the last follow-up was 13.1± 2.8 (about 80% of the patients got a score of CCOS≥ 13). Therefore, FMMD was effective for CM-I patients with syringomyelia in terms of improving neurological symptoms in the current study and is considered to be an effective surgical technique for not only syringomyelia resolution but also helping in cervical sagittal realignment. We speculate that this benefits from the elimination of intradural obstruction through FMMD, which might be conducive to a more complete restoration of CSF circulation, thereby reversing the adverse effects of chronic SCI and sagittal alignment imbalance caused by unrestricted enlargement of the syrinx (
Fig. 5A,
B).
The above parameters with statistically significant differences before and after surgery were then compared among 4 groups of different syrinx types. Compared with other groups, the syrinx with moniliform type showed the most significant changes in ΔC2–7A, and ΔS/C ratio after surgery, and the postoperative CCOS of it is relatively better. In addition, the cervical sagittal realignment of the moniliform syrinx is the most obvious after surgery in the 4 different morphological kinds of syringomyelia (
Fig. 4E).
Therefore, we propose that the moniliform type of syrinx may be a special kind of configuration. Its particularity lies in that the reactivity following the change of CSF circulation seems to be more sensitive, and it shows stronger adaptability and reducibility than other types of syringomyelia, which not only reflects in the greater changes in the cervical lordosis during the syrinx formation with a shorter natural history but also in the more obvious syrinx resolution and a relatively better prognosis after removing the intradural obstruction and dredging the circulation of CSF. In terms of syrinx width, both moniliform type and distended type belong to the category with a larger S/C ratio; from the perspective of syrinx morphology, syrinx separation is the typical imaging manifestation for moniliform type, and some studies have preliminarily confirmed syrinx separation might be related with the ependymal cells surrounding the central canal of the spinal cord [
29,
30], which would be a source of endogenous stem cells, heralding a potential endogenous approach to SCI repair. While it is still unknown the specific pathophysiological mechanism of syrinx separation in chronic SCI.
Correspondingly, further analysis suggested that the ΔS/C ratio was positively correlated with both ΔC2–7A and CCOS in the moniliform syrinx. With the restoration of the CSF circulation following FMMD, the resolution of syringomyelia also coexisted with the improvement of cervical lordosis angle. Our results proposed that some relationships exist among syringomyelia, cervical sagittal alignment, and neurological prognosis, revealing that both syrinx resolution and cervical sagittal alignment might be predictive factors for prognosis, especially in the typical moniliform type.
Given that musculoskeletal abnormalities are part of the initial presentations of intraspinal lesions in a significant proportion of cases, the pre- and postoperative multifactorial analysis should be taken into account in patients with syringomyelia, especially for the sagittal plane presented on lateral plain radiographs of the spine. Spinal deformities may also serve as a certain reference value for spinal cord dysfunction in the pathogenesis and clinical manifestations. For example, Zhu et al. [
31] found the convexity of thoracic vertebrae in idiopathic syringomyelia patients associated with the deviated side of the syrinx. Ono et al. [
17] concluded that scoliosis could be a predictor of the prognosis in CM-I patients with syringomyelia. Ouellet et al. [
32] also proved that thoracic hyperkyphosis in the sagittal plane may be used as an indicator of the presence of syringomyelia. All of them reinforced the necessity of assessing sagittal plane deformity for the predisposition to syringomyelia [
33]. Therefore, from the overall prognosis of surgical decompression for syringomyelia in CM-I patients, the multivariate factors including CCOS, syrinx characteristics on MRI, and cervical sagittal alignment could evaluate the surgical outcome more comprehensively, especially for the specific syrinx phenotype.
In our opinion, the syringomyelia configuration on MRI and cervical sagittal alignment on x-ray radiographs might complement each other in the clinical diagnosis and treatment of CM-I with syringomyelia. To evaluate the prognosis of CM-I patients with syringomyelia following PFD, attention should be given only to the change of the intramedullary syrinx but also to the improvement of the cervical sagittal alignment.
Our findings have supplemented and improved based on the previous studies. Some of the propositions we put forward have a certain significance in clinical and mechanism research. On the one hand, we should pay attention to the significance of the cervical sagittal alignment in the process of syringomyelia development or disappearance after decompression, such as evaluating the prognosis of patients with syringomyelia through sagittal plane presented on lateral plain radiographs; in addition, for some patients with the cervical sagittal deformity (straight or lordotic), they may be able to return to normal on their own to a certain extent as the syrinx resolution following decompression surgery. On the other hand, we should attach importance to the special type of moniliform syringomyelia. It is characterized by typical syrinx separation on imaging morphology, which we have initially confirmed in rat models of syringomyelia [
30]. However, that still needs to be better understood whether it belongs to a transitional state of syringomyelia or a special configuration, which will certainly lead us to potential implications for the exploration of the hydrodynamics and biological mechanisms during syringomyelia formation and resolution.
But as a retrospective analysis, selection bias is hard to avoid in this study. The duration of clinical observation was not long enough, and the results cannot provide the relationship between the syringomyelia resolution and cervical sagittal alignment over a longer period of follow-up. In addition, it is necessary to conduct a more specific analysis for patients in different age groups. However, given the limited number of patients, it seems not realistic to conduct randomized controlled trials.