Surgical Strategies and Perioperative Considerations for Cervical Deformity With Cerebral Palsy: A Comprehensive Review of the Literature
Article information
Abstract
The complex nature of the cervical spine makes surgical intervention challenging when treating cervical deformity in patients with cerebral palsy (CDCP). However, few studies have investigated the unique characteristics of cerebral palsy that create the need for surgery, the most effective surgical strategies, and the possible perioperative complications. The intended benefit and the potential risk of postoperative complications must be considered when deciding to operate for CDCP. Because the approach and correction strategy depend on the type of cervical deformity, as well as the patient’s comorbidities and functional status, a customized strategy is needed. Perioperatively, botulinum toxin injections and muscle division techniques can help control excessive involuntary movements and improve the spinal fusion success rate. Surgical intervention for CDCP requires a multidisciplinary approach, and the information presented in this article is intended to help in the perioperative management and surgical treatment of CDCP.
INTRODUCTION
Patients with cerebral palsy (CP) demonstrate involuntary and repetitive neck movements and are more likely to develop cervical deformity (CD) or cervical myelopathy (CM) [1-5]. The excessive involuntary movements in CP cause early degenerative changes (degenerative discs, herniated intervertebral discs, osteophytes) as well as spinal instability [6,7]. Accelerated degeneration and continuous motion in the cervical spine eventually result in CM, which can further complicate a patient’s already compromised neurological function and severely limit their autonomy [1,8-11]. In addition, static anatomical factors (i.e., bones or ligaments) can result in problems with stenosis, and dynamic factors (i.e., nerves or muscles) are related to problems of incoordination [6,10,12-16]. Since the cervical spine has a wide range of motion and complex functions, these factors can generate a wide range of disorders and alignment pathologies necessitating surgical treatment [3,6,7]. Although cervical spine deformities in CP have unique characteristics and substantially impact patients’ quality of life, few comprehensive studies have focused on CD in patients with CP (CDCP). In a previous report, spinal deformities were present in 20% to 70% of CP patients, depending on the severity of the disease [17]. Once neurological deterioration due to CM or CD has developed in patients with CP, conservative treatments are ineffective and surgical intervention is required [10,18]. However, the diagnosis of CDCP and surgical interventions for CDCP are challenging, and the incidence of postoperative complications is 2–3 times higher than that of CD in patients without CP [19]. Therefore, we reviewed the existing literature on the characteristics and surgical strategies for CDCP including preoperative and postoperative management.
ETIOLOGY AND PATHOPHYSIOLOGY
CDCP usually presents with a rigid and severe curve of the cervical spine with dystonic muscle characteristics. Furthermore, the characteristics of CDCP differ from those of non-CP patients with degenerative CM [6]. The primary pathologic factors that lead to serious disability include: (1) compression of neural elements caused by canal stenosis from excessive spondylotic changes and (2) severe dynamic instability of the spine induced by sustained involuntary movements and malalignment of the cervical spine [20]. Studies have found that CM occurs at a younger age in patients with CP (generally in their 40s) whereas it is most common in non-CP patients in their 50s [21,22]. Although the precise incidence of CDCP has not been reported, the incidence of CDCP varies according to the type of CP [23]. There are 3 major types of CP: spastic (70%–80%), dyskinetic (athetoid/dystonic, 10%–20%), and ataxic (5%–10%). A mixed combination of the 3 types can also occur [14,24-27]. Spastic CP is the most common type, but those with dyskinetic (athetoid/dystonic) CP are at much higher risk for cervical canal narrowing in the early years of the disease [22]. In some studies, cervical spinal stenosis (CSS) and instability causing CD or CM were found to be more frequent in patients with dyskinetic (athetoid) CP than in a control group [3,21,28-30]. The authors hypothesized that increased muscle tone, poor head control, and abnormal gait patterns lead to abnormal shearing forces that contribute to development of CSS and a much higher prevalence of symptomatic CSS in patients with dyskinetic (athetoid) CP [21,28,30]. Guettard et al. [31] reported that 31% of adults with dyskinetic CP developed CM or CD, all after the age of 36 years, and another recent study found that 7.5% of adults with spastic CP had symptomatic CSS [32]. Radiological studies demonstrated that patients with dyskinetic CP exhibited an 8-fold higher frequency of cervical disk degeneration, spondylosis, and significant canal narrowing than control subjects [3,33].
ANATOMICAL CONSIDERATIONS
Because severe degenerative changes occur with CDCP, the normal anatomical structure is greatly altered, which may cause difficulties for the spine surgeon. Therefore, caution is required when using instruments and with screw insertion. Since it is important for the operator to be aware of any anatomical changes created by the CDCP, many spine surgeons depend on continuous fluoroscopy or a navigation system during surgery to ensure accurate screw insertion and prevent neurovascular complications.
The early onset of degenerative lesions in CDCP was well described by Harada et al. [3] in a radiological study of over 180 patients with CP compared with control subjects. Disc degeneration occurred in 51% of the patients, an 8-fold higher frequency than in the control group. In addition, at the C3–4 and C4–5 levels, there was listhetic instability in 17% and 27% of the patients, respectively, with a 6-fold and 8-fold higher frequency than in the control subjects. There was also a significantly higher incidence of cervical canal narrowing in the patients with CP, especially at the C4 and C5 levels. In a recent study by Kim et al. [15], disc/facet degeneration was more progressed and lateral mass (LM) height was smaller in the CP group. However, the LM thickness and width were larger in the CP group at the midcervical level. The pedicle inner diameter, which we defined as the inner cancellous diameter, was significantly smaller in patients with CP. In addition, pedicle sclerosis was more frequent, and the range of cervical motion was smaller in the CP group than in the control group. Kato et al. [10] reported that pedicle sclerosis, a wide transverse angle, and a LM deformity were frequently observed in patients with CP. Since the deformation of the cervical spine anatomy, which is the target during screw insertion, can cause a critical breach during surgery, understanding the various anatomical changes is most important in CDCP surgery.
SURGICAL DECISION PROCESS
A basic and important point in planning the surgical intervention is determining the position of the spinal deformity [34]. Lee et al. [35] proposed a surgical treatment strategy based on the T1 slope (T1S) and cervicothoracic junction (CTJ) angle (Fig. 1). When the T1S is normal and the CTJ angle is normal, the deformity is located in the cervical spine [35]. The correction should be at the lower cervical spine (including pedicle subtraction osteotomy) when the T1S is normal and the CTJ angle is kyphotic [35]. A high T1S and kyphotic CTJ angle mean the deformity is at the upper thoracic spine, and a high T1S and normal CTJ imply that the correction should be performed at the middle or lower thoracic spine [35]. Furthermore, in cervical deformities, evaluation of the flexibility and rigidity of the cervical spine should be performed preoperatively, as the results may determine the approach, technique, and range of the surgery [36]. If the cervical spine is flexible and is not ankylosed, based on a clinical examination and imaging studies including dynamic x-rays, an anterior-alone or posterior-alone correction strategy may be used [36]. If the cervical spine is rigid without ankylosed facets or has prior instrumentation, an anterior-alone strategy may be sufficient [36]. Although anterior-alone surgery can be considered in CDCP, ankylosed facets are often present; therefore, it is rare to perform anterior-alone surgery. If the anterior spinal column is rigid with ankylosed facets, a combination of anterior and posterior strategies may be needed to correct the deformity [36]. It is also important when planning deformity surgery in cervical kyphosis to locate the apex of the cervical kyphosis (C0–2 or C3–T1) [6]. In a craniocervical junction (C0–1–2) deformity, craniocervical junctional osteotomy is indicated when the deformity is irreducible and results in severe pain, functional impairment, or neurological impairment that cannot be relieved with a surgical decompression and/or stabilization procedure alone.6 When the apex of the cervical spine deformity is localized at the subaxial spine (C3–T1), surgeons may choose one of several surgical options according to curve flexibility (flexible vs. rigid) as well as the location of the apex of kyphosis (C3–C6 vs. C7–T1) [6].
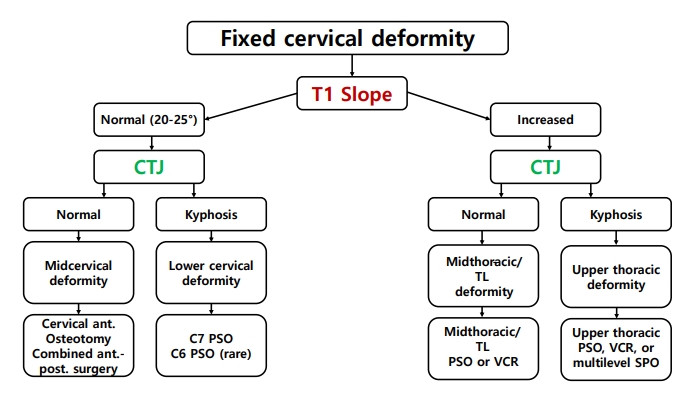
Surgical planning for fixed cervical deformities based on the location of the deformity using the T1 slope and the cervicothoracic junctional (C5–T3) angle. CTJ, cervicothoracic junction; ant., anterior; post., posterior; TL, thoracolumbar; PSO, pedicle subtraction osteotomy; VCR, vertebral column resection; SPO, Smith-Petersen Osteotomy [35].
SURGICAL METHODS
The most important surgical objectives for treatment of CDCP are adequate decompression of the spinal cord and nerve roots, stabilization of the cervical spine, alignment correction, and a good postoperative clinical outcome [6,37,38]. Recent advances in medical technology have led to the development of improved internal fixation methods that promote stronger initial mechanical stability with anterior plating or posterior screw fixation [39]. However, surgery for CDCP remains a challenge due to the risk of perioperative instrumentation failure, nonunion, deformity progression, poor bone quality, and neurological deterioration caused by repetitive involuntary neck movements and deformity of the cervical spine [38,40]. Several surgical procedures have been described, including posterior decompression without fusion and spinal arthrodesis via anterior, posterior, or circumferential approaches [2,28,41,42] In our review of the literature, most surgeons agreed that strong fixation was essential for the surgical treatment of CDCP. However, there was not a consensus on the appropriate surgical method.
1. Combined Anteriorposterior Approach With Instrumented Fusion
The anterior approach generally included releasing the disc, osteophytes, and uncovertebral joints, as well as corpectomies if indicated [36]. When the CD is rigid with ankylosed facets, a combined anteriorposterior (AP) fusion strategy may be applied [43]. Kim et al. [44] reported that combined AP fusion resulted in a superior fusion rate at 3 years postoperatively compared to posterior-alone fusion in patients with CP (26 of 28 patients, 93% vs. 22 of 35 patients, 63%; p = 0.02). Visual analogue scale (VAS) scores for postoperative posterior neck pain (5.7 vs. 2.8, p = 0.02) and the incidence of instrument-related complications (21% vs. 60%; p = 0.01) were also significantly lower in the combined AP fusion group 3 years postoperatively compared to posterior-alone fusion in patients with CP. Onari et al. [41] demonstrated that combined AP fusion can effectively improve neurological function in patients with CP and cervical spondylotic myelopathy (CSM) (CP-CSM), even in those with severe involuntary movements. Lee et al. [7] demonstrated that patients with CP-CSM who underwent deformity correction had better clinical outcomes than patients who did not undergo deformity correction. In addition to adequate cord decompression, stabilization of the cervical spine is the most important surgery-related goal in CP-CSM, and highly rigid fixation is required [30,44]. Some authors have argued that correction of CP-CSM deformities, including translational and angular deformities, is important and may require the reinforcement of posterior structures [7,45]. In a retrospective review of 36 patients with CP and myelopathy who underwent CD correction surgery, Grosso et al. [46] found that a greater degree of focal kyphosis correction was associated with improved neurological outcomes.
2. Posterior Approach With or Without Instrumented Fusion
Combined AP fusion surgery has the advantage of correcting sagittal alignment and promoting solid fusion, but for CDCP, this method may also carry a significant medical comorbidity burden. Consequently, some authors reported that a single operation with a posterior approach rather than a staged operation was also an effective treatment for CDCP [40,47-49]. Moreover, a report that autofusion inside the disc or anterior vertebral bony bridging was observed in 86% of intervertebral levels without anterior surgery also supports this view [47]. In a retrospective study of 31 patients with CP and cervical disorder, Watanabe et al. [40] showed that posterior cervical fusion alone using pedicle screw constructs had a high fusion rate and good clinical outcomes without correction loss Furuya et al. [49] reported that subaxial pedicle instrumentation achieved good surgical outcomes for patients with CP. Demura et al. [47] demonstrated that laminoplasty and pedicle screw fixation for CDCP had contributed to favorable stability and clinical outcomes at > 10 years of follow-up. The authors also reported that a C2–7 Cobb angle (from 11.9° of kyphosis to 0.8° of lordosis) could only be corrected with posterior surgery [47]. In addition, Zhou et al. [48] reported that laminoplasty with LM screw fixation was an effective treatment for CSM in patients with athetoid CP. Clinical outcomes such as the mean VAS score (p < 0.01) and Neck Disability Index score (p < 0.01) had significant decreases after surgical intervention. Several studies reported that decompression without fusion or laminoplasty is not recommended because of repetitive abnormal cervical movement, adjacent segment instability, and progression of spondylosis [15,20,30,41]. However, Harada et al. [50] suggested that cervical laminoplasty may be an effective and less invasive surgical method for selective patients, especially for those with a low level of involuntary movements and no remarkable cervical kyphosis or instability. In that study, the recovery rate based on Japanese Orthopedic Association (JOA) scores in the laminoplasty group was significantly higher than that of the fusion group (p = 0.02), whereas the C2–7 Cobb angle did not improve postoperatively.
3. Additional Perioperative Procedures
CDCP can demonstrate a poor course during and after surgery due to involuntary repetitive movements and an abnormal increase in muscle tone of the cervical spine. Even after a successful operation, CDCP is associated with a higher incidence of postoperative complications such as pseudoarthrosis or instrument failure (broken or dislodged screw) due to involuntary movements [6]. In order to partially compensate for these limitations and to improve spinal stability after the operation, additional techniques can be performed before and after surgery for CDCP [6].
1) Splenius and sternocleidomastoid muscle cutting
Muscle division aims to reduce involuntary movement by directly destroying overactive muscles. Matsuo [51] performed muscle release for catatonic torticollis and neck strain in patients with CP and showed good clinical outcomes after the procedure. Ueda et al. [42] reported that cervical laminoplasty combined with muscle release for the treatment of CM due to CP was effective in simplifying postoperative therapy and improving JOA scores. These muscle release methods were performed by cutting the splenius capitis and semispinalis muscles at the attachments to the occipital bone posteriorly. Anteriorly, the left and right splenius and sternocleidomastoid (SCM) muscles (including the sternal and clavicular branches) were cut 2 cm central to the clavicle.
2) Botulinum toxin injection
Botulinum toxin injection was the most widely used intervention in patients with CP [52,53]. Pharmacokinetically, botulinum toxin binds to the cholinergic nerve endings of the neuromuscular junction, thereby reducing the release of acetylcholine, blocking neuromuscular transmission and reducing muscular overactivity [6]. Thus, botulinum toxin injection can be very effective in controlling spasmodic torticollis perioperatively in patients with cervical dystonia [54]. Anderson et al. [55] reported improvement in 89% of patients receiving injections. The median time after injection to the onset of benefit was 7 days, with a peak benefit at day 14. The median duration of the benefit was approximately 9 weeks. The use of botulinum toxin to control cervical movements perioperatively has been reported in the surgical literature since 1996 [56]. Previous studies demonstrated that botulinum toxin decreased involuntary neck movements, facilitating postoperative spinal fusion and prevention of possible complications and reoperation [6,57,58]. Kim et al. [38] reported that botulinum toxin injections significantly lowered the incidence of a second operation in a 5-year follow-up study of 24 patients with athetoid CP.
3) Cervical traction
Prior to surgical correction of a deformity, cervical traction may be tried [59]. A trial of 3 to 5 days of traction may be sufficient to reduce the deformity [59,60]. However, if the deformity does not reduce with traction after 5 days, additional traction time or weight is unlikely to benefit the patient [59].
PERIOPERATIVE COMPLICATIONS AND RISK FACTORS
The decision to pursue surgery in CDCP should balance the intended outcome with the potential risk of complications. Providing accurate and up-to-date information on the known complications of cervical surgeries for CDCP will allow for improved informed consent and better standards for reimbursement [19]. The postoperative complications for correction of CDCP were various, including neurologic deterioration, instrument failure, pseudoarthrosis or nonfusion, and revision. Samdani et al. [61] recently reported a 39% complication rate in 127 patients with CP who underwent spinal fusion. Yaszay et al. [19] reported that spinal deformity surgery in 257 patients with CP with > 2 years of follow-up had a 36% rate of major postoperative complications with a spine-related reoperation rate of 14.0%. When compared to CD without CP, surgical treatment in patients with CP was associated with higher rates of perioperative and postoperative complications. This is likely due to differences in the comorbidities and surgical complexities of the 2 populations. Kim et al. [27] demonstrated that a CSM-CP group had significantly more overall postoperative complications than the control group (45.7%, 16 of 35 patients vs. 20.0%, 7 of 35 patients; p = 0.021). Specifically, the incidence of adjacent segment disease (20.0%, 7 of 35 patients vs. 2.9%, 1 of 35 patients; p = 0.018] and of revision (17.1%, 6 of 35 patients vs. 0%, 0 of 35 patients; p = 0.003) were significantly higher in the CP group. Moreover, more postoperative complications occurred in the fixed CD group than in the control group (31.3%, 5 of 16 patients vs. 5.3%, 1 of 19 patients; p = 0.037). Scheer et al. [62] reported significant differences in complication rates for different approaches (anterior approach, 27.3%; posterior approach, 68.4%; combined approach, 79.3%). Among patients with CP, these results were likely because surgical invasiveness, need for surgical release, and utilization of osteotomies significantly increased in those with fixed cervical kyphosis compared to those with semi-rigid or flexible kyphosis [27]. The occurrence of a major perioperative complication lengthened both intensive care unit (ICU) and hospital stays [61]. In addition, most patients with CP have substantial comorbidities and postoperative medical complications can impact the patient’s prognosis. These considerations should be adequately addressed before and after surgery. In an observational study by Yaszay et al. [19], the most common postoperative medical complications in 257 patients with CP related to wound healing (n = 16, 6.2%), pulmonary issues (n = 28, 10.9%), and prolonged ventilator support (n = 21, 8.2%). Samdani et al. [61] reported complications categorized as pulmonary 29.9% (38 of 127 patients), gastrointestinal 18.9% (24 of 127 patients), other medical (coagulopathy and severe hypotension) 11.8% (15 of 127 patients), and wound infection 4.7% (6 of 127 patients). Since the incidence of postoperative complications is much higher in a CDCP group than in a general patient group, and because unexpected complications are also more likely to occur, serious consideration is required before surgery and a more detailed observation of the patient’s symptoms is required after surgery.
The factors that affect the postoperative prognosis for CDCP are diverse and are different from those of non-CP patients with CD. In previous studies, the risk factors for postoperative complications in CDCP were analyzed. Better clinical outcomes were reported if early surgical therapy was conducted [20]. In general, it was reported that CM or CD in patients with CP often progresses in the 30- to 40-year age range. However, if unexplained neurological deterioration or changes occur before then, the clinician should suspect myelopathy even at a relatively young age [20,30]. Greater clinical attention to neurological deterioration, even subtle symptoms in individuals with CP, contributes to better outcomes [24]. Conversely, a decrease in abnormal movements must be considered an alarming sign, even though it could be interpreted as an improvement [22]. Other retrospective studies have found a significant negative correlation between developmental cervical spinal canal stenosis and recovery rate based on the modified JOA score (p = 0.01) [48]. Samdani et al. [61] reported that the risk factors for postoperative complications in CDCP included larger preoperative kyphosis (p = 0.05), staged procedures (p < 0.05), a lack of antifibrinolytic use (p < 0.05), and increased estimated blood loss (p < 0.05), with the latter being an independent predictor of a major perioperative complication. Jackson et al. [63] showed that staged and combined AP fusions were associated with longer operative times, hospital stays, ICU stays, and days intubated. Although some studies have reported that combined AP fusion increases complications [64-66], a recent study by Jackson et al. [63] demonstrated no difference in major complication rates according to the type of approach.
ADDITIONAL CONSIDERATIONS
Strict postoperative immobilization should be maintained for 3 months with a Philadelphia collar or a cervicothoracic orthosis to prevent sustained abnormal tonicity or involuntary movement of the neck [20,22,42,67]. Some authors recommend a halo vest for up to 6 months for postoperative immobilization with particular attention to the potential risk of skull fractures [42,68]. Even when the postoperative prognosis is good, long-term follow-up is essential in CDCP [18,22]. In a radiographic analysis, Demura et al. [47] found that 35% (proximal) and 21% (distal) of the adjacent segments showed a progression in degeneration of more than one grade after 10 years. More than 90% of the patients who underwent magnetic resonance imaging showed progressive disc degeneration on either side after 10 years.
CONCLUSION
Surgical treatment of CDCP can be challenging. Early diagnosis and intervention, as well as planning an appropriate surgical approach can improve the neurological function and clinical outcomes in CDCP. Understanding the unique characteristics of CDCP helps the surgeon decide on the appropriate surgical procedure. The decision to operate in CDCP should consider the intended benefits and the potential risk of postoperative complications. Additional perioperative management, such as the appropriate use of SCM cutting or botulinum toxin injections, can be effective, and long-term follow-up can help the patient’s postoperative progress.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: HCK, JKO, YH; Data curation: HCK; Formal analysis: HCK; Methodology: HCK, JKO; Project administration: HCK, YH; Visualization: HCK; Writing - original draft: HCK; Writing - review & editing: HCK, SHO, JKO, YH.
Acknowledgements
Thank you to my supervisor, Prof. Ha, for providing guidance and feedback throughout this project.
