Pediatric Spinal Hemangioblastomas: Clinical Features and Surgical Outcomes of 39 Cases
Article information
Abstract
Objective
Spinal hemangioblastomas (HBs) are a rare pathology, especially in the pediatric population. The natural history and long-term outcomes of pediatric patients with spinal HBs remain unclear due to their scarcity.
Methods
A retrospective review of the clinical data and treatment outcomes of children with spinal HBs in our institution from 2012 to 2021 was conducted.
Results
Thirty-nine pediatric patients were included, with an average age of 15.9 ± 2.9 years (range, 8–18 years), and 51.3% were female. Children were more likely to have von Hippel-Lindau (VHL) disease (p < 0.001), a family history of VHL (p < 0.001), multiple symptoms (p = 0.006), a shorter duration of symptoms (p < 0.001), and a larger lesion size (p = 0.004) and volume (p = 0.008) than their adult counterparts. The VHL-associated group of patients was more likely to present with multiple symptoms (p = 0.026), have a family history of VHL (p < 0.001), have multiple HBs (p < 0.001) and have synchronous intracranial lesions (p < 0.001) than the sporadic group. After surgery, 15 patients (38.5%) showed improved clinical outcomes, 17 patients (43.6%) remained unchanged, 4 patients (10.2%) worsened, and 3 patients (7.7%) died of tumor progression. During follow-up, there was a high rate of recurrence and repeated surgery, especially for children in the VHL-associated group.
Conclusion
Pediatric patients with spinal HBs appear to have a higher relapse risk than their adult counterparts. Therefore, life-long follow-up of these patients is necessary, especially for VHL-associated cases. Surgery can benefit children with HBs and should be considered early to avoid irreversible neurological deterioration.
INTRODUCTION
Spinal hemangioblastomas (HBs) are benign, hypervascular entities that may either present sporadically or be associated with von Hippel-Lindau (VHL) disease [1-4]. HBs, together with an accompanying cyst/syrinx, may trigger symptoms as they expand and mechanically compress the spinal cord or the nerve root. HBs account for 2%–6% of all spinal cord neoplasms and usually affect adults in their 3rd–4th decades [5,6]. Patients may experience disease progression in the form of local recurrence, distant tumor progression or formation of new HBs after surgery [7,8].
Pediatric patients with spinal HBs are rarely encountered, and these cases are usually reported conjointly with adult or intracranial HBs [9-12]. Moreover, despite being biologically identical, children with HBs may be different from their adult counterparts [11,12]. The optimal management strategy, prognostic factors, and surgical outcome are still controversial, and the proper surgical timing and follow-up strategy are unclear. Since the optimal management decision-making and follow-up strategy is mainly based on realizing the natural history of the disease, this study was undertaken to identify the clinical features and prognosis of pediatric patients with spinal HBs.
MATERIALS AND METHODS
Pediatric patients (≤ 18 years) treated with spinal HBs from January 2012 to January 2021 in our institution were identified and enrolled in this study. Clinical characteristics, magnetic resonance imaging (MRI) features, treatment strategies, and follow-up data were collected and analyzed. Patient charts were collected after obtaining permission from the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University (KY 2022-112-02-2). All human studies were approved by the appropriate ethics committee and therefore performed in accordance with the ethical standards defined in the 1964 Declaration of Helsinki.
VHL was determined through genetic analyses with the presence of established clinical diagnostic criteria (≥ 1 VHL-associated HBs with a family history of VHL, or ≥ 2 VHL-associated HBs regardless of family history) [2]. The Modified McCormick Scale (MMCS) was used to evaluate neurological function [13] (Table 1). The tumor size was defined as the largest diameter of solid part, not including the extratumoral cysts or edema. The extent of resection (EOR) was defined as total, subtotal, or partial. Disease progression was divided into local recurrence or formation of new HBs on MRI during the follow-up period.
After discharge, all patients were routinely followed at clinics at 3, 6, and 12 months, and subsequently assessed on a yearly basis either by telephone or outpatient visits. MRI was routinely performed in the first 2 years after discharge, then according to patients’ compliance or for new onset of symptoms to assess for tumor recurrence. Neurological changes and imaging features were evaluated at each visit and compared with previous data. The last follow-up was performed in May 2022.
Baseline characteristics were summarized using descriptive statistics. χ2 test (parametric) or Fisher exact test (nonparametric) were used to compare categorical variables, and Student t-test (parametric) or the Wilcoxon rank-sum test (nonparametric) were used to compare continuous variables between the 2 groups by using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). Kaplan-Meier and cox proportional hazards methods were used for time to event analysis for recurrence on follow-up. Probability values < 0.05 were considered statistically significant. All p-values were reported as 2 sided.
RESULTS
1. Baseline Characteristics
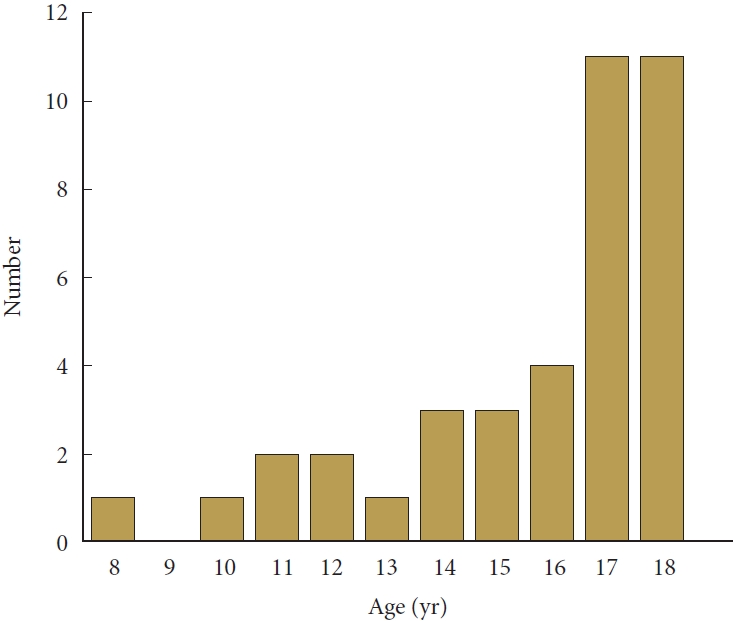
Thirty-nine eligible pediatric patients treated with HBs were included; 157 adults with HBs were also included for the comparison of clinical characteristics. Five children (12.8%) underwent 3-dimensional computed tomography angiography preoperatively, and 7 patients (17.9%) underwent cerebral HB resection before admission. The mean age of the children was 15.9 ± 2.9 years (range, 8–18 years) (Fig. 1); female predominance (51.3%) was found among the pediatric patients, which was different from the male predominance (54.8%) in the adult group. Pediatric patients had a higher prevalence of VHL (p<0.001), family history of VHL (p<0.001), and multiple HBs (p=0.049) than adult patients.
Symptoms in children on admission were mainly sensory disturbance (n=26, 66.7%), limb weakness (n=23, 58.9%), and pain (n=25, 64.1%), followed by urinary/bowel disturbance (n=8, 20.5%), and spinal spondylosis or scoliosis (n=5, 12.8%), and these symptoms were consistent with those in the adult patient population. Pediatric patients had a much shorter duration of symptoms (7.1 months vs. 33.3 months, p<0.001) and more symptoms (p=0.007) than adults. On admission, neurological disability was mostly MMCS grade 1 and grade 2 in both pediatric and adult patients.
The lesion location was mostly in the thoracic (n=24, 54.6%) segment in the pediatric group, followed by the cervical (n=14, 31.8%) segment, which was different from an obvious cervical predominance (n=106, 58.2%) in the adult group. Pediatric patients had a larger lesion size (p=0.004) and volume (p=0.008) as well as a higher proportion with lesion size ≥ 2 cm (p=0.003) and volume ≥ 2 cm3 (p=0.001) than adults. No statistically significant differences were found between the 2 groups in terms of sex, lesion site, or associated cerebral lesion signals on presentation (Table 2). A typical MRI features of HBs can be seen in Fig. 2.
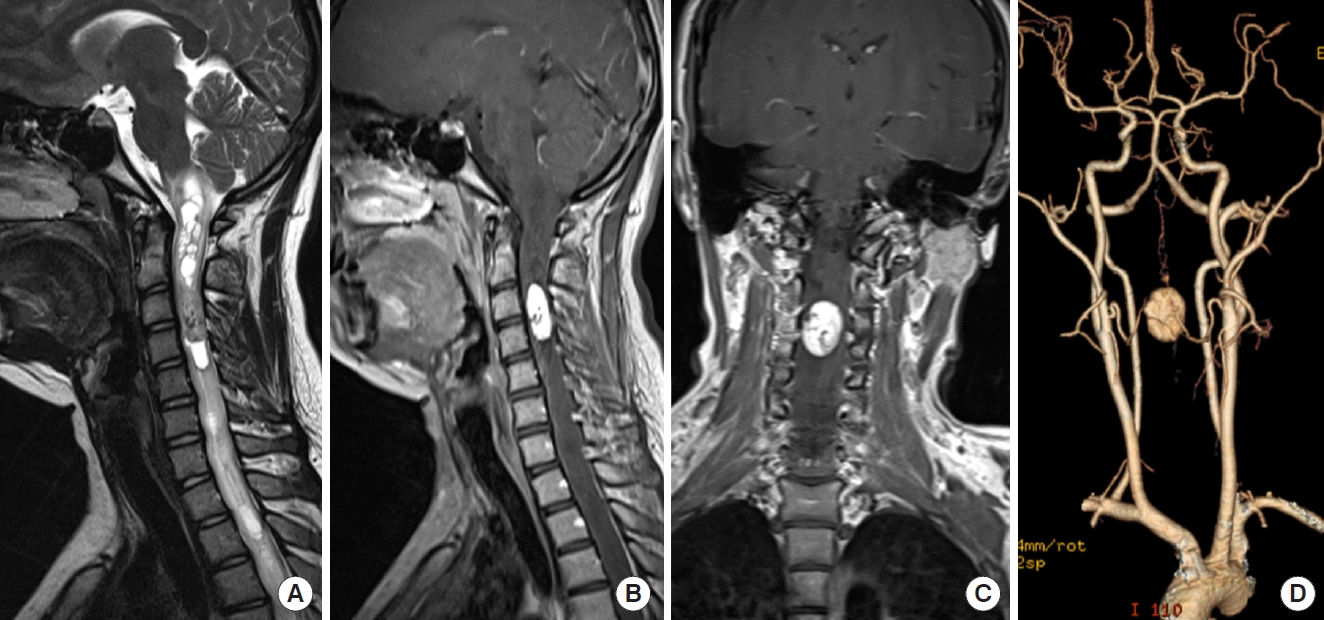
Magnetic resonance imaging (MRI) features of a 15 years old boy with spinal hemangioblastomas (HBs). Preoperative MRI shows a solid HBs at C3–4 level with iso-hyperintensity on T2-weighted images (A), and significant heterogeneous enhancement on sagittal (B) and coronal (C) view. The HB is associated with complex syrinx and perilesional edema. Computed tomography angiography can reveal the feeding arteries that comes from branches of left vertebral artery and the anterior spinal artery.
2. Sporadic and VHL Associated Spinal HBs
Children in the VHL-associated group were more likely to present with multiple symptoms (p=0.026), have a family history of VHL (p<0.001), have concurrent cerebral HBs (p<0.001), and have multiple spinal HBs (p<0.001). The mean maximum size and volume of the lesion were larger in the VHL associated group than in the sporadic group (p=0.035 and 0.010, respectively), and there was a higher proportion of lesions ≥ 2 cm (p=0.012) and ≥ 2 cm3 (p=0.001) in the VHL associated group. No difference in age, sex distribution, symptom duration, function status, lesion level or site between the sporadic and VHL associated groups was observed (Table 3). Additionally, children in the VHL-associated group had a higher recurrence rate than children in the sporadic group (p=0.008).
3. Surgical Details and Intraoperative Findings
The operation was performed via a conventional posterior midline approach, and somatosensory-evoked and motor-evoked potentials were routinely used to monitor neurological function. Removal of the solid HBs abided by the principles of arteriovenous malformation dissection. After laminectomy/myelotomy, the feeding arteries and dark-red or purplish-red HBs can usually be found. The HBs were circumferentially microdissected along the tumor-pial margin, and the feeding arteries were coagulated to reduce blood flow. The HBs were removed en bloc, rather than piecemeal, to avoid extensive bleeding. For cystic HBs, the nodule of HBs was excised microsurgically, but the peritumoral cyst walls and the associated syringes were left unmanipulated.
Indocyanine green videoangiography was used intraoperatively in 6 patients to show the feeding arteries, abnormal venous drainage, and the border of lesions. For patients with multiple spinal HBs, the principal goal was to remove the larger lesion that was assumed to be responsible for patients’ present symptoms, and adjacent HBs were also removed in one procedure if possible. Subtotal resection was achieved in 4 lesions (9.1%), and these resection processes were technically challenging. One lesion was a large cystic lesion, and total resection was deemed unsafe. One lesion with the feeding artery located ventrically and 2 lesions with obvious and tortuous feeding arteries were adhered tightly to the spinal cord without an obvious gliotic plane, and total resection was unachievable. Gross total resection confirmed by postoperative MRI was achieved for the remaining 40 HBs (90.9%).
4. Clinical Outcomes
Five patients (12.8%) experienced neurological deterioration immediately after surgery, and 3 of them returned to their preoperative status before discharge. Five patients (12.8%) had meningitis and were cured by antibiotics, and no patients experienced cerebrospinal fluid leakage or wound infection.
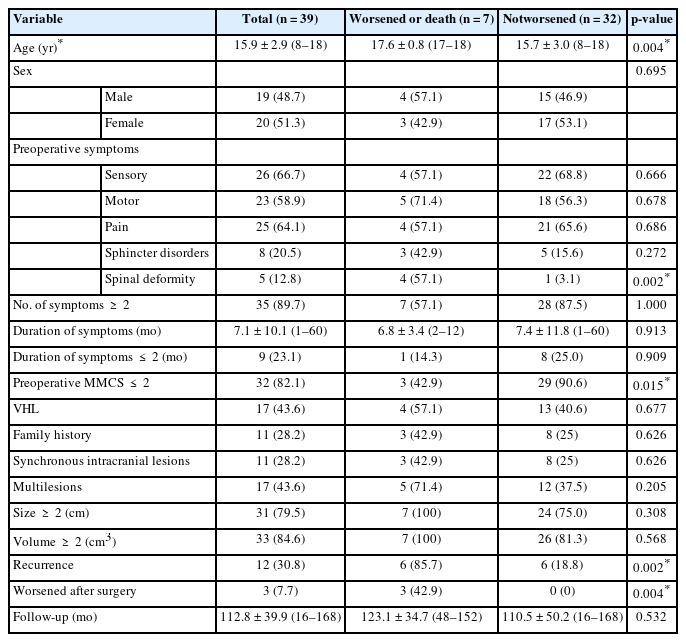
During a mean follow-up period of 99.4± 37.4 months (range, 16–124 months), 12 patients (30.8%) experienced spinal HBs recurrence. Most patients (n=9, 75%) who experienced recurrence were in the VHL group, and 5 of these patients underwent repeated surgery. Four children did not undergo surgery due to their poor health conditions, and 3 of them died of lesion recurrence for multifocal spinal cord or cerebellar HBs after an average of 77.6 months (24–121 months) after the first surgery. Three patients in the sporadic group experienced recurrences at the site of the original tumor and received a second surgery after lesion relapse. Children in the VHL group underwent more repeated surgeries for progressive spinal HBs as well as cerebellar HBs or other VHL-associated tumors beyond the central nervous system (CNS). The mean recurrent free-survival time was 84.6 ± 43.5 months (12–124 months), which was longer in the sporadic groups than VHL-associated groups (Table 3; Figs. 3, 4). The distribution of VHL-associated tumors is summarized in Table 4.
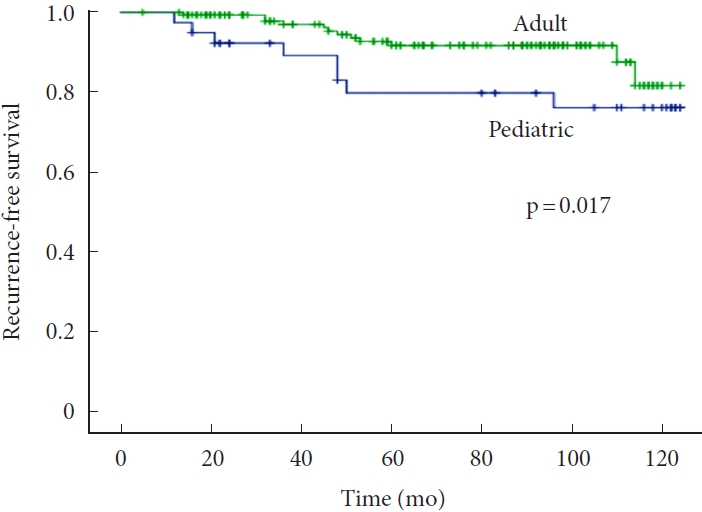
Kaplan-Meier analyses of recurrence-free survival in pediatric and adult patients with spinal hemangioblastomas.

Kaplan-Meier curve for recurrence-free survival (A) and overall survival (B) of sporadic and von Hippel-Lindau (VHL) hemangioblastomas in pediatric patients.
Pain completely resolved in all but 1 patient (4%), and other symptoms, such as weakness, sphincter disturbance, and sensory disorders, improved in 73.3%, 50.0%, and 37.5% patients, respectively, at the last follow-up. One patient (2.8%) that underwent orthopedic surgery experienced postoperative worsening of scoliosis, but an MRI confirmed no regrowth of the residual lesion 2 years after surgery. Disability calculated by the MMCS was grade 1 in 24 patients (66.7%), grade 2 in 9 patients (25.0%), and grade 3 in 3 patients (8.3%) at the last follow-up.
Compared with children whose functional status was stable at the last follow-up, children who experienced worsened postoperative functional status or death tended to be older (p=0.004), had a poorer preoperative functional status (p=0.015), had a higher likelihood of having a baseline spinal deformity (p=0.002), had a higher rate of recurrence (p=0.002), were more likely to be worsened after surgery (p=0.004), and were more likely to undergo repeated surgeries (p=0.001) (Table 5).
DISCUSSION
This study was one of the largest series focusing on the clinical presentation, natural history, management strategies and outcomes of pediatric patients with HBs. A comparison with adult patients was made, and we found that pediatric patients usually had VHL disease, with a family history of VHL, multiple symptoms, a shorter duration of symptoms, a larger lesion size, an increased incidence of multifocal tumors, and a higher rate of recurrence. Children in the VHL-associated group tended to have more symptoms at presentation and experienced a higher rate of repeated surgery than children in the sporadic group.
1. Demographics
HBs are rarely encountered in children, and the true incidence of pediatric HBs in a population-based cohort remains unclear. The mean age to receive spinal HBs surgery was 15.9 years in our cohorts, and most patients were aged between 10 and 18 years old, which was similar to previous reports on pediatric patients with brain HBs [3,11]. The youngest onset age was 8 years in our patients and 6 years in previous reports [10], which indicated that the occurrence of spinal HBs increased with age and rarely occurred before 10 years.
The sex distribution and lesion location of pediatric HBs are debatable. A slight female predominance was observed in our case series (51.3%), which was different from the male predominance for the adult patients (54.8%). The thoracic predominance in the children (54.6%) in our cohort was different from the significant cervical predominance (58.2%) in the adult patients with HBs. Additionally, we found that the average size and volume of HBs in pediatric patients were larger than those in adults.
2. VHL-Associated HBs
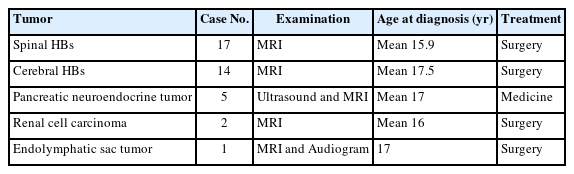
A germline mutation of the VHL gene located on chromosome 3p25-26 that encodes the VHL protein of 213 amino acids is a feature of VHL disease [2,10,14,15], and 60%–80% of VHL patients will develop CNS HBs, as previously reported [3]. In this study, we found that a higher proportion of pediatric patients had VHL than adult patients, and the most common early manifestations were associated with CNS HBs, followed by renal tumors, pancreatic tumors, and endolymphatic sac tumors. These results were inconsistent with previous reports and indicated that CNS HBs may be highly suggestive of VHL disease in childhood; therefore, germline mutations of the VHL gene should be screened. Furthermore, radiological evaluations of the brain and entire spine, abdomen, and urological as well as an ophthalmological examination should be conducted to rule out VHL-associated lesions [3,10].
Adult patients with VHL-associated spinal HBs are thought to have early onset and multiple symptoms [16,17]. We found that pediatric patients in the VHL-group had multiple symptoms and a much shorter duration of symptoms than pediatric patients in the sporadic-group. This may indicate that tumor and/or tumor-associated pseudocysts may produce more symptoms and progress more rapidly in VHL-associated children since symptoms are caused by these space-occupying components [18]. The mean size and volume of HBs were larger in children, which highlighted the growth patterns of HBs in different age groups, and HBs in children may grow more rapidly than those in adults.
3. Management Strategies and Outcomes
Complete resection is currently the best modality for management of adult HBs since surgery can result in improvement in more than half of adult patients [5,9,19-23]. However, the experience of managing pediatric ISCM is inadequate and has mostly been derived from experience with managing adult patients. However, given that pediatric patients have a higher proportion of VHL and the unpredictable growth pattern of HBs, the indications and best approach for surgical timing in this subset of patients should be clarified [3].
Our results demonstrated that surgery led to good outcomes in children in the sporadic group before significant neurological deficits occurred; all 22 children improved or were stable after surgery. However, the optimal surgical timing in patients with multiple VHL-associated tumors remains debatable. Resection of HBs that were responsible for the symptoms seems reasonable, and adjacent HBs can be removed in one procedure. However, multiple laminotomies in one surgery may cause spinal instability, thereby worsening neurological function [3]. Staged procedures can be considered for initial asymptomatic lesions [3,11,12] since no sudden deterioration is encountered in our patients and neurosurgical intervention can be postponed when radiological progression occurs. In our series, 4 patients underwent resection for multiple HBs at once and experienced no deterioration after surgery. Two patients had initially nonresected spinal HBs that did not grow significantly in size during a relatively long-term follow-up, and these patients underwent repeated surgery 36 months (12–58 months) after the first surgery. However, 4 patients with multispinal HBs did not undergo resection of all lesions, and long-term observation was needed because the growing pattern of these nonoperative HBs was unpredictable.
In general, the functional outcome after microsurgery for pediatric HBs was good, and 82.1% of children were stable. Preoperative neurological status was an important factor associated with long-term outcomes. Worsened functional status immediately following surgery was a predictor of poor outcomes. The high recurrence rate in children was problematic, especially for children in the VHL-associated group, and these recurrences did not seem to correlate with the EOR. Patients who experience recurrence can still benefit from surgery. However, an increase in neurological deficits was associated with lesion relapse and multiple surgeries due to the cumulative damage to the spinal cord as the lesion progressed. Additionally, patients exhibited a poorer functional status before a second surgery.
4. Limitations
There were several limitations in this study. First, this study involved a small case series due to the rarity of this disease, and future multicenter large-scale studies are needed to further elucidate the natural history of this disease. Second, genetic screening was not routinely performed in all family members of the children, and extensive radiological, ophthalmological, and urological screening was performed for children according to their compliance and relevance of symptoms. Therefore, the true incidence of familial and VHL-associated tumors of other systems in these pediatric patients may be underestimated. Third, the natural history of children with spinal HBs is unclear; further studies must have long enough follow-up periods to assess the lifelong risk of tumor development in these patients.
CONCLUSION
Pediatric patients with spinal HBs are different from their adult counterparts in terms of clinical features, tumor features, and recurrence rates. Pediatric patients with HBs appear to have a higher relapse risk than their adult counterparts, thus indicating a necessity for life-long follow-up, especially for VHL-associated patients. Surgery can achieve better satisfactory outcomes, even in patients with recurrent lesions, and should be considered early to avoid irreversible neurological deterioration.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (XMLX202109).
Author Contribution
Conceptualization: BH, LZ, WJ; Formal analysis: W Jia; Methodology: BH, LZ, WJ; Project administration: LZ; Visualization: WJ; Writing - original draft: BH, LZ; Writing - review & editing: BH, LZ, WJ.