The Incidence, Changes and Treatments of Cervical Deformity After Infection and Inflammation
Article information
Abstract
A healthy cervical spine with normal movement is the basis of many daily activities and is essential for maintaining a good quality of life. However, the alignment, fusion, and structure of the cervical spine can change for various reasons, leading to cervical deformity, mainly kyphosis. Approximately 5%‒20% of spinal infections in the cervical spine cause cervical deformity. The deformity can recover early; however, the disease's long-term existence or the continuous action of abnormal stress may lead to intervertebral fusion and abnormal osteophytes. Many gaps and controversies exist regarding infectious cervical deformities, including a lack of clear definitions and an acceptable classification system thereby requiring further research. Moreover, there is no consensus on the indications for postinfectious cervical deformity associated with Mycobacterium tuberculosis, Staphylococcus aureus, and Brucellosis. Therefore, we reviewed and discussed the incidence, clinical manifestations, changes, and treatment of infectious and inflammatory secondary cervical deformities from common to rare to provide a theoretical basis for clinical decision-making.
INTRODUCTION
The cervical spine is a complex and vital spinal alignment that transmits axial load from the skull, maintains horizontal gaze and average head and neck movement, protects vital neurovascular structures and achieves a maximum range of motion compared to that of the rest of the spine [1]. Therefore, a healthy cervical spine with normal movement is the basis of many daily activities and is essential for maintaining a good quality of life [2].
The alignment, fusion, and structure of the cervical spine can change for various reasons, causing cervical deformity, especially kyphosis. Cervical kyphosis can be congenital, surgical, or traumatic. In addition, cervical degenerative changes, tumors, ankylosing spondylitis, and other factors may cause cervical kyphosis.
Infection is also a cause of cervical kyphosis, although spinal infection is not very common, accounting for 2%‒7% [3,4] of systemic skeletal infections. However, with the aging of the population and increased number of immunosuppressant users, the incidence of spinal infections has increased [5], with 5%‒20% accounting for cervical spine infections [6-8]. In addition, factors that impair a patient's immune system, such as malignant tumors, malnutrition, diabetes, and acquired immune deficiency syndrome (AIDS), are risk factors for cervical spine infection, similar to those of other infectious diseases [9].
The deformity can recover early, but the disease’s long-term existence or the continuous action of abnormal stress may cause intervertebral fusion and osteophytes [10,11]. This may result in kyphosis, and the anterior edge of the spinal cord will show neurological symptoms due to compressions, such as Hoffman’s sign and tendon hyperreflexia. Some patients have permanent neurological impairment despite antibiotic treatment [12]. In addition, the cervical spine may impinge on the spinal cord owing to kyphosis during extension and flexion, resulting in cervical spinal cord injury [13].
Many gaps and controversies exist regarding infectious cervical deformities, including a lack of clear definitions and an acceptable classification system thereby requiring further research. Moreover, there is no consensus on the indications for conservative treatment, surgical methods, or postoperative drug treatment for its various types. Therefore, this article reviewed and discussed the primary classification, clinical manifestations, diagnosis, and treatment of infectious secondary cervical kyphosis to provide a theoretical basis for clinical decision-making.
CERVICAL DEFORMITY ASSOCIATED WITH MYCOBACTERIUM TUBERCULOSIS
1. Mycobacterium tuberculosis
Mycobacterium tuberculosis (MTB), the etiologic agent of tuberculosis (TB), is a severe global public health challenge, causing significant morbidity and mortality worldwide [14]. In India, Charaka and Sushruta named it “Yakshama” in the oldest medical paper ever written from 1,000 to 600 BC [15]. DNA evidence suggests that MTB was contemporary with early hominids in East Africa; therefore, it has coevolved with Homo sapiens [16]. MTB is mainly found inside immune cells that house or destroy most other bacteria [17]. MTB can counteract a complex and dynamic range of host defenses, including acidification, reactive oxygen, nitrogen intermediates, and antimicrobial peptides [18]. In addition, MTB can escape into the cytoplasmic matrix and may encounter additional environmental pillars [19].
2. Cervical Spine Tuberculosis
Spinal tuberculosis is considered secondary, caused by the hematogenous dissemination of TB from the primary lesion [20]. It mostly affects the thoracolumbar junction, followed by the lumbar and cervical vertebrae [21]. In 2011, Sabat et al. [22] reported Os odontoideum at the craniovertebral junction (CVJ), a rare preference site for MTB. Cervical tuberculosis has a greater likelihood of neurological deterioration, instability and progressive malalignment owing to its smaller canal dimension, proximity to the vertebral artery and other vital structures, unique faceted architecture, higher mobility, and lordotic alignment [23,24]. Managing cervical spine tuberculosis (CSTB) with kyphosis and delayed presentation is a great challenge [25]. With the accumulating evidence, more surgeons have focused on cervical tuberculosis deformity changes in recent years. Many surgical strategies and approaches have been undertaken to treat CSTB kyphosis.
3. Epidemiology
In 2021, the World Health Organization (WHO) estimated 10 million new TB cases. They mostly occurred in the WHO regions of Southeast Asia (43%), Africa (25%), and the Western Pacific (18%). Among all patients with TB, 8.0% were people living with human immunodeficiency virus/AIDS. The global number of TB deaths has increased from 1.2 to 1.3 million compared to that in 2019 (Global tuberculosis report 2021. Geneva: WHO; 2021. License: CC BY-NC-SA 3.0 IGO). Of the 6.3 million new TB cases confirmed by the WHO in 2017, 16% were extrapulmonary, ranging from 8% in the Western Pacific region to 24% in the Eastern Mediterranean region (Global tuberculosis report 2018. Geneva: WHO; 2018. License: CC BY-NC-SA 3.0 IGO).
According to the National Tuberculosis Clinical Center in China, skeletal tuberculosis is the main form of extrapulmonary tuberculosis in hospitalized patients, accounting for 41% of all extrapulmonary TB cases [26]. However, spinal TB remains the most common form of skeletal TB, representing 50%‒62.2% of all osteoarticular locations [27,28]. CSTB is divided into CVJ tuberculosis (CVJTB) and subaxial cervical tuberculosis (SACTB) constituting 0.3%‒1% and < 3% of all spinal TB cases, respectively [29]. Three patients (5%) who underwent ambulatory chemotherapy for spinal tuberculosis developed a deformity exceeding 60° [25]. The deformity was the chief complaint in 22 of the 27 patients (81.5%) who underwent cervical dorsal junction operation [30].
4. Pathophysiology
CSTB in adults is more localized and less purulent than in pediatric patients [31]. Tuberculosis is characterized by granulomatous inflammation. Granulomas are organized aggregates of lymphocytic infiltrates and epithelioid cells, which may coalesce to form classic Langhans giant cells, eventually leading to affected tissue necrosis and cold abscesses formation [32]. Regarding the deformity of CVJTB, the disease progresses to involve the atlantoaxial joint by destructive necrosis and inflammation, resulting from the extension of the inflammatory reaction [33]. Regarding the deformity of SACTB, the cervical spine eventually develops instability and deformity due to progressive destruction of the vertebral body caused by TB [34].
5. Clinical Presentation
CSTB involves the anterior part of the cervical vertebral body, with fewer posterior elements [35]. CSTB kyphosis tends to be less of a concern than that of chest or thoracolumbar spine TB because the weight transmission line in the cervical spine is posterior to the vertebral body, and vertebral loss is well tolerated in the cervical spine [36]. Regarding the deformity of CVJTB, severe torticollis is a characteristic presentation of atlantoaxial TB, which was occasionally reported as occipital condyle syndrome or postinfectious atlantoaxial rotary instability [33,37]. Regarding the deformity of SACTB, Luan et al. [38] measured the local kyphosis angle of 25.1° ± 8.3° in 23 SACTB patients. In contrast, Chen et al. [39] measured the local kyphosis angle of 73.6° ± 13.1° in 10 patients with upper thoracic or cervicothoracic junction. Cervical spine immaturity and flexibility are the reasons why children are prone to rapid and severe malformation progression after a vertebral collapse. An unstable spine was classified as retropulsion, subluxation, lateral translation, or toppling [40]. Children younger than 8 years had more significant deformities at presentation than that of older children and adults, meaning they were more prone to collapse in the acute phase of the disease and the progression of the deformity after widespread disease. Before age 8, the fulcrum of normal cervical motion is in the C2–3 disc space, where kyphosis progresses owing to gravity, macrocephaly, and increased flexion moments [30].
A medical team in China reported that the prevalence of neurological deficits was 73.8% in SACTB and 45% in CVJTB [41]. In the early stage, abscess, inflammatory tissue, sequestrum, and instability lead to direct compression causing a neural compromise in the active stage [42]. At the healed stage, myelomalacia, traction, or compression injury to the cord at the apex of the deformity, pseudoarthrosis, and intervertebral instability contribute to neurological deficit [43]. The Japanese Orthopedic Association (JOA) score was 11.5 points on average, and the mean visual analogue scale score was 4.5, indicating that patients with CSTB kyphosis often experience limitations in neck mobility and pain [44]. Tenderness had a high sensitivity of 97.6% for identifying abscess, which meant that if there was an abscess, tenderness was likely to occur at the abscess location [41]. The systemic complaints were also a common presentation in CSTB patients with a cervical deformity, such as fever (18%), night sweats (24%), cervical lymphadenopathy (17%), dysphagia (5%), wheezing (7.5%), and airway impairment [29,45].
6. Sagittal Alignment Changes of CSTB Deformity
As early as 1995, spine surgeons were aware of the importance of cervical and thoracic sagittal alignment in the adolescent population [46], which prompted researchers to study the complex area of cervical alignment further. For asymptomatic normal children, Lee et al. [47] proposed that the C2–7 Cobb angle of Asian asymptomatic children was -4.8° ± 12°, showing a large variation in the cervical spine curvature. We believe that cervical lordosis is a normal physiological curvature; however, studies have found that kyphosis or a straight neck is common in asymptomatic children [48].
The C2‒7 Cobb angle (8.15° ± 26.62° vs. -3.00° ± 15.96°) of patients with CSTB deformity who underwent surgery was more significant than those who could be treated conservatively. At the same time, there was no significant difference in the C2‒7 sagittal vertical axis (SVA) (16.10 ± 7.74 mm vs. 16.18 ± 13.22 mm) between them [49]. The whole sagittal alignment changes in patients with CSTB kyphosis who underwent staged combined posterioranterior surgery were reported by Luan et al. [50]: The SVA (35.19 ± 10.69 mm) and coronal balance distance (22.58 ± 7.59 mm) was greater than the normal values, indicating a coronal and sagittal imbalance. Surgery can improve the cervical spine sagittal alignment; the C0–2, C2–7, and local Cobb angles, T1 slope, C2–7 SVA, and CGH-C7 SVA were corrected remarkably after surgery [38].
7. Management of Cervical Deformity Associated With Mycobacterium tuberculosis
1) Multidrug antitubercular treatment
The fundamental principle in treating spinal tuberculosis is obtaining culture samples to develop optimal protocols [51]. Prompt antitubercular chemotherapy is required to prevent complications [52]. Multidrug antitubercular treatment (ATT) is the mainstay treatment for complicated and uncomplicated CSTB [42], and the development and combination of anti-TB chemotherapy have made it possible to reduce mortality. Multidrug ATT remains the cornerstone of spinal tuberculosis treatment is essential, as varying categories of bacilli exist in a lesion [53]. The first-line ATT include isoniazid, rifampicin, pyrazinamide, ethambutol, and streptomycin. The WHO recommends 9 months of treatment via 2 phases—intensive phase (isoniazid, rifampicin, pyrazinamide, ethambutol, or streptomycin administered for 2 months) and continuation phase (isoniazid and rifampicin for 7 months) [54]. There is still no consensus on the definition of the healing standard of spinal tuberculosis, and the time to stop ATT treatment. The consensus of Chinese experts for the diagnosis and treatment of tuberculosis recommends the antituberculosis treatment of 12–18 months [55], while India’s guidelines for the diagnosis and treatment of tuberculosis recommend the anti-tuberculosis treatment of 10–16 months [56]. Kanamycin, amikacin, capreomycin, levofloxacin are recommended, as the second-line ATT drugs, are recommended to be used judiciously due to more side effects and cost [57].
However, it is inadequate for treating CSTB kyphosis in the presence of spinal instability, progression of neurological deficits, and conservative treatment failure [58]. CSTB kyphosis management is divided into conservative and surgical treatments, which are challenging and controversial. The spinal immobilization was also considered for patients with CSTB kyphosis during the chemotherapy to obtain the stability and prevent long-term deformity [33,59].
At least 2–4 weeks of antituberculosis therapy (isoniazid 300 mg, rifampicin 450 mg, ethambutol 1,200 mg, and pyrazinamide 1,500 mg) is recommended before spinal tuberculosis patients undergo surgery. Its duration may make it possible to stabilize the disease status and restore body temperature, erythrocyte sedimentation rate (ESR), C-reactive protein levels (CRPs), and other indicators to their acceptable ranges [60]. The symptoms of TB poisoning were significantly controlled, when ESR < 50 mm/hr, and CRP <30 mg/L, surgical treatments of CSTB would be performed.
2) Surgical treatments and technique of CSTB kyphosis
The operation of cervical deformity after infection and inflammation were outlined in Fig. 1. Indications for surgery of patients with CSTB include progressive neurological worsening, significant static neurological deficits, kyphotic deformity, spinal instability, bowel bladder involvement, no response to chemotherapy, and large paraspinal abscess [24,36,38,50,61].
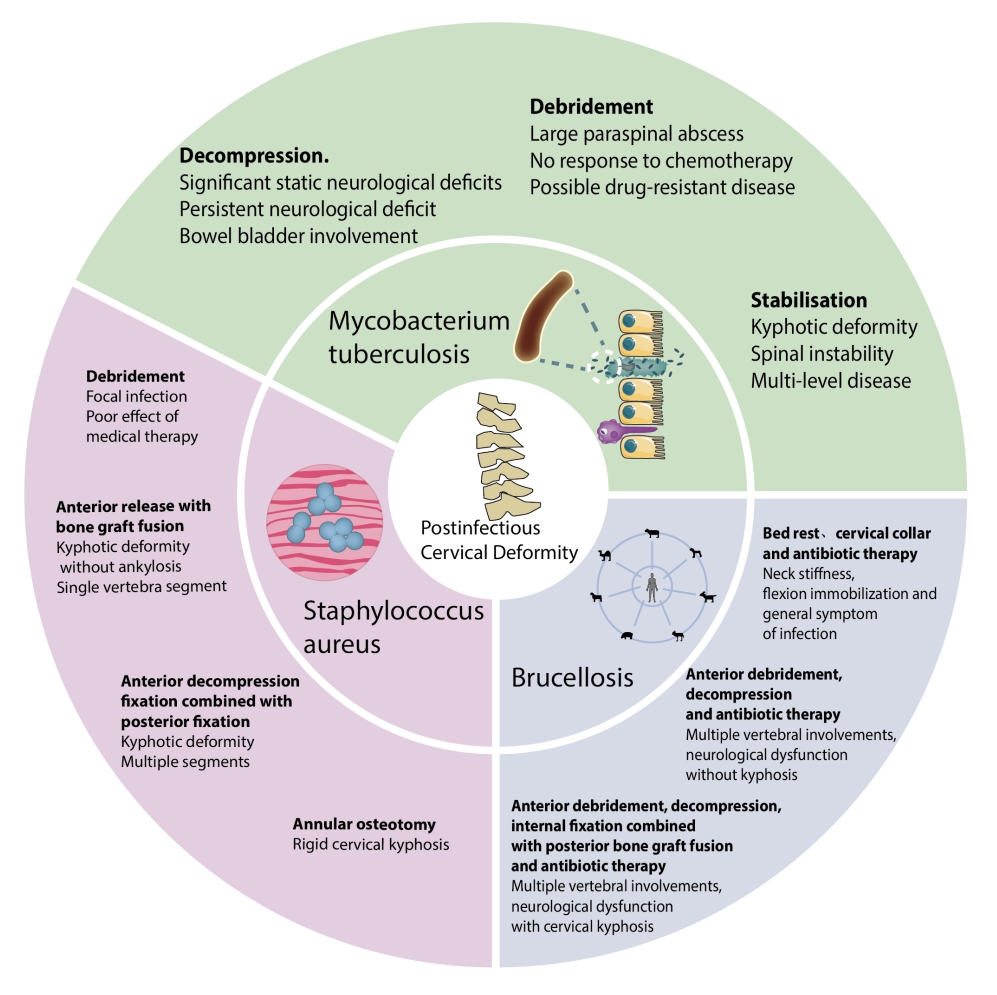
From common to rare: causes and indications of the operation of cervical deformity after infection and inflammation.
Regarding atlantoaxial TB, atlantoaxial and occipitocervical fusion are the most preferred globally [62]. If atlantoaxial TB is irreducible or rotatory, posterior distraction with stabilization or a combined anteroposterior approach should be undertaken. Transoral debridement, lesion drainage, fusion with bone graft with stabilization via external fixation, and other minimally invasive surgeries are the primary surgical treatments [60]. Twenty patients with atlantoaxial TB who underwent anterior transoral debridement combined with posterior fixation and fusion were analyzed to find that the satisfaction rate was 100%, and no severe complications were documented during follow-up [61].
Regarding SACTB, 1-stage anterior debridement, instrumentation and fusion, and single posterior instrumentation followed by chemotherapy are practical to correct the cervical deformity of the patient, whose JOA score improved to 9‒12 postoperatively [44]. In 2020, Jia et al. [43] retrospectively analyzed the safety and efficacy of early surgical management of spine tuberculosis in patients with neurological deficits. They found that standard anti-TB treatment for < 4 weeks may relieve spinal cord compression and benefit early recovery. Anterior debridement and bone grafting with fusion using internal fixation combined with anti-TB chemotherapy could eradicate the lesion, decompress spinal cord compression, and correct kyphotic deformity to restore spinal sagittal balance [38]. Using titanium cages, plates, and screws for spinal fixation stabilizes the spine and corrects the deformity. Studies have reported no risk of graft rejection or inflammation [51]. Pan et al. [36] suggest that more attention should be paid to realigning the cervical spine, particularly to restore the C2–7 SVA, the most influential factor correlated with outcome improvement when debridement, decompression, and reconstruction were performed. Fifteen articles with a total of 456 patients were evaluated in a meta-analysis, and the results showed that radical debridement might cause progressive kyphosis during children’s growth [58].
3) Surgical approaches for CSTB kyphosis
Direct access to the lesion, better decompression, and tissue sampling in the anterior approach provides biomechanically robust options for stabilization in the posterior approach. Anterior debridement and decompression followed by bone grafting and instrumentation have been widely applied as the gold standard treatment [58]. Garg et al. [25] presented an anterior-posterioranterior procedure for severe, rigid, posttubercular cervical kyphosis, which included an anterior approach to osteotomize the fused vertebral body mass and decompress the spinal cord, a posterior approach to osteotomize the fused facets and decompress the cord dorsally, and an anterior approach to replace the corpectomy cage with a larger one supplemented.
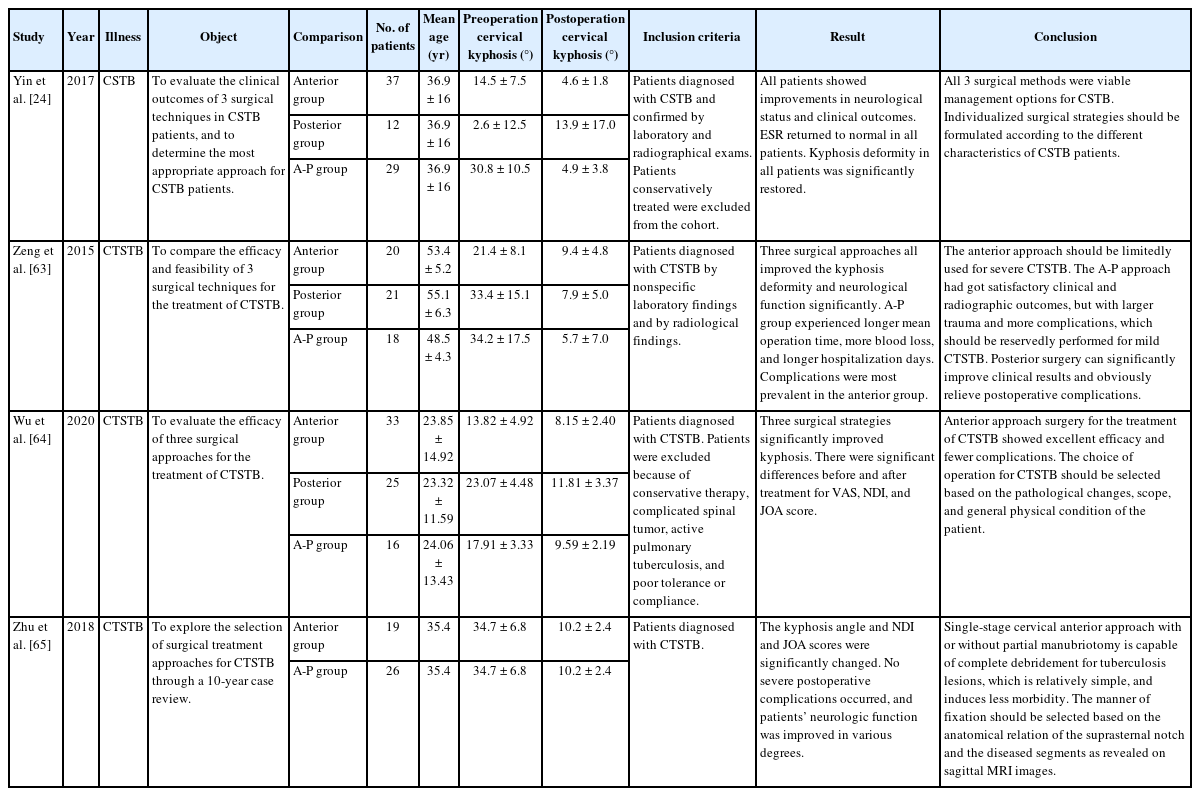
For the choice of surgical approach, spine surgeons mainly focus on the anterior, posterior, and posterioranterior combined approaches [33,36-39,42,48,61]. Table 1 summarizes the four studies that evaluated the efficacy of different surgical approaches in CSTB patients [24,63-65]. The average operation time, blood loss, and length of hospital stay for patients with CSTB who underwent the posterioranterior combined approach were greater than those who underwent the anterior or posterior approach [25]. Zhu et al. [65] compared patients with CSTB who underwent a single-stage anterior debridement and instrumentation approach with or without additional posterior fusion and reported that either approach could complete debridement for tuberculosis lesions. Yin et al. [24] found that postoperative deformities and neurological deficits significantly improved as did the visual analog pain scale at the last follow-up in the anterior, posterior instrumentation, anterior, and posterior groups (p<0.05). Direct access to the lesion enables better decompression, and tissue sampling in the anterior approach provides biomechanically robust options for stabilization in the posterior approach. Complications were most common in the anterior and posterioranterior combined approaches and least common in the posterior approach [25]. There was no significant difference among the three approaches in correction loss and bone fusion at the last follow-up (p>0.05) [64].
CERVICAL DEFORMITY ASSOCIATED WITH STAPHYLOCOCCUS AUREUS
1. Epidemiology
Staphylococcus aureus is the main pathogenic bacterium causing cervical spine infection, with an insidious onset and a lack of specificity in clinical manifestations. Pathogens can reach and infect related vertebrae in three ways: (1) hematogenous spread from the source of infection, (2) external infection caused by trauma (injury or surgery), and (3) diffusion of neighboring tissues [66]. S. aureus has the unique ability to invade, customize, and grow in the bones. Once the bone is infected, it activates osteoclasts, increases bone resorption, and destroys the vertebral body bone, further destroying the vertebral body and causing cervical instability. In severe cases, this can lead to cervical kyphosis [67]. S. aureus vertebral infections are common in the lumbar vertebrae but are rare in the cervical vertebrae, accounting for only 11% of spinal infections [6]. Other pathogenic bacteria include Escherichia coli, Streptococcus, Pneumococcus, Salmonella, etc. E. coli can be found in patients with urinary tract infections but rarely invades the cervical spine. Streptococcus and Anaerobes are commonly found in patients with diabetes, AIDS, malignant tumors, malnutrition and other diseases that damage the immune system [67-69].
2. Pathophysiology and Clinical Presentation
The rich blood supply of the spine makes the spine highly susceptible to infection. Since the arteries of one segment can supply both the lower part of the upper vertebra and the upper part of the lower vertebra, spinal infections usually involve 2 adjacent vertebrae. The spinal venous system has slow blood flow, which can be stagnant or retrograde, so the pathogenic bacteria can also spread through the venous system. In addition, the prevertebral pharyngeal vein can be a potential route for bacterial transmission during head and neck infections, thereby invading the cervical vertebra. As the most dominating pathogen of cervical spine infection, S. aureus has multiple pathogenic mechanisms during bone infection. An article from the University of Rochester, USA, published in Nature [70], identified intracellular infections within osteoblasts, osteoclasts, and osteocytes as possible sources of S. aureus persisting during osteomyelitis. S. aureus can also achieve immune escape by invading the osteocyte-lacunar tubule network. In addition, S. aureus has the unique ability to chronically infect the bone marrow by forming robust SACs during osteomyelitis and soft tissue. SACs at the bone infection site are often used to diagnose and classify osteomyelitis stages because they can significantly increase the severity of the infection by restricting blood flow to the area [71]. The possible multiple pathogenic mechanisms of bone infection by S. aureus were showed in Fig. 2.
Neck or back pain, including fever, chills, weight loss, and other common symptoms associated with bacterial infection, are the general manifestations of cervical S. aureus infection [9]. Within 2 weeks to 3 months after infection, the affected vertebral endplate is irregularly destroyed or loses its normal contour, which may progress to vertebral collapse in the later stage, leading to local kyphosis of the cervical spine [72]. In 2010, Walter et al. [73] presented five patients with cervical suppurative infection, one of whom had a 2.7° cervical kyphosis, while the other four patients had varying degrees of loss of lordosis (0.2°–3.1°). However, Abumi et al. [74] reported a case of cervical kyphosis caused by a suppurative infection of the cervical spine, with a local kyphosis Angle of 35° at the affected site. In addition, in the study of O'Shaughnessy et al. [75] of patients with rigid cervical kyphosis, they included a patient with cervical kyphosis secondary to a suppurative infection, with a kyphosis angle of 38°. With the progress of cervical kyphosis, patients often complain of different degrees of neurological symptoms such as upper limb numbness and pain, lower limb numbness, walking instability due to changes in the cervical spine force line, pathological changes in soft tissues around the forehead caused by infection, and spinal nerve roots compression [76,77].
3. Diagnosis
The diagnosis of cervical kyphosis secondary to infection is based on clinical features, imaging, bacteriology, and pathology. The literature shows that 42.6%–81.3% of the patients with suppurative spondylitis do not have an increase in white blood cells, so it can be used as a general examination but has little effect on the diagnosis of suppurative spondylitis [78,79]. After infection, 90% of patients show a rapid increase in CRP, which is more specific than ESR and is a sensitive marker of bacterial infection.
X-ray examination is the most common screening method for cervical spine infection. In the early stage of infection, narrow intervertebral space and damaged vertebral endplate can be seen in x-ray. In the late stage of infection, x-ray can reveal vertebral collapse, segmental kyphosis, and bony ankylosis of the affected part [72]. Magnetic resonance imaging (MRI) is the first choice for imaging examination and is the detection method with the highest sensitivity (93%‒96%) and specificity (92.5%‒97%) in the early diagnosis of suppurative spondylitis [80]. Most bone infection cases show diffuse and uniform enhancement on contrast-enhanced MRI. The accumulated pus showed a low signal in the T1 and a high signal in the T2 phase [81]. X-ray of the cervical spine is not evident in the early stages of infection (2‒8 weeks), and significant bone destruction can be seen after 8‒12 weeks. Computed tomography (CT) can further observe the narrowing of the intervertebral space and erosion of the intervertebral disc and endplate of the vertebral body [6]. Bacteriological and pathological examinations are the gold standards for detecting cervical suppurative infections. Local tissue puncture culture and pathological biopsy can be performed under CT guidance, but bacterial culture has a negative rate of 39% [82,83]. In addition, the consistency between the results of the bacterial blood culture and local tissue culture was 95.7% [84].
4. Management of Cervical Deformity Associated With Staphylococcus Aureus
1) Antibiotic treatment of suppurative cervical spondylitis
The diagnosis of the pathogen should be confirmed as soon as possible. According to the results of CT-guided needle biopsy and blood culture, the pathogen and drug sensitivity should be determined. And Broad-spectrum antibiotics should be used in patients with unknown pathogenic bacteria. It was believed that antibiotics such as rifampicin and levofloxacin can be used when spinal stability is not destroyed. Rifampicin can eliminate S. aureus in osteoblasts [85]. Studies have shown that rifampicin and levofloxacin have good therapeutic effects against S. aureus, the most common pathogen, and rifampicin is considered to be able to completely eliminate S. aureus in osteoblasts. Most studies recommend 6 to 8 weeks of intravenous antibiotics followed by 6 weeks of oral antibiotics [86-88]. However, Seyman et al. [87] found that intravenous antibiotics for more than 6 weeks, followed by oral antibiotics for another 8 weeks, could significantly reduce the recurrence of infection.
2) Surgical approaches for kyphosis associated with S. aureus
Medical therapy alone is less effective when patients present with progressive neurological impairment, with or without cervical instability (cervical kyphosis), and surgical treatment is needed. The objectives of surgical treatment include deformity correction, horizontal gaze restoration, the release of neurospinal compression, and restoration of sagittal balance [75,77]. Table 2 summarizes the three studies that evaluated the efficacy of different surgical approaches in patients with cervical spine infection [75,89,90]. Anterior debridement with bone graft fusion can be performed for cervical kyphosis with focal infection. Talia et al. [90] indicated that 1-stage debridement and fusion has the dual benefits of eliminating infection and stabilizing the spine. And the implantation of titanium cages after debridement is safe and effective. Hann et al. [91] also proposed that cervical kyphosis without ankylosis should be preferentially treated with anterior release and bone grafting, with or without posterior fusion. However, when the infection involves multiple segments, complete lesion removal, and the anterior bone graft fusion destroy the growing ability and stability of the anterior spine [92]. The combination of the anterior and posterior approaches can remove the lesion and resolve spinal instability, preventing the recurrence and aggravation of cervical kyphosis caused by the imbalance of anterior and posterior cervical growth in the long term after the operation [89].
3) Surgical treatments and technique of kyphosis associated with S. aureus
In the study by Papavero et al. [93], 23% of cervical spine surgery patients underwent revision surgery due to infection. Therefore, it is crucial to avoid the recurrence of infection after surgery. Chen et al. [94] describe a case of cervical kyphosis secondary to pyogenic infection who underwent anterior C5 and C6 vertebral resection due to progressive kyphosis and neurological impairment. In addition, it is the first report on the use of antibiotic polymethylmethacrylate (PMMA) strut in suppurative spondylitis. The PMMA strut mixed with antibiotics was inserted into the injured cavity before the end of the surgical procedure, and the wound was closed primarily without drainage. Antibiotics mixed with PMMA strut are released into the surrounding soft tissue, increasing the local antibiotic concentration. After the surgery, the patient’s cervical spine was stable, and the symptoms were relieved. During follow-up, no recurrence of infection occurred in the patients, but 9.8% of the patients experienced subsidence of the struts.
Annular osteotomy is required for rigid cervical kyphosis, where the Cobb angle changes by < 10° between flexion and extension. In the study of Abumi et al. [74], 13 patients with rigid cervical kyphosis underwent circular osteotomy and posterior fusion, and the kyphotic angle was corrected from +31° preoperatively to +1° at the last follow-up. No complications related to internal fixation and bone graft occurred in all patients after the operation. Brian et al. [75] also performed circular osteotomy for rigid cervical kyphosis patients in the study. The average improvement was 48°, from +38° to -10°, accompanied by a low incidence of internal fixation-related complications. Although this is a successful surgical strategy, the high risks associated with osteotomy limit the application of this technique.
Nerve injury is the most severe complication of the surgical treatment of cervical kyphosis. Therefore, intraoperative electrophysiological detection plays an essential role in reducing the occurrence of nerve injury [95]. Once there is a change in the somatosensory evoked potential and motor evoked potential, the compression site of the spinal cord should be actively searched for decompression. Supposing that the high spinal cord tension leads to electrophysiological changes, the scope of distraction should be appropriately reduced to avoid the excessive pursuit of the kyphosis correction effect. It has been shown that excessive pursuit of kyphosis correction cannot effectively increase surgical efficacy but can increase the risk of surgical complications such as nerve injury [96]. In recent years, with the continuous progress of minimally invasive techniques, surgeons have treated cervical spinal epidural abscesses with local kyphosis using minimally invasive endoscopic surgery [97]. Nevertheless, minimally invasive endoscopic cases with moderate-to-severe spinal deformities have not been reported.
The use of postoperative antibiotics remains controversial. Shiban et al. [98] suggest oral antibiotic treatment for 3 months after CRP being reduced by more than half, and clinical symptoms are significantly relieved. If inflammation markers do not show signs of infection on reexamination after 3 months of oral antibiotics and no recurrence occurs within 12 months after surgery, the infection is considered completely cleared.
CERVICAL DEFORMITY ASSOCIATED WITH BRUCELLOSIS
1. Epidemiology
Brucellosis is a systemic disease caused by certain species of Brucella that can be transmitted to humans through infected animals or dairy products. Brucellosis can damage various tissues and organs, especially the reticuloendothelial and musculoskeletal systems, leading to arthritis, bursitis, and spondylitis [99]. Osteoarthritis accounts for 20%‒60% of the cases, and spondylitis was about 8%‒13% [100]. The infection occurs most frequently within the spine in the lumbar and thoracic regions and, more rarely, in the cervical location [101,102].
2. Pathophysiology and Clinical Presentation
After invading the human body through damaged skin, gastrointestinal mucosa, or the respiratory tract, Brucella bacteria can grow and reproduce in nearby lymph nodes and are then killed by macrophages. Those that fail to be killed continue to grow and multiply to form infection foci and eventually break through the lymph node barrier into the blood to develop bacteremia, followed by violation of the reticuloendothelial system. Brucella spondylitis occurs alternately with three pathological changes: exudation, hyperplasia, and granuloma [103]. When Brucella invades the cervical spine, the patient may develop neck stiffness, flexion immobilization, and reduced range of motion with common symptoms such as fatigue, fever, and night sweats [104,105]. With the increasing number of osteoporosis patients, cervical kyphosis is more likely to occur when combined with brucellosis, which challenges its treatment [106].
3. Changes in Cervical Deformity Associated With Brucellosis
Brucella spondylitis was first reported by Tekkok et al. [107], but it is more common in the lumbar spine and rarely occurs in the thoracic or cervical spine [108]. There are two main types of Brucella infection: focal and diffuse. The diffuse type can lead to the softening of the involved vertebral endplate and the instability of the intervertebral disc, and segmental cervical kyphosis may occur with the progression of the disease [109]. A study in 2022 involving 22 patients with Brucella cervical infection showed a mean kyphotic cobb Angle of 11.5° in 22 included patients. The mean kyphosis angle of these 22 patients improved from 11.5° preoperatively to 0.2° postoperatively. At the last follow-up, 15 of the 20 patients with neurological dysfunction had fully recovered. No implant failure or pseudarthrosis was reported, and bone fusion was achieved in all patients [106].
4. Diagnosis
Brucella spondylitis can present with elevated white blood cell count and ESR. However, routine clinical and laboratory evaluations cannot precisely diagnose vertebral involvement in brucellosis, and x-rays do not reveal spondylitis or spinal discitis at an early stage. Therefore, CT, MRI, and bone imaging techniques are required for further diagnosis [100]. Its imaging features present as endplate lesions resembling Schmorl nodes and disc gas [109]. With the progress of the disease, the affected part may appear endplate destruction, intervertebral space narrowing, and even collapse of the vertebral body, and then local cervical kyphosis. It is worth noting that there are about 3 months from the involvement of the cervical spine to the occurrence of vertebral body collapse [110].
The etiological diagnosis included blood cultures and specific serological tests (Rose Bengal, agglutination, and Coombs) against Brucella. Because Brucella can migrate into cells over time [111], the positive rate in blood cultures of patients with acute and chronic brucellosis is 40%‒70% and 25% [112], respectively, which means that the longer the disease course, the lesser the likelihood of a positive blood culture result.
5. Management of Cervical Deformity Associated With Brucellosis
When cervical brucella has not yet caused kyphosis or nerve compression, conservative treatment with bed rest and wearing a cervical collar can be adopted, and antibiotic therapy should be carried out following the principle of “long-term, sufficient, and combined.” A recent study [113] showed that a long-term (at least 24 weeks) triple antibiotic regimen of doxycycline, rifampicin, and aminoglycosides was effective. All patients achieved complete remission with no recurrence or sequelae. But Ulu-Kilic et al. [114] suggest that the duration of treatment appears to be more important than the choice of antibiotic.
Surgery is necessary for patients with multiple vertebral involvements, neurological dysfunction, or significant cervical kyphosis. Khan et al. [115] reported a patient with an epidural abscess and secondary cervical kyphosis caused by Brucella infection, who underwent an anterior approach alone. Postoperative follow-up results showed that the patient developed cervical kyphosis, one of the drawbacks of anterior approach alone surgery. However, the patient did not have neck pain or neurological impairment. The authors believed that if the kyphosis developed, additional posterior fixation surgery should be considered.
It was suggested by Li et al. [106] that patients with cervical kyphosis should undergo anterior debridement, decompression, bone graft fusion, and internal fixation combined with posterior bone graft fusion. After surgery, all patients were treated with an antibiotic therapy of doxycycline 100 mg twice a day + gentamicin 5 mg/kg one a day + rifampicin 10 mg/kg up to 900 mg for at least 3 months. Clinical and radiographic results showed satisfactory surgical results without internal fixation failure and good bony fusion. de Divitiis O and Elefante [116] also concluded in a review of brucellosis that surgery is the best treatment for patients with cervical kyphosis secondary to brucellosis, and a satisfactory prognosis can be achieved in all patients with drug therapy.
CONCLUSION AND PROSPECT
In conclusion, early detection, diagnosis and treatment should be advocated for treating cervical infection. Early anti-infection therapy and debridement can often achieve good results. However, as the disease progresses, a cervical infection may cause cervical kyphosis and neurological impairment. Due to its possible injury to the spinal cord and neurological function, the method and timing of surgery for postinfectious cervical kyphosis are still controversial, which is the mainstream research direction in the future. Given the development of minimally invasive technology, its advantages of less trauma and low surgery risk will make it widely used in treating infectious cervical kyphosis. This review describes the common and rare postinfectious cervical kyphosis and reviews the relevant literature comprehensively, which provides theoretical guidance for the clinical decision-making of the disease. However, further exploration of personalized treatment for patients is still needed in future clinical work.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: JW, YH; Formal analysis: BH; Methodology: BH, JW, YH; Project administration: YH; Visualization: BH, JW, DS; Writing - original draft: BH, JW, HD; Writing - review & editing: YH, WL, PY.