INTRODUCTION
Thoracic myelopathy caused by ossification of the ligamentum flavum (OLF) is uncommon. Decompression surgery is required in patients with neurological symptoms of thoracic myelopathy. Traditional surgical techniques for the treatment of thoracic OLF include open microscopic laminectomy. However, these techniques are traumatic to the normal structures of the thoracic vertebrae, and posterior instrumentation is necessary in cases of wide laminectomy [
1]. Compared to open laminectomy, minimally invasive surgery reduces perioperative morbidity [
2].
Endoscopic spine surgery, including full endoscopy and biportal endoscopy, has evolved from lumbar discectomy to the current treatment of a wide range of degenerative diseases [
3-
8]. Spinal endoscopic techniques have also been developed to reduce the need for additional instruments [
4,
9,
10]. Full endoscopic thoracic approaches have been used to treat thoracic stenosis and have shown good surgical outcomes [
11-
15]. Biportal endoscopic surgery has also been described as a surgical technique to treat cervical and thoracic spondylotic myelopathy [
16-
18].
Additional pressure on a compressed spinal cord can induce irreversible neural injury. Therefore, a novel surgical technique that minimizes dural manipulation is necessary to safely remove thoracic OLF. Biportal endoscopic surgery is suitable for this requirement as it can perform delicate procedures intimated with the dura. A small-diameter endoscope can be used to visualize each corner of the spinal canal through a narrow extra space created by precise drilling.
We have developed a biportal endoscopic OLF removal techniques for treating thoracic myelopathy and analyzed the surgical outcomes according to different approaches.
MATERIALS AND METHODS
1. Study Patients
This prospective study analyzed patients who underwent posterior thoracic laminectomy using a biportal endoscopic system to treat thoracic spondylotic myelopathy due to OLF between January 2020 and December 2021 at 2 spinal centers. A single surgeon with 2 years of experience in biportal endoscopic lumbar spinal surgery performed all procedures. All consecutive patients who met the following inclusion criteria were included in this study.
(1) The presence of myelopathy symptoms in the back and legs for more than 6 weeks failing to respond to conservative treatment.
(2) Thoracic OLF, with or without spinal cord signal changes, confirmed using magnetic resonance imaging (MRI) and computed tomography (CT).
(3) Posterior thoracic OLF removal performed for one or 2 consecutive levels.
Patients were excluded based on following criteria:
(1) More than 3 consecutive levels of decompression surgery were necessary.
(2) Thoracic OLF was accompanied by segmental instability at the operating level.
(3) Thoracic OLF was accompanied by ossification of the posterior longitudinal ligament, involving more than 50% of the spinal canal and prominent disc herniation.
(4) A broad extent of dural ossification was identified on the preoperative CT.
2. Surgical Procedures
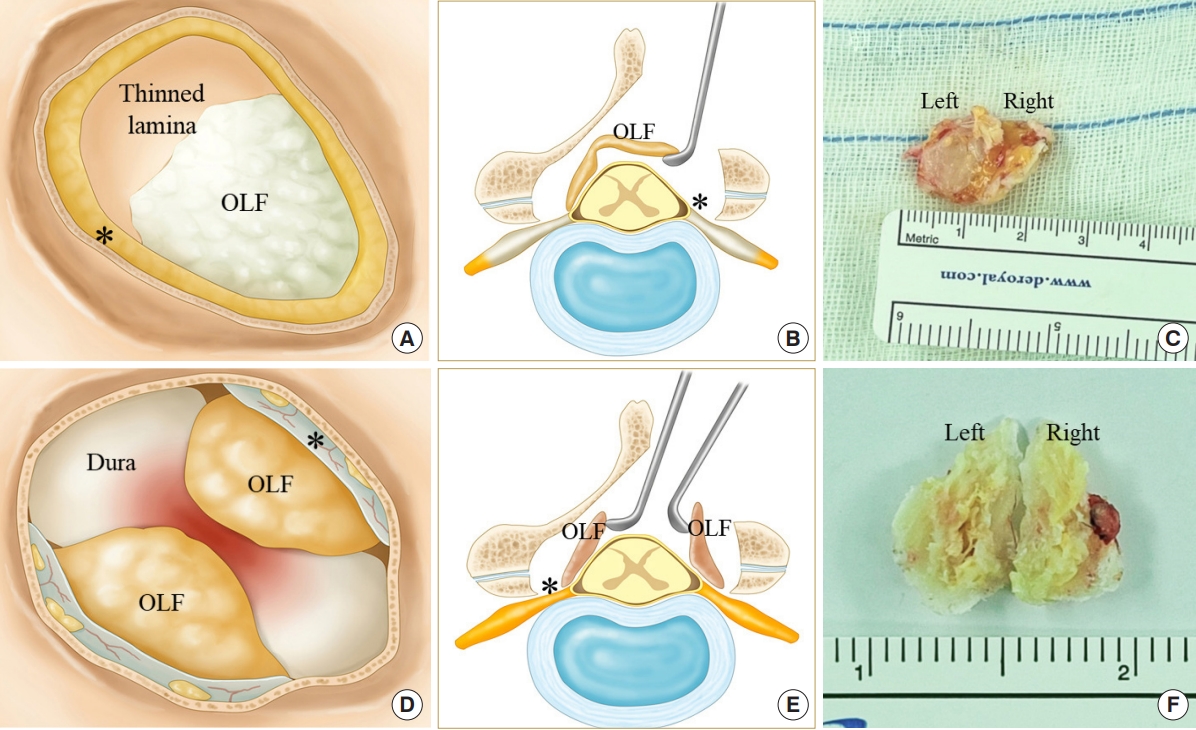
Surgical methods were divided into 3 types: inside-out piecemeal, outside-in
en bloc removal as a single flap, and bisection flaps. Inside-out piecemeal removal referred to early exposure of the dura after partial laminotomy and flavectomy. Punches and dissectors were inserted into the space between the dura and the OLF to remove the ossified mass. Serial removal of the lamina and ossified flavum was repeated using a piecemeal removal pattern with a drill and punches. Outside-in decompression involved removing the lamina and ligamentum flavum by layer-by-layer drilling to expose the entire contour of the OLF (
Fig. 1). The identified OLF was cut using a diamond drill and extracted with an
en bloc pattern as a single piece (
Fig. 1A-
C) or bisected flaps (
Fig. 1D-
F). The 2 outside-in
en bloc removal techniques consisted in the following steps (
Supplementary video clip 1).
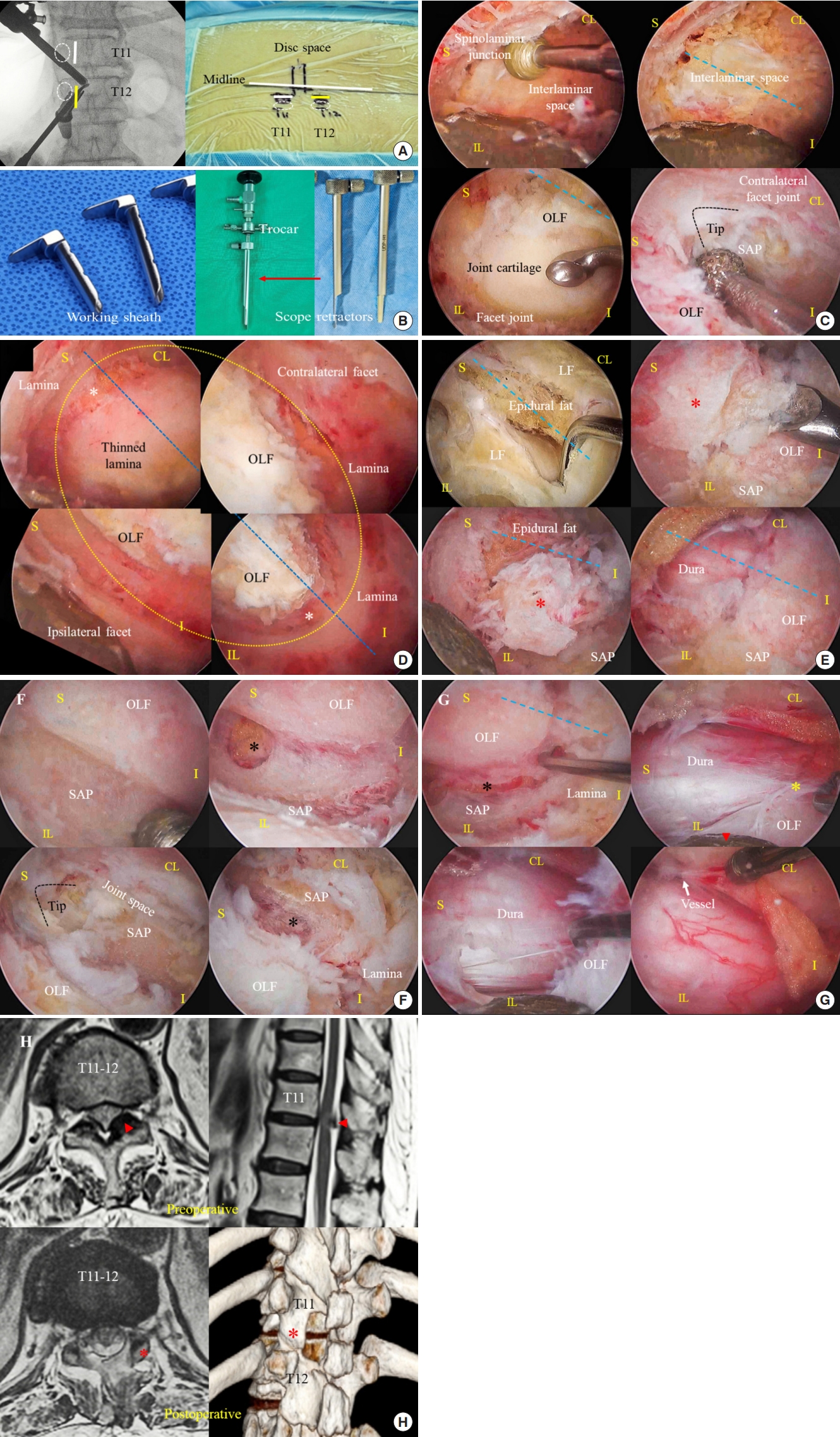
Patients underwent surgery under general anesthesia in the prone position on a chest bar or a radiolucent Wilson frame. The characteristics of the OLF determined the position of the surgeon. Under image intensification, 2 paramedian skin incisions were made at the medial border of the target-level pedicles to access the spinal canal bilaterally (
Fig. 2A). A biportal endoscopic system (4 mm, 0┬░ endoscope), a toolkit set, a customized scope retractor, and a working sheath (MD & Company, Seoul, Korea) were used for the procedure [
17,
19,
20]. The working sheath was essential for the smooth insertion of surgical instruments and drainage of irrigation fluid (
Fig. 2B).
The ipsilateral target lamina, upper part of the lower-level lamina, facet joint, and the bilateral interlaminar window were exposed after soft tissue dissection using a radiofrequency probe and forceps. An ipsilateral laminotomy, including the spinous process base, was performed using an endoscopic diamond drill to expose the bilateral interlaminar window (
Fig. 2C).
Cranial laminotomy was extended until the proximal end of the OLF was exposed through the space created by sublaminar drilling. The cranial end of the laminotomy could be determined by identifying the tip of the superior articular process (SAP) or by confirming the intervertebral foramen under C-arm guidance, as the extended-type OLF is usually located in the foraminal area (
Fig. 2C). Subsequently, circumferential bony drilling was performed over the estimated boundary of the OLF, laterally with the bilateral medial facet joints (
Fig. 2C,
D). The medial region of the SAP should be exposed after the initial bony drilling because the OLF is commonly fused with the SAP.
The midline of the ligamentum flavum was removed to divide the ossified mass into bilateral flaps. We confirmed the extent of OLF and dural ossification (
Fig. 2E). After identifying the contour of the OLF, the remaining lamina and the bulky OLF were thinned using a diamond drill for easy manipulation during removal. The extended-type OLF commonly presents another bony ossification attached to the isthmic part, which is separated from the capsular OLF region. The extended part of the OLF was removed first and the capsular part was completely exposed (
Fig. 2E). The capsular part of the OLF is fused to the SAP and is usually larger than the extended part. The bulky OLF was thinned using layer-by-layer pattern drilling (
Fig. 2F). Subsequently, the medial part of the SAP was drilled bilaterally along the lateral dural border, until the epidural space was exposed (
Fig. 2F). The secured OLF flap was removed from the bony edge using a fine dissector. The flap was gradually floated from the dura using a fine hook. Epidural dissection was performed safely using fine dissectors while holding the OLF flap with a scope retractor (
Fig. 2G). Subsequently, the detached OLF flap was removed
en bloc using forceps. A sufficiently decompressed thecal sac with free dural pulsation was also observed (
Fig. 2G). The illustrated case shows the successful removal of bilateral OLF at the T11ŌĆō12 level while preserving the facet joint (
Fig. 2H).
If bisection of the ossified mass was difficult, the OLF and ligamentum could be lifted as a single flap (
Fig. 1AŌĆō
C). At this time, more attention should be paid to epidural dissection to prevent dural tear (
Supplementary video clip 1).
A foamy hemostatic agent was used to achieve hemostasis of diffuse epidural bleeding. However, we recommend coagulating the epidural vessel if bleeding is noticeable, to prevent surgical site hematoma (
Fig. 2G). The skin wounds were closed by inserting a drainage catheter through the working portal. Blood loss during surgery was minimal, with a total surgical duration of 80 minutes.
3. Data Collection
1) Collection of clinical data
This study was approved by the Ethics Committee of Wiltse Memorial Hospital (IRB No. 2022-W13). Patient characteristics including sex, age, and symptoms were recorded. The level of operation, operating time, and length of hospital stay, as well as any postoperative complications, were also documented. Physicians collected clinical information before, after and at the final follow-up in the ward and at outpatient departments. Visual analogue scale (VAS) for back and leg and MacNab criteria were used to assess clinical improvement. Neurological results were evaluated using the Japanese Orthopaedic Association (JOA) score.
2) Radiologic evaluations
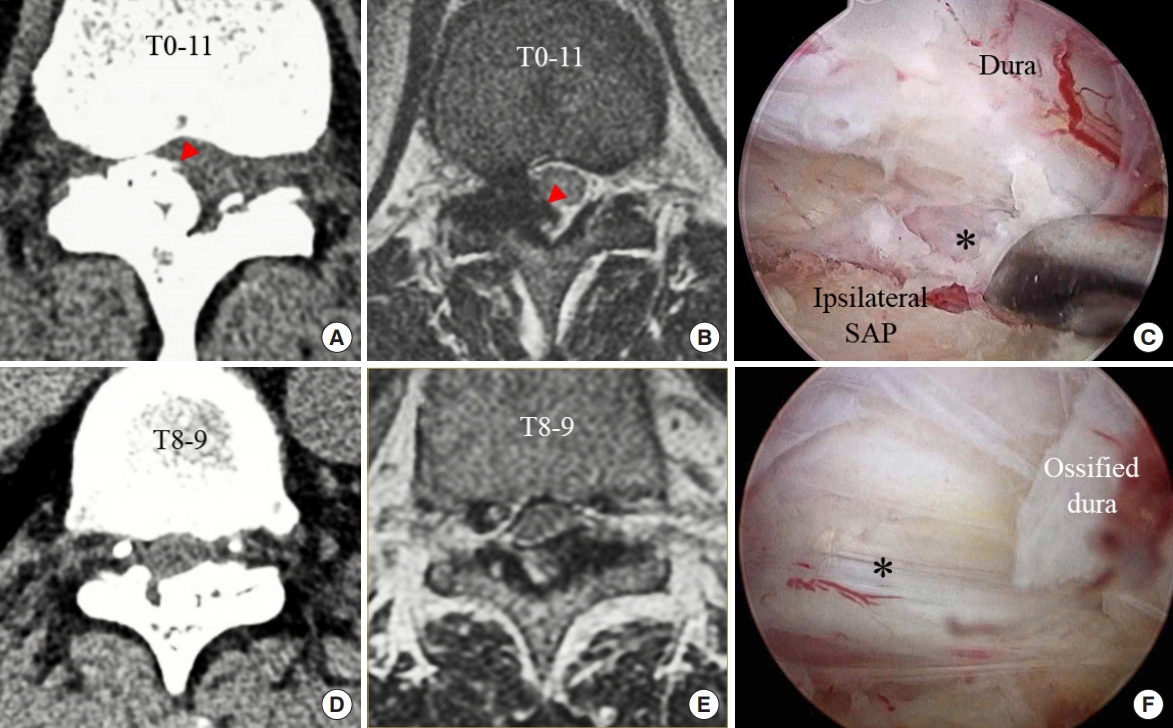
Preoperative CT and MRI were obtained to analyze dural ossification [
21] (
Fig. 3) and to categorize the Sato classification [
22]. On postoperative day 1, MRI was performed to analyze the suitability of neural decompression and complications. The endoscopic operating video was thoroughly reviewed to observe intraoperative dural ossification (
Fig. 3) and to determine the OLF removal pattern. In the video, the procedures that caused spinal cord injury were analyzed. Statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
We included 16 patients (9 men and 7 women) who underwent posterior thoracic laminectomy using the biportal endoscopic system. The mean age was 60.4 ┬▒ 9.7 years (
Table 1). The mean duration of follow-up and hospital stays was 17.4 ┬▒ 4.4 months and 7.1 ┬▒ 2.9 days, respectively (
Table 1). The operating levels are listed in
Table 1. Eleven patients underwent single-level decompression and 5 underwent 2-consecutive-level operations. The mean operation time per segment was 106.6 ┬▒ 38 minutes (
Table 1). All included patients had neurological deficits caused by thoracic spondylotic myelopathy, and the most common symptoms were mild sensory loss in the lower extremities and lack of gait stability (
Table 1).
The type of OLF was classified using the Sato classification [
22] according to the progression of ligamentum flavum ossification. The lateral type refers to the ossification of only the capsular portion of the ligamentum flavum. The extended type refers to the extended ossification of the interlaminar portion. The enlarged type indicates anteromedial thickening and enlargement of the ossification. The fused type refers to fusion of the bilateral ossified masses at the midline. The tuberous type refers to anterior growth of the fused mass of ossification. Three patients had a lateral type; 10 patients had an extended type, 1 had an enlarged type, and 2 had a fused type. The tuberous type was excluded from this study.
Three patents presented a dural ossification ŌĆ£tram tract signŌĆØ [
21] on the preoperative CT. We analyzed the intraoperative videos and observed 5 cases of dural ossification. Two patients with observed intraoperative dural ossification did not show signs of dural ossification on preoperative CT. We detached the ossified dura from the inner layer of the dura under magnified endoscopic vision but did not observe any dural laceration.
We differentiated the 3 surgical approaches according to the OLF removal pattern. Three patients in the early experience stages underwent OLF removal using the ŌĆ£inside-out piecemeal removalŌĆØ method. At the middle and late experience stages, ŌĆ£outside-in bisectionŌĆØ and ŌĆ£outside-in single pieceŌĆØ
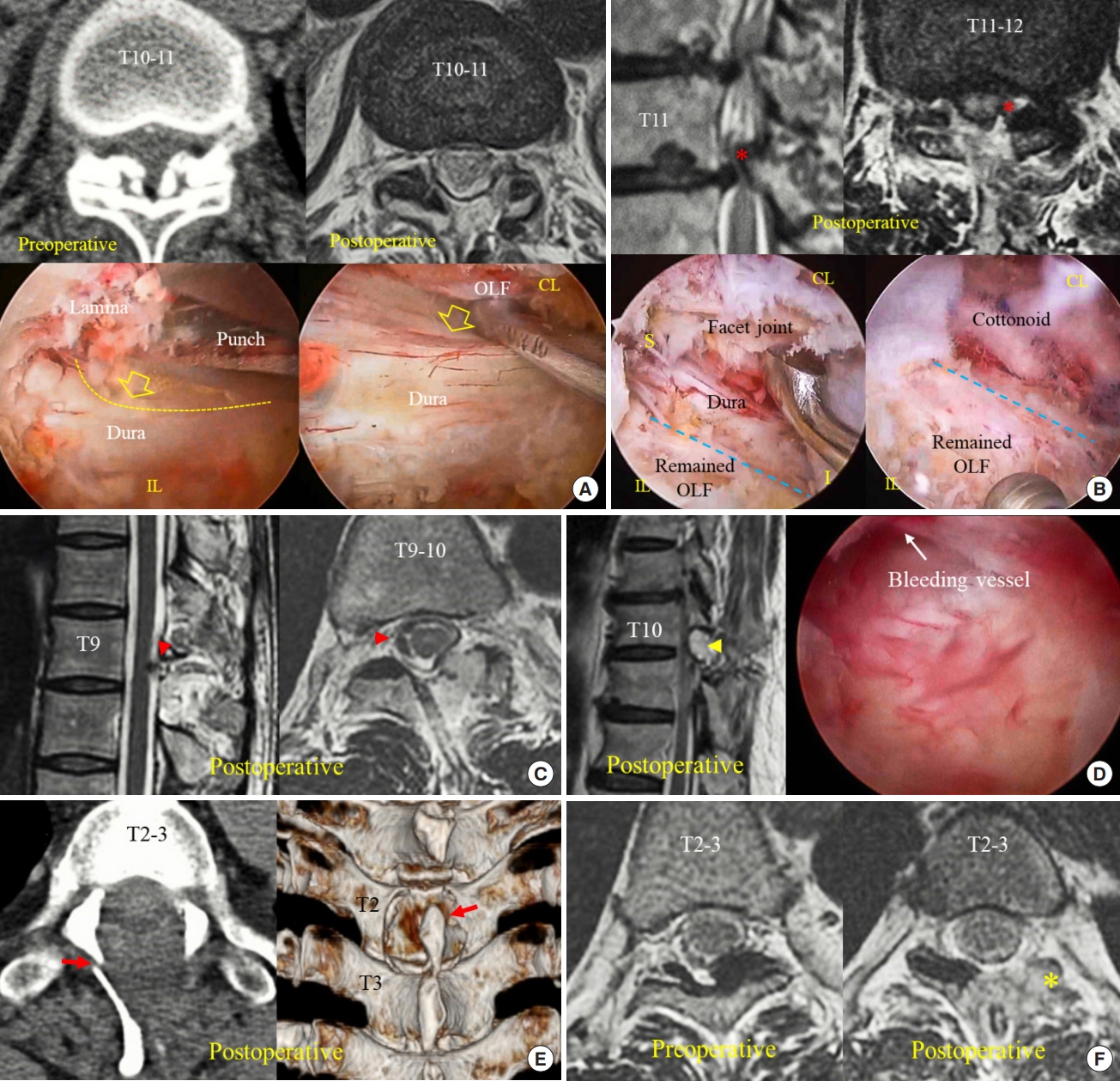
en bloc removal techniques were performed in 7 and 6 patients, respectively. Of the 3 patients who underwent the ŌĆ£inside-out piecemeal removalŌĆØ method, 2 exhibited spinal cord injury (
Fig. 4A) and 1 presented insufficient decompression on the postoperative MRI (
Fig. 4B). The 2 spinal cord injury patients could not stand alone without help and required a walking tool on the flat floor postoperatively. However, before surgery, they could walk without assistance even though they lacked gait stability. Of these, one patient recovered quickly from the illness and was able to walk without assistance; however, the other patient did not recover and needed walking assistance at the final follow-up. The patient with insufficient decompression showed marked improvement of myelopathy symptoms, but complained of persistent pain in the legs and back.
One patient who underwent a 2-level operation showed an asymptomatic subdural hematoma. We recommended 3 days of bed rest to prevent progression of the hematoma and the patient did not present any further neurologic symptoms (
Fig. 4C). One patient showed an epidural hematoma on postoperative MRI and was treated with conservative treatment. An uncontrolled epidural bleeding vessel was observed on the intraoperative video, which might cause the epidural hematoma (
Fig. 4D). Three patients with T2ŌĆō3 and T3ŌĆō4 level decompression had spinous process fractures on postoperative CT (
Fig. 4E). One patient in the T2ŌĆō3 level operation had an entirely removed facet joint (
Fig. 4F). The upper thoracic vertebrae have smaller facet joints, lamina, and spinous processes. Therefore, preserving the midline bony structures was technically demanding, and excessive facet resection was inevitable for complete OLF removal. However, the patient did not complain of mechanical back pain during the follow-up period. Furthermore, none of the included patients showed any postoperative segmental instability on lateral flexion-extension x-ray images. Infused water-related complications were not observed, such as epidural fluid collection and increased intracranial pressure.
VAS scores for back and leg pain improved significantly after surgery (
Table 2). Pain at the operating site was not noticeable because of the well-preserved muscle ligament structures. Neurological function of thoracic myelopathy was improved and confirmed with an increase in the JOA score (preoperative JOA score:12.6 ┬▒ 1.0, final follow-up:15.6 ┬▒ 1.2;
Table 2). The results of the MacNab criteria showed satisfaction with the surgery (8 excellent, 10 good, 1 poor). The patient with a severe spinal cord injury showed poor response.
DISCUSSION
In patients with cervical or thoracic spondylotic myelopathy, the compressed spinal cord is vulnerable to intraoperative injury even with slight pressure exerted by the surgical instruments. If piecemeal removal procedures are used in patients with myelopathy, repetitive insertion of instruments between the dura and the ligamentum flavum can cause irreversible spinal cord injury. In this study, 2 patients who underwent OLF removal using the ŌĆ£inside-out piecemeal removalŌĆØ method experienced intraoperative spinal cord injuries (
Supplementary video clip 2). In reviewing the operating videos, punches repeatedly pressed on the spinal cord during laminectomy and the dissectors pushed onto the dural sac during contralateral sublaminar dissection (
Fig. 4A). Furthermore, early dural exposure limited the free use of instruments and may have induced insufficient decompression. One patient who underwent inside-out decompression showed a noticeable residual mass, which prevented the improvement of symptoms (
Fig. 4B). Therefore, the surgical techniques commonly used in lumbar decompression surgery may not be suitable for thoracic decompression surgery.
Novel techniques that minimize dural manipulation are necessary for the safe removal of OLF. Recently, Kim et al. [
16,
17] described biportal endoscopic
en bloc removal techniques for treating cervical spondylotic myelopathy and cervical epidural cysts without complications. The key procedures of this technique include over-the-top decompression using layer-by-layer drilling and
en bloc lesion removal; this technique was considered safe and efficient for removing thoracic OLF while minimizing dural manipulation. Full endoscopic surgery has also been used as an
en bloc removal technique to treat thoracic OLF and has shown favorable surgical outcomes [
13]. In this study, no neurological complications occurred after using the outside-in decompression method.
Before thinning and completing marginal drilling of the OLF, any inserted instruments between the OLF and dura have a high risk of spinal cord compression. It is impossible to completely control the punch not to hit the dura while punching the hard bone (
Supplementary video clip 2). Incidental pressing on the dura can induce irreversible spinal cord injury, even if only once. Therefore, we should try to eliminate the risk of spinal cord injury during OLF removal procedures, and outside-in
en bloc removal can decrease the chance of spinal cord compression.
Outside-in decompression and
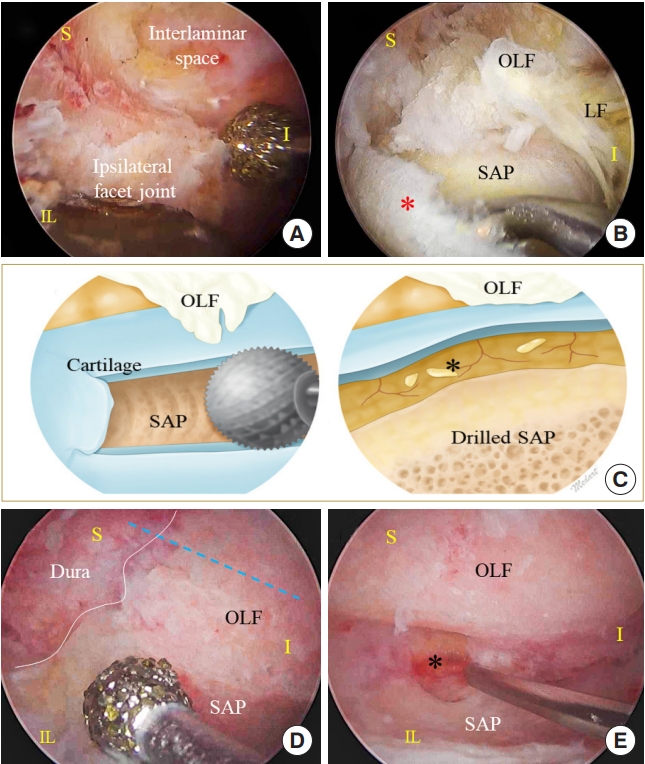
en bloc OLF removal can be technically demanding and attention should be paid to critical surgical steps. First, in the initial laminotomy step, the circumferential drilling boundary contains the ossified mass. The cranial and lateral endpoints of bone drilling should be determined prior to bone work. As the capsular part of the ligamentum flavum is ossified, the ossified mass fuses with the SAP. Therefore, the medial aspect of the SAP should be exposed after drilling the facet joint. The SAP can be differentiated from the ossified mass by confirming joint cartilage (
Fig. 5). If the joint cartilage is not visible during lateral laminotomy, drilling may be directed to the OLF and spinal cord. An ossified mass commonly extends to the neuroforaminal area as the foraminal extended ligamentum flavum becomes ossified (
Fig. 2E). Therefore, cranial drilling must be extended to the foraminal level. The foraminal location can be confirmed by identifying the SAP tip (
Fig. 2C) or by using an intraoperative x-ray image.
Second, the bulky OLF mass consists of hypertrophied SAP and an ossified ligamentum flavum. A diamond drill can slide along the outer surface layer of the OLF and can be peeled off in a layer-by-layer pattern. The thinned OLF is easy to manipulate and minimizes the risk of additional neural compression during the removal procedure (
Fig. 1B,
E). The thinned OLF can be cut into the SAP, which is covered by joint cartilage along the lateral border of the dural sac. This cutting location is critical for removing the OLF without any residual ossified mass, and the cutting tract is determined by an imaginary line of the dural lateral edge (
Fig. 5).
Third, the half-and-half removal technique may be safer than the single-piece removal method for bilateral nonfused OLF. Smaller halved OLFs offer easier manipulation and reduce the risk of spinal cord compression. However, the midline portion of the OLF is fused to the lamina. Inserting the instruments into the epidural space to halve the ossified mass also exerts pressure on the vulnerable spinal cord. In this case, the single-piece removal technique could be a better surgical option than the half-and-half removal, although a more careful epidural dissection is necessary to prevent dural laceration (
Fig. 1). If the OLF is elevated at the lateral border, the contralateral part of the OLF compresses the spinal cord due to the ŌĆ£seesaw phenomenon.ŌĆØ Resected OLF flap should be pulled outward from the medial edge using angled hooks or dissectors (
Fig. 2G). Remained attachments between the OLF and bony structures can induce the ŌĆ£seesaw phenomenonŌĆØ while manipulating the OLF. Therefore, we should remove the OLF flap after completely detaching it from surrounding bony structures.
Fourth, if the thinned OLF severely adheres to the dura, the endoscope moves more medially to visualize the dissecting plane, and careful dissection is performed between the ossified outer dural layer and intact inner dural layer using the sharp hook or dissectors. This technique should be performed under clear, magnified endoscopic vision to prevent dural laceration. However, in the case of whole-layer dural ossification, the OLF should not be excessively separated from the dura but should be floated and left on the dura.
Two customized surgical instruments and an endoscopic diamond drill were essential for a successful operation (
Fig. 2B). The working sheath maintained good saline outflow and kept the epidural water pressure low. A low epidural water pressure allows safer long-time surgery at the thoracic spinal level. The scope retractor was installed on the endoscopic trocar. It acts as a neural tissue protector during bony drilling and retracts the ossified mass while detaching it from the dura.
The upper thoracic vertebrae, such as the cervical vertebrae, have smaller laminae, facet joints, and short spinous processes. Therefore, during the removal of bilateral OLF from the unilateral side of the upper thoracic level, the preservation of the spinous process and the contralateral lamina may be technically more challenging than at the lower thoracic level. In this study, excessive facet resection and delayed spinous process fracture occurred at levels T2ŌĆō3 and T3ŌĆō4 levels; however, these complications did not induce noticeable surgical site pain. Biportal endoscopic surgery preserves muscle ligament structures and may maintain segmental stability.
Although this technique has remarkable advantages, biportal endoscopic removal of OLF from the thoracic vertebrae should be considered in selected patients after gaining significant experience with endoscopic spinal surgery. Open surgery with wide laminectomy should be considered first in patients with severe signs of dural ossification [
21] and Sato classification of tuberous type [
22] on the preoperative CT scan. Continuous high-pressure saline infusion may increase epidural pressure and induce spinal cord injury [
23]. The patency of the saline outflow should be monitored carefully and a saline infusion pressure below 30 mmHg is recommended. If incidental durotomy occurs during surgery, the hole should be repaired using a fibrin-sealant patch. However, if the endoscopic repair fails, open microscopic surgery should be performed to achieve complete dural repair [
24]. Intraoperative electrophysiological monitoring is necessary to prevent iatrogenic spinal cord during surgery.