Early Experience With Uniplanar Versus Biplanar Expandable Interbody Fusion Devices in Single-Level Minimally Invasive Transforaminal Lumbar Interbody Fusion
Article information
Abstract
Objective
To compare the early radiographic and clinical outcomes of expandable uniplanar versus biplanar interbody cages used for single-level minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF).
Methods
A retrospective review of 1-level MIS-TLIFs performed with uniplanar and biplanar polyetheretherketone cages was performed. Radiographic measurements were performed on radiographs taken preoperatively, at 6-week follow-up, and 1-year follow-up. Oswestry Disability Index (ODI) and visual analogue scale (VAS) for back and leg at 3-month and 1-year follow-up.
Results
A total of 93 patients (41 uniplanar, 52 biplanar) were included. Both cage types provided significant postoperative improvements in anterior disc height, posterior disc height, and segmental lordosis at 1 year. No significant differences in cage subsidence rates were found between uniplanar (21.9%) and biplanar devices (32.7%) at 6 weeks (odds ratio, 2.015; 95% confidence interval, 0.651–6.235; p = 0.249) with no additional instances of subsidence at 1 year. No significant differences in the magnitude of improvements based on ODI, VAS back, or VAS leg at 3-month or 1-year follow-up between groups and the proportion of patients achieving the minimal clinically important difference in ODI, VAS back, or VAS leg at 1 year were not statistically significantly different (p > 0.05). Finally, there were no significant differences in complication rates (p = 0.283), 90-day readmission rates (p = 1.00), revision surgical procedures (p = 0.423), or fusion rates at 1 year (p = 0.457) between groups.
Conclusion
Biplanar and uniplanar expandable cages offer a safe and effective means of improving anterior disc height, posterior disc height, segmental lordosis, and patient-reported outcome measures at 1 year postoperatively. No significant differences in radiographic outcomes, subsidence rates, mean subsidence distance, 1-year patient-reported outcomes, and postoperative complications were noted between groups.
INTRODUCTION
Minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) has become a mainstay procedure in the treatment of degenerative spinal conditions. Compared to other interbody techniques, MIS-TLIF allows for decreased soft tissue injury, reduced nerve root manipulation, and lower risk of neurologic complications [1-3]. Additionally, minimally invasive approaches have been associated with less postoperative pain, shorter hospitalizations, and earlier mobilization [4,5]. Specialized interbody devices are utilized during MIS-TLIF to restore lumbar lordosis (LL) and facilitate bony fusion. However, the small operative window offered by MIS-TLIF limits the size of spacers that may be inserted, restricting the extent of postoperative lordosis correction, and potentially resulting in suboptimal patient outcomes [6,7]. A recent advancement in the field of TLIF interbody cages has been the introduction of devices that are expandable following deployment into the intervertebral space.
Expandable cages have been designed with a variety of favorable characteristics. Earlier generation devices were predominantly expandable in a single plane (caudal-cranial), allowing greater restoration of disc height and segmental lordosis (SL) when compared to static cages [8]. However, uniplanar devices are not risk free. The theoretical increase in distraction forces during cage expansion may increase the risk of endplate violation, resulting in iatrogenic endplate damage and cage subsidence [9]. Though controversial, cage subsidence has been associated with suboptimal clinical outcomes, with sinking of the interbody device resulting in narrowing of intervertebral disc height, diminishing anterior support to the spine, and hindering successful fusion [10]. In comparison, newer biplanar expandable cages expand in both the medial-lateral and cranial-caudal directions, thus increasing their surface area on the end plate plates prior to intervertebral disc space distraction, which may mitigate the risk of cage subsidence. Despite this advantage, biplanar devices may also contribute to endplate damage through cortical “cutting” during lateral distraction.
Though studies have compared uniplanar and biplanar expandable cages in isolation, no studies exist comparing the performance of uniplanar and biplanar expandable spacers. Thus, the primary goal of this study is to evaluate the radiographic and patient-reported outcome measures (PROMs) of uniplanar versus biplanar expandable interbody devices in patients who underwent single-level MIS-TLIF. The secondary goal was to evaluate the incidence of subsidence and complication rates between the 2 cages.
MATERIALS AND METHODS
1. Study Design and Demographics
After obtaining Institutional Review Board approval, a retrospective review of patients undergoing single-level MIS-TLIF with a bullet-shaped expandable polyetheretherketone (PEEK) interbody spacer at a single academic institution was performed. Demographic data including age, body mass index (BMI), sex, history of osteoporosis, and device details were obtained from electronic medical records for patients who underwent elective 1-level MIS-TLIF by 3 surgeons from 2014 to 2020. All patients attempted and failed a conservative treatment regimen including nonsteroidal anti-inflammatory drugs, corticosteroid injections, and physical therapy for a minimum of 3 months prior to surgery. Patients with less than 3 months of radiographic follow-up, 1 year of clinical follow-up, and those treated for trauma, tumor, or infection were excluded. Selection of cage type and size was based on surgeon preference.
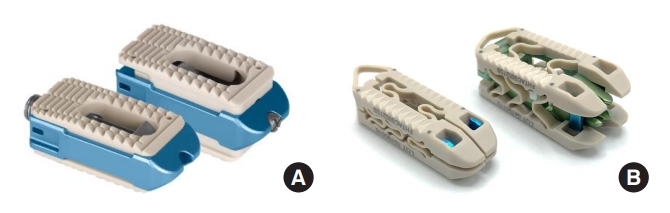
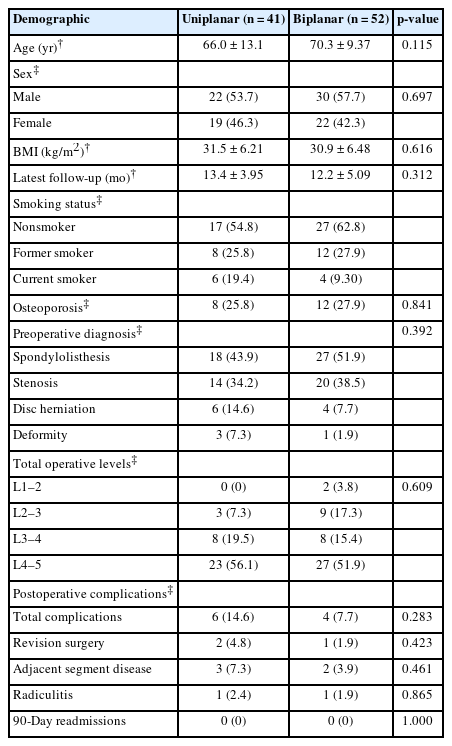
Images of cages used in this study may be seen in Fig. 1, with pre- and postoperative lateral lumbar films of each device presented in Fig. 2.
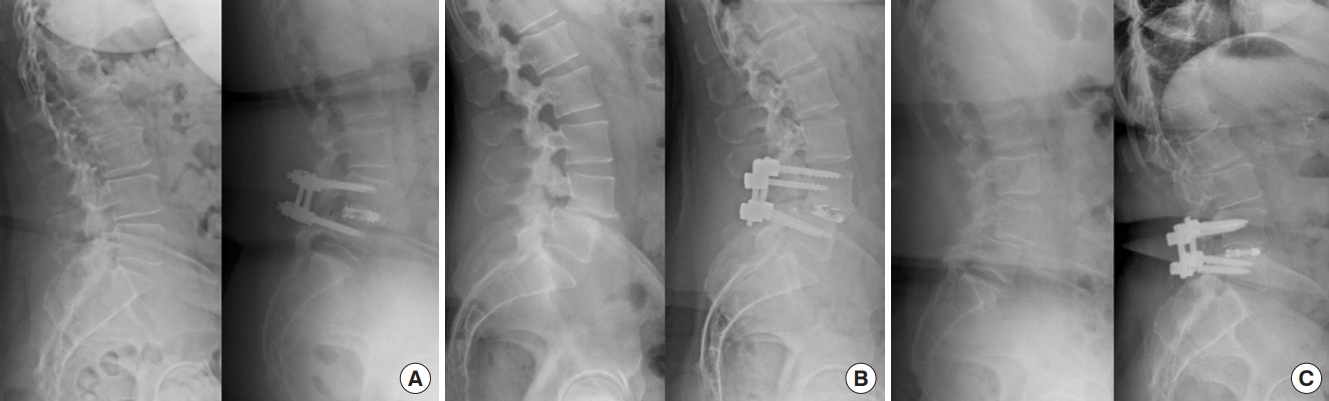
Pre- and postoperative lateral spine radiographs and photographs of various interbody devices used including Globus Caliber (A), Medtronic Elevate (B), and Accelus Flarehawk (C).
Uniplanar cages included the Elevate cage (Medtronic, Minneapolis, MN, USA) and the Caliber cage (Globus, Audobon, PA, USA), while the biplanar cage used in this study was the Flarehawk cage (Accelus, Palm Beach Gardens, FL, USA). The Medtronic Elevate and Globus Caliber are uniplanar expandable PEEK, tantalum, and titanium alloy interbody devices consisting of various lengths and starting heights. Medtronic Elevate is offered in 2 implant options. The standard implant offers posterior expansion and up to 8° of lordosis when fully expanded, while the ultralordotic implant offers fixed posterior height with various degrees of lordosis when fully expanded. The hollow geometry of the implants allows the interbody to be packed with autogenous and/or allogenic bone graft comprised of cancellous and/or corticocancellous bone.
The Accelus FlareHawk is a biplanar expandable PEEK, tantalum, and titanium alloy interbody device consisting of a Shim, Shell, and Core component. When the device is deployed, these components lock together to create one complete FlareHawk9 device. The dimensions of the final deployed device are determined by the dimensions of the selected Shim and Shell. The FlareHawk is offered in various lengths and starting heights, then expands in width, height, and lordosis based on Shim selection. Additional information regarding the selected cages may be found in Supplementary Table 1.
2. Surgical Technique
All surgeries were performed by fellowship trained spine surgeons (MFK, KER, DGA) who were familiar and proficient with the procedure. At our institution, MIS-TLIF is performed using a posterior paramedian incision of approximately 2 cm. Unilateral exposure of the disc space through a standard MIS approach was performed, which included sequential tubular dilation. Hemilaminotomies at the index surgical level(s) were then performed, followed by medial facetectomies and foraminoties. Following bony decompression, the remaining ligamentum flavum was identified and resected to reveal the underlying thecal sac. The intervertebral space was then prepared with removal of the intervertebral disc and cartilaginous portion of the endplate without violating the cortical bone, which should minimize the risk of cage subsidence. The disc space was then dilated, and a trial expandable implant deployed to determine optimal implant size. Following selection of the appropriate trial, the disc space was filled with local autograft and allograft chips. The implant was then packed with local autograft and inserted into the disc space via a transforaminal approach. Implants were then back filled with additional graft material. Bilateral percutaneous pedicle screws are then inserted over K-wire. Finally, intraoperative fluoroscopy was used to confirm appropriate screw placement.
3. Radiographic and Clinical Outcome Measures
Standing lateral lumbar spine radiographs were evaluated at 150% magnification to assess anterior and posterior disc height, fusion status at 1 year, SL, LL, pelvic tilt (PT), sacral slope (SS), pelvic incidence (PI), and PI-LL mismatch (PI-LL) preoperatively, 6 weeks postoperatively, and 1 year postoperatively. Preoperative values were subtracted from postoperative values to calculate a Δ value for each measurement. Anterior and posterior disc height were measured from the inferior endplate of the superior vertebral body to the superior endplate of the inferior vertebral body (Fig. 3). Successful fusion at 1 year was defined as evidence of bridging bone and no screw “haloing” present on anteroposterior and lateral plain radiographs [11,12]. Radiographic measurements were performed using IDS 7 imaging software for Windows (Sectra, Linköping, Sweden).

Preoperative lateral radiograph demonstrating anterior disc height (a), posterior disc height (b), segmental lordosis (c), and lumbar lordosis measurements (d).
SL was measured as the lateral Cobb angle from the inferior endplate of the superior vertebral body relative to the superior endplate of the inferior vertebral body. LL was measured as the lateral Cobb angle from the superior endplate of the L1 vertebral body to the inferior endplate of the L5 vertebral body. PT was measured as the angle between a reference vertical line and the line joining the bicoxofemoral axis with the midpoint of the S1 endplate. SS was measured as the angle between the superior endplate of S1 and a horizontal reference line. PI was measured as the angle between the line orthogonal to the midpoint of superior sacral endplate and the line connecting the midpoint of the sacral endplate with the center of bicoxofemoral axis. Postoperative radiographs were assessed for evidence of device subsidence, defined as vertical breach of the margin of the interbody device into the superior or inferior endplate of the vertebral body > 2 mm as described by previous studies (Fig. 4) [13,14].
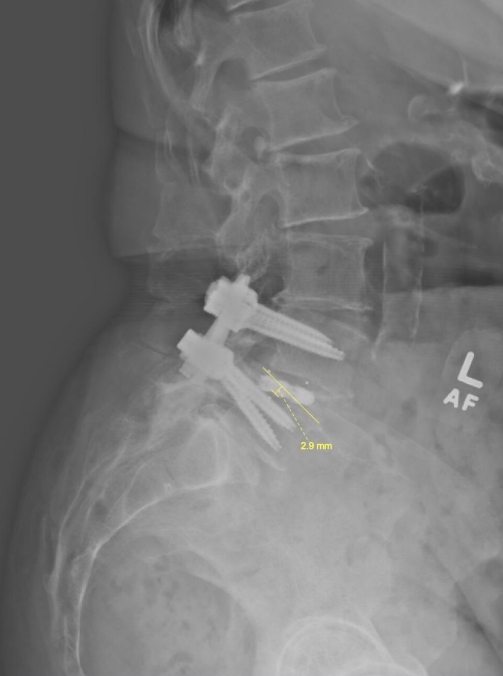
Measurement of cage subsidence defined as vertical breach of the margin of the interbody device into the superior or inferior endplate of the vertebral body > 2 mm.
PROMs were obtained from the OBERD software system (Columbia, MO, USA) using Oswestry Disability Index (ODI), visual analogue scale (VAS) back, and VAS leg pain scores. A Δ value was calculated for each PROM, as described above. The minimally clinically important difference (MCID) for each PROM was determined using previously established cutoffs: ODI 8.2 points, VAS back 2.2 points, and VAS leg 5.0 points [15,16]. Complications assessed included rates of 90-day readmissions, revision surgery, development of adjacent segment disease, dural tear, and radiculitis. Radiculitis was defined as the recurrence of radicular symptoms after postoperative resolution, with no evidence of neurologic involvement on follow-up magnetic resonance or computed tomography (CT) imaging.
4. Statistical Methods
Statistical analysis was performed using IBM SPSS Statistics ver. 27.0 (IBM Co., Armonk, NY, USA). Comparison of means for continuous variables between groups was performed using independent Student t-test. Mann-Whitney U-test was used to compare means for nonparametric variables and distributions that did not pass the Shapiro-Wilk test for normality. Preoperative and postoperative variables for the same patients were compared using paired Student t-test; Wilcoxon signed-rank test was used for nonparametric variables. Multivariate linear regression analysis was performed to determine the effect of cage type on change in PROMs perioperatively, controlling for age, biological sex, BMI, and perioperative diagnosis. Statistical significance was set at p < 0.05 for all cases.
RESULTS
1. Patient Demographics and Surgical Characteristics
This study included 93 patients, of which, 41 had uniplanar and 52 had biplanar expandable PEEK cages (Table 1). No significant differences in patient age (uniplanar: 66.0±13.1 years vs. biplanar: 70.3±9.37 years, p = 0.115), BMI (uniplanar: 31.5±6.21 kg/m2 vs. biplanar: 30.9±6.48 kg/m2, p = 0.623), sex (uniplanar: 53.7% male vs. biplanar: 57.7% male, p = 0.697) and average follow-up time (uniplanar: 13.4±3.95 months vs. biplanar: 12.2±5.09 months, p = 0.312) was noted between groups. No significant differences were noted in proportion of patients diagnosed with osteoporosis preoperatively (uniplanar: 25.8% vs. biplanar: 27.9%, p = 0.841). One instance of postoperative infection occurred in the uniplanar group, which was treated with an irrigation and debridement. There was no difference in the total complication rate (uniplanar 14.6% vs. biplanar: 7.7%, p = 0.283), rate of radiculitis (uniplanar: 2.4% vs. biplanar: 1.9%, p = 0.865), or rate of revision surgery (p = 0.423) (Table 1). No patients were readmitted within the 90-day postoperative period (p = 1.000) and there were no instances of incidental durotomies.
The most common indication for MIS-TLIF was a symptomatic spondylolisthesis (uniplanar: 43.9% vs. biplanar: 51.9%). There were no significant differences in preoperative diagnosis between groups including similar surgical indications of lumbar stenosis, disc herniation, and deformity (p = 0.392). Patients in both groups underwent surgery most frequently at the L4–5 level (uniplanar: 56.1% vs. biplanar: 51.9%, p = 0.609).
2. Radiographic Outcome Measures
No significant differences were noted in the preoperative anterior (p = 0.831) or posterior (p = 0.456) disc height between groups. Both groups had significant increases in anterior and posterior disc height at 6 weeks and 1 year. No significant differences in postoperative anterior disc height, posterior disc height, and Δ anterior and posterior disc height were noted at 6-week and 1-year (p> 0.20 for all) follow-up between groups (Table 2). No significant differences in percentage of patients with endplate subsidence were noted at 6 weeks (uniplanar: 9 of 41 [21.9%] vs. biplanar: 17 of 52 [32.7%], p = 0.249) with no additional instances of subsidence at 1 year. There were no significant differences in mean subsidence distance between cage types at 6 weeks (uniplanar: 3.96±1.49 mm vs. biplanar: 4.62±2.32 mm) and 1 year (uniplanar: 4.04±1.35 mm vs. biplanar: 4.83±2.08 mm). No significant differences in fusion rate at 1 year were noted between groups (uniplanar: 35 of 41 [85.4%] vs. biplanar: 47 of 52 [90.4%], p = 0.457).

Comparison of radiographic parameters including fusion status at 1-year, pre-, and postoperative anterior disc height, posterior disc height, and subsidence parameters at 6-week and 1-year follow-up
At 1-year follow-up, no significant differences between and within groups were noted in SS, PT, LL, SL, and PI-LL mismatch (p> 0.250 for all) (Table 3). Significant improvements in SL compared to baseline were noted within each group at 1-year follow-up (p ≤ 0.002 for both cages). Furthermore, no significant differences in anterior disc height, posterior disc height, cage subsidence (Supplementary Table 2) or sagittal parameters (Supplementary Table 3) were observed at 3 months and 1 year in patients who underwent surgery at the L4–5 level with a preoperative diagnosis of spinal stenosis or spondylolisthesis.
3. Functional Outcome Measures
Significant improvements in all PROMs within groups were noted at 3 months and 1 year (p < 0.001 for all) (Table 4). No significant differences in ODI (3-month postoperative: p = 0.738, 3-month Δ: p = 0.068; 1-year postoperative: p = 0.574, 1-year Δ: p =0.454), VAS back (3-month postoperative: p =0.982, 3-month Δ: p = 0.126; 1-year postoperative: p = 0.574, 1-year Δ: p = 0.454), and VAS leg (3-month postoperative: p = 0.825, 3-month Δ: p = 0.591; 1-year postoperative: p = 0.356, 1-year Δ: p = 0.142) were noted between groups. No significant differences were noted in the proportion of patients who reached the MCID at 1 year for ODI, VAS back, and VAS leg between uniplanar and biplanar cages (Table 5). Multivariate linear regression demonstrated no significant correlation between cage type and changes in any PROMS at 3 months and 1 year when controlling for age, sex, BMI, and perioperative diagnosis. When evaluating patients who underwent surgery at the L4–5 level with a preoperative of diagnosis of spinal stenosis or spondylolisthesis, no significant differences in ODI, VAS back, and VAS leg were noted between groups at both follow-up time points (Supplementary Table 4). Multivariate linear regression controlling for age, sex, and BMI in this same subgroup demonstrated no significant correlations between cage type and changes in PROMs at either follow-up point.
DISCUSSION
Progressive age-related spondylosis can result in a myriad of spine disease including spondylolisthesis, adult degenerative scoliosis, disc herniation, and spinal stenosis. These conditions can subsequently lead to neurogenic claudication, axial back pain, and/or radiculopathy that may prohibit participation in even the simplest of activities. MIS-TLIF offers a surgical solution to degenerative spinal pathologies through adequate neural decompression and correction of spinal alignment with minimal adjacent soft tissue disruption, thus generating significant improvements in short and long-term pain, physical function, and disability [1,2,4]. Expandable interbody spacers have been introduced in response to the size constraints imposed by the narrow operative corridor utilized in MIS-TLIF. Uniplanar cages comprised the earliest iterations of expandable devices and have demonstrated favorable results when compared to static implants in several prior studies [8,17,18]. More recently, biplanar expandable cages have been developed that enlarge horizontally, effectively increasing the contact surface area with the vertebral endplate and decreasing point expansion pressures, thus theoretically reducing the risk of implant subsidence. Despite this potential advantage, our study found no significant differences in postoperative anterior disc height, posterior disc height, sagittal balance parameters, subsidence rates, or PROMs between uniplanar or biplanar expandable implants at 1 year.
Consistent with previous studies, patients receiving uniplanar or biplanar cages experienced significant improvements in disc height, SL, and all PROMs at 3 months and 1 year [8,17-19]. A retrospective study consisting of 48 MIS-TLIFs reported larger improvements in disc height, neuroforaminal height, and SL in patients receiving expandable cages when compared to static cage [8]. These results parallel an observational study performed by Boktor et al. [20] who noted similar overall radiographic and clinical improvements amongst his patients who underwent TLIFs with expandable cages. Tan et al. [21] conducted a retrospective review of 13 consecutive patients who underwent MIS-TLIF with a biplanar PEEK cage. All patients experienced significant improvements in anterior disc height, posterior disc height, foraminal height, SL, VAS back, and VAS leg scores at the 1-year postoperative visit. Though the radiographic and clinical performance of both expandable cage types falls within the expected range of improvement as denoted by the literature, the current study failed to identify any significant differences in radiographic or clinical outcomes between uniplanar or biplanar expandable cages.
With regards to secondary outcomes, no significant differences were noted in subsidence rates or mean subsidence distance at 6-week follow-up. Previous studies remain inconclusive regarding the likelihood of subsidence for expandable spacers. A biomechanical study comparing expandable and static interbody cages in 10 human cadavers revealed a trend towards higher subsidence in expandable cages despite greater contact surface area [19]. These results were mirrored by a larger retrospective comparative study using a consecutive series of 178 patients in which cage subsidence was more frequent in expandable versus static cages (19.7% vs. 5.4%, p = 0.0017) [22]. However, several other studies have described no differences in subsidence rates between cage types and low subsidence rates with expandable devices. A small retrospective review by Massie et al. [23] found a low subsidence rate of 6% with their initial experience of expandable cages in 39 patients undergoing 1- and 2-level MIS-TLIF. Gelfand et al. [24] conducted a larger comparative study consisting of 133 fused segments and found a clinically significant subsidence rate (> 4 mm) of 26.3% with no significant difference noted between groups. The present study revealed no significant differences in subsidence rates at 6 weeks between expandable cage types. Additionally, uniplanar and biplanar expandable cages provided similar maintenance of anterior and posterior disc height at 1-year. Our results suggest that the minimization of point contact stresses provided by biplanar expandable devices may not provide additional clinical utility, even in the presence of continuous axial loading moments in the postoperative period. Our data is further supported by our subsidence rates falling well within those previously established in the literature, which range from 6% to 33% [13,23]. Furthermore, the prevalence of osteoporosis was similar between groups, suggesting that bone density did not significantly influence our observed subsidence rates. Therefore, our exploratory comparison of uniplanar and biplanar cages does not provide justification for the use of biplanar expandable cages solely to minimize the risk of subsidence.
Evaluation of sagittal balance revealed no significant changes at 1-year follow-up between uniplanar and biplanar groups, or within either group, for all radiographic parameters except for SL which improved significantly compared to baseline. Concerns persist regarding the ability to achieve sufficient lordosis correction through TLIF, with some studies demonstrating either no significant changes or worsened kyphosis postoperatively [18,24]. However, the consensus regarding the lordotic capabilities of TLIF remains a subject of debate. In line with our results, a large meta-analysis by Alvi et al. [17] consisting of 706 patients revealed a significant improvement in SL with expandable cages at final follow-up (15.55 months); however, no significant changes in LL were appreciated. The increased distraction forces exerted during in situ cage expansion may account for greater gains in SL. However, these modest gains may induce changes at adjacent levels with potentially negative overall effects on LL or may comprise such a small portion of overall LL that the magnitude of SL change is not large enough to significantly alter the overall LL [17]. Regardless, Vaishnav et al. [8] reported maintenance of segmental and whole LL in those with low (< 15°) preoperative SL with static and expandable cages. However, those with moderate (> 15° to < 25°) and high (> 25°) preoperative SL who received expandable cages only demonstrated maintenance of lordosis compared to those receiving static cages. The results of this study suggest that both uniplanar and biplanar devices provide significant improvements in SL with no change in LL or any other sagittal parameters consistent with prior studies [8,17,21].
The findings of this study should be interpreted with the following limitations. The retrospective nature of this study raises concern for inherent biases, such as restriction of the number of patients in each treatment arm, availability of clinical data, and implant selection bias by the surgeons. In response, regression analysis controlling for demographic and surgical factors was performed to reduce confounders associated with our results. However, we are unable to control for surgeon implant selection bias, which we acknowledge is a main limitation of our data. Additionally, longer follow-up is necessary to evaluate whether cage types influence long-term PROMs, which may differ based on construct-related factors such as gradual cage subsidence and fusion quality [25]. Finally, radiographs rather than CT scans were used to evaluate fusion status at 1 year, which we are aware has lower sensitivity and specificity than CT scans. However, our institution does not routinely obtain postoperative CT scans in patients undergoing lumbar fusion unless the patient has new-onset axial back pain with or without radiculopathy that appears consistent with a potential pseudarthrosis.
CONCLUSION
Overall, our experience with uniplanar and biplanar expandable PEEK cages indicate that both cage types are safe and efficacious at improving anterior disc height, posterior disc height, SL, and PROMs at 1 year postoperatively. No significant differences in clinical and radiographic outcomes including subsidence rates and 1-year fusion rates were observed between uniplanar and biplanar expandable cages. Further, no differences in postoperative complication rates, 90-day readmissions, and revision surgery rate were noted in either group. This study supports the need for larger, preferably randomized studies, with longer follow-up durations to validate our experience.
Supplementary Material
Supplementary Tables 1-4 can be found via https://doi.org/10.14245/ns.2244870.435.
Breakdown of interbody cages used sorted by manufacturer, surface material, starting anteroposterior (A-P) length, starting and maximum medial-lateral (M-L) widths, maximum height, maximum lo rdosis, and count
Comparison of anterior disc height, posterior disc height, and subsidence parameters preoperatively, at 6-week follow-up, and 1-year follow-up for procedures performed at L4–5 in patients with diagnosis of spinal stenosis or spondylolisthesis
Comparison of radiographic sagittal parameters preoperatively and at 1-year follow-up for procedures performed at L4–5 in patients with diagnosis of spinal stenosis or spondylolisthesis
Patient-reported Outcomes at 3-month and 1-year follow-up for procedures performed at L4–5 in patients with diagnosis of spinal stenosis or spondylolisthesis
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: JAL, MJL, MFK, KER, DGA; Data curation: JAL, JCO, MJL, AD, TLT, MFK, KER, DGA; Formal analysis: JAL, JCO, MJL, AD, TLT, MFK, KER, DGA; Methodology: JAL, MJL, MFK, KER, DGA; Project administration: JAL, MJL, MFK, KER, DGA; Visualization: JAL, MFK, KER, DGA; Writing - original draft: JAL, JCO, MJL, AD, TLT, MFK, KER, DGA; Writing - review & editing: JAL, JCO, MJL, AD, TLT, MFK, KER, DGA.