Global Research Trends of Exosomes in the Central Nervous System: A Bibliometric and Visualized Analysis
Article information
Abstract
Objective
Exosomes in the central nervous system (CNS) have become an attractive area of research with great value. However, few bibliometric analysis has been conducted. The study aimed to visualize the scientific trends and research hotspots of exosomes in the CNS by bibliometric analysis.
Methods
All potential articles and reviews on exosomes in the CNS published in English from 2001 to 2021 were extracted from the Web of Science Core Collection. The visualization knowledge maps of critical indicators, including countries/regions, institutions, authors, journals, references, and keywords, were generated by CiteSpace and VOSviewer software. Besides, each domain's quantitative and qualitative analysis was also considered.
Results
A total of 2,629 papers were included. The number of exosomes-related publications and citations regarding CNS increased yearly. These publications came from 2,813 institutions in 77 countries/regions, led by the United States and China. Harvard University was the most influential institution, while the National Institutes of Health was the most critical funding source. We identified 14,468 authors, among which Kapogiannis D had the most significant number of articles and the highest H-index, while Théry C was the most frequently co-cited. The cluster analysis of keywords generated 13 clusters. In summary, the topic of biogenesis, biomarker, and drug delivery will serve as hotspots in future research.
Conclusion
Exosomes-related CNS research has gained considerable attention in the past 20 years. The sources and biological functions of exosomes and their promising role in diagnosing and treating CNS diseases are considered hotspots in this field. The clinical translation of the results from exosomes-related CNS research will be of great importance in the future.
INTRODUCTION
The central nervous system (CNS), which consists of the brain and spinal cord, influences bodily functions through innervation and neurotransmitters [1]. Due to several significant factors, including inflammation, infection, trauma, neurodegeneration, heredity, and tumor, CNS diseases have become a leading cause of death worldwide [2]. Although significant improvements have been made in neuroscience, challenges such as the undetermined etiology of some CNS disorders [3], the lack of novel early diagnostic biomarkers and precise therapeutic targets [4], more importantly, the restriction of the blood-brain barrier (BBB) on therapeutic responses to drugs still remain [5]. Fortunately, the emergence of exosomes holds much promise for the treatment and research of CNS diseases [6].
Exosomes were defined as vesicles of endosomal origin secreted from reticulocytes in the 1980s [7]. With deepening research, scientists have discovered that exosomes bear a variety of substances, including RNA, DNA fragments, proteins, and lipids, regulating intercellular communication between dissimilar cell types in vivo, thus affecting both normal and pathological conditions [8]. To step further, increasing studies have shown that exosomes are involved in CNS diseases [9]. Roughly, the cargo of exosomes can be used as noninvasive diagnostic biomarkers for CNS diseases [10], alternatively or be chosen as drug delivery vehicles that can cross BBB [11]. Therefore, exosomes-related CNS research is challenging and promising.
Bibliometric analysis has been broadly employed to understand knowledge structure and explore research trends through qualitative and quantitative analysis of included literature [12]. This analytical approach is crucial in identifying research hotspots and developing guidelines [13]. In this study, we have collated exosomes-related CNS research over the past 20 years and drawn maps of scientific knowledge by means of CiteSpace and VOSviewer software. Additionally, we examined the hotspots and developmental trends with a view to providing the basis and direction for scientific research in this field.
MATERIALS AND METHODS
1. Data Sources and Search Strategies
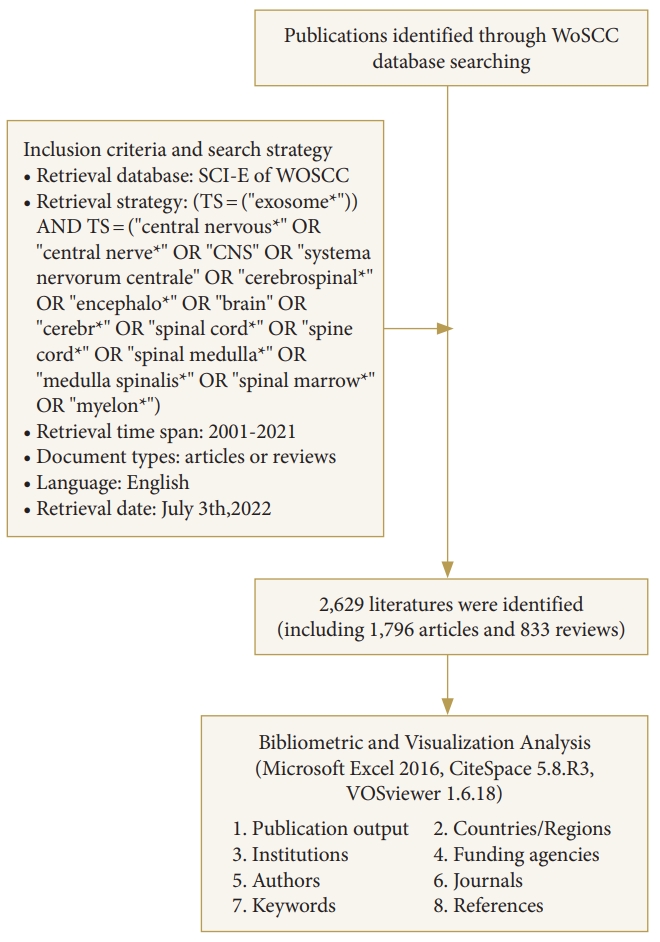
The literature extracted from the Science Citation Index Expanded in the Web of Science Core Collection (WosCC) database was downloaded within one day on July 3, 2022. The search formula was as follows: (TS= ("exosome*")) AND TS= ("central nervous*" OR "central nerve*" OR "CNS" OR "systema nervorum centrale" OR "cerebrospinal*" OR "encephalo*" OR "brain*" OR "cerebr*" OR "spinal cord*" OR "spine cord*" OR "spinal medulla*" OR "medulla spinalis*" OR "spinal marrow*" OR "myelon*"), while the timespan for literature retrieval was set from 2001 to 2021. Only articles or reviews written in English were included. Ultimately, a total of 2,629 records, including 1,796 articles (68.31%) and 833 reviews (31.69%), were identified for analysis.
2. Data Extraction and Bibliometric Analysis
The included records were exported and saved as plain text files with “Full Record and Cited References” for further bibliometric analysis. The data from the Web of Science, including publication outputs, citation trends, countries/regions, institutions, authors, journals, funding sources, and the Hirsch index (H-index), was imported to Microsoft Excel 2016 (Microsoft Corp., Redmond, WA, USA) for quantitative and qualitative analysis. Additionally, the impact factor (IF) and quartile rank of each journal from the Journal Citation Reports (JCR) 2021 were also considered. Two trained researchers performed the information extraction process independently, while any disagreements were resolved by consensus or by seeking a third one for help.
Subsequently, the CiteSpace 5.8.R3 (Drexel University, Philadelphia, PA, USA) and VOSviewer 1.6.18 (Leiden University, Leiden, The Netherlands) were employed to perform the bibliometric and visualization analysis. The CiteSpace was applied to extract keywords and references from publications with high citation bursts, produce a keywords cluster network and visualization map of timeline viewer, and generate a dual-map overlay for journals. The parameters were set as follows: time slicing (from 2001-01 to 2021-12), years per slice (1), links (strength=cosine; scope=within slices); selection criteria (top 5%), pruning (pathfinder + pruning the merged network + pruning sliced network). The clusters were labeled by keywords, and the log-likelihood rate (LLR) was applied to the clustering algorithm. The VOSviewer was used to produce knowledge maps of the identified influential authors, contributing countries and institutions, core journals, high-quality papers, keywords co-occurrence, and cocited references. The flow diagram of the retrieval strategy and analysis was shown in Fig. 1.
RESULTS
1. The Trends of Publication Outputs and Citations
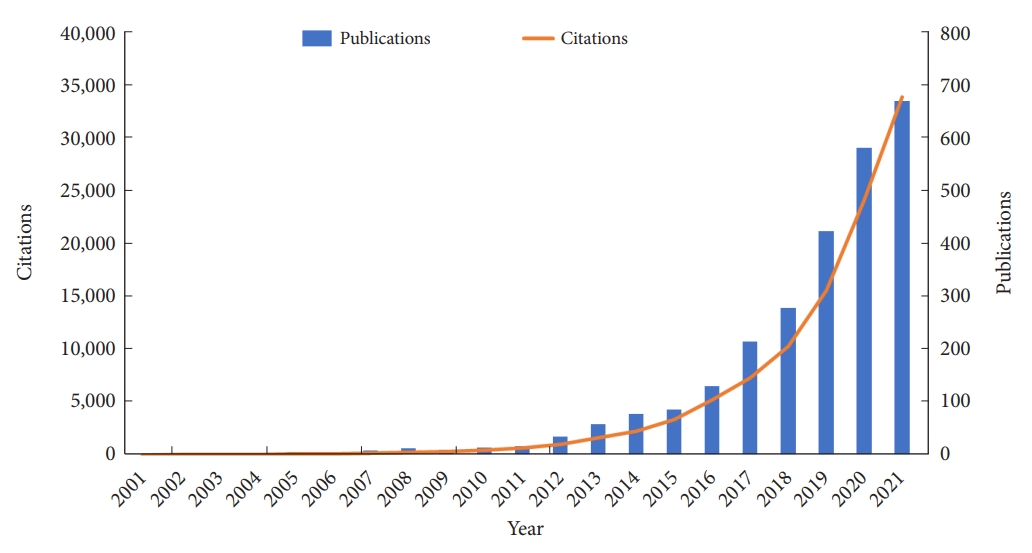
The annual number of publications and citations reflects the research trends in a field [14]. From 2001 to 2021, 2,629 studies published on exosomes in the CNS field were identified. These publications received 20,633 citations by the search date, an average of 40.64 citations per publication. Generally speaking, the annual number of publications and citations has shown an overall upward trend during these years. Specifically, the evolution of exosomes-related CNS research can be separated into 3 stages. In the embryonic stage from 2001 to 2007, the number of publications and citations was relatively low. Then, there was a steady growth phase between 2008 and 2016. From 2017 to 2021, the number of publications and citations on exosomes in the CNS field increased significantly, and the total number of publications in 2021 reached 670, while citations of 33,868. This implies that more attention has been paid to the potential of exosomes-related CNS research so that it can be regarded as a rapid and high-yield growth phase (Fig. 2).
2. Distribution of Countries/Regions, Institutions, and Funding Sources
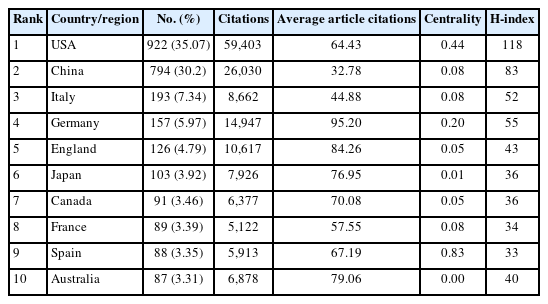
Exosomes-related CNS research has been a significant hotspot worldwide [15]. A total of 2,629 studies were published by 2,813 institutions in 77 countries/regions (Fig. 3A). The top 10 most productive countries/regions were displayed in Table 1 and Fig. 3B. The United States published the largest number of articles (922, 35.07%), followed by China (794, 30.20%) and Italy (193, 7.34%). In addition, the United States accounted for 59,403 citations with an H-index of 118, which both ranked first among all involved countries/regions. The number of citations of publications from China was 26,030 with an H-index of 83, which both ranked second. This means that the United States and China are the most contributing countries in this field. The total number of publications from these 2 countries was more than half of the total. In terms of average article citations, Germany (95.20 per publication) ranked first, followed by England (84.26 per publication), and Australia (79.06 per publication). However, the average number of article citations in China was relatively the lowest (32.78 per publication), which meant Germany, England, and Australia was the forerunner in this field with high-quality research, China as a latecomer should improve its research impact in this field. Centrality has been regarded as a significant turning point that may lead to transformative discoveries [16]. Spain held the highest level of centrality (0.83), followed by the United States (0.44), and Germany (0.20). As illustrated in Fig. 3C, among the 77 countries/regions, 50 have published more than 5 articles in this field. The United States and China occupied the center of the network visualization of co-authorship between countries/regions, which reflected the leadership position in this field.

(A) Geographical distribution map of global publications on exosomes-related central nervous system (CNS) research. (B) The changing trend of the annual publication counts in the top 10 countries/regions. (C) The co-authorship map of countries/regions involved in exosomes-related CNS research. (D) The co-authorship map of institutions involved in exosomes-related CNS research. The size of the node indicates the number of documents in countries/regions or institutions, and the thickness of the line between the nodes indicates the collaborative intensity between countries/regions or institutions.
As displayed in Fig. 3D, the institutional co-authorship network map showed there was positive cooperation among leading institutions (minimum of 15 publications). To some extent, Chinese institutions preferred domestic partnerships, while European and American institutions preferred international collaboration. The top 10 most productive institutions were listed in Table 2, 7 from the United States, 2 from China, and 1 from Sweden. Harvard University contributed the most articles (84, 3.20%), followed by the University of California San Francisco (50, 1.90%), and Zhejiang University (45, 1.71%). Harvard University accounted for 87,92 citations with an H-index of 44 and a centrality of 0.21, which both ranked first among all involved institutions. In terms of the average number of citations, Karolinska institution ranked first (143.05), followed by Henry Ford Hospital (142.67), and Oakland University (133.55).
The top 10 most active funding sources were listed in Table 3, 6 from the United States, 3 from Europe, and 1 from China. The National Institutes of Health was the largest funding source for exosomes-related CNS research, which covered 596 studies (22.67%) with an H-index of 97. The National Natural Science Foundation of China ranked second (502 studies, 19.09%) with an H-index of 71, followed by the European Commission (197 studies, 7.49%) with an H-index of 50.
3. Authors and Co-cited Authors
A total of 14,468 authors contributed to the publications on exosomes-related CNS research, with 218 of these scholars publishing 5 or more articles. Thus, the co-authorship network map of prominent authors was drawn and shown in Fig. 4A. Every node represents an author, with larger nodes representing more published papers. Thicker lines indicate stronger collaboration between authors, and the different colors mean different clusters. It has been found that there was a relatively strong collaboration between authors in the same cluster. In contrast, association from dissimilar clusters was weak, implying that collaboration between different research teams should be strengthened.
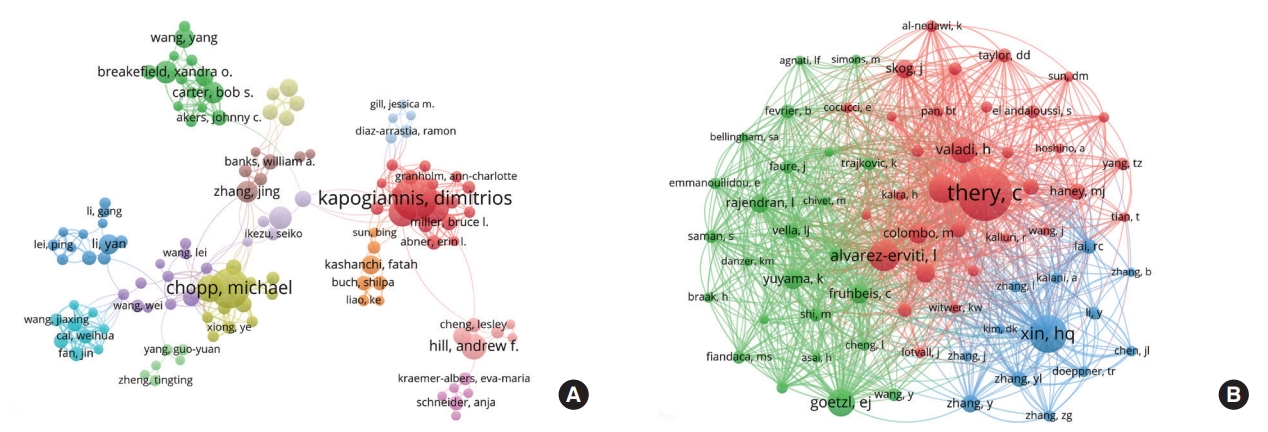
The visualization map of authors’ co-authorship (A) and co-citation (B) involved in exosomes-related central nervous system research. In the visualization map of authors’ co-authorship, the nodes' size represents the number of papers published by the author, and the thickness of the line between the nodes indicates the collaborative intensity between authors. In the visualization map of authors’ co-citation, the size of the node represents the citation frequency, and the line between 2 nodes means that both were cited by 1 author.
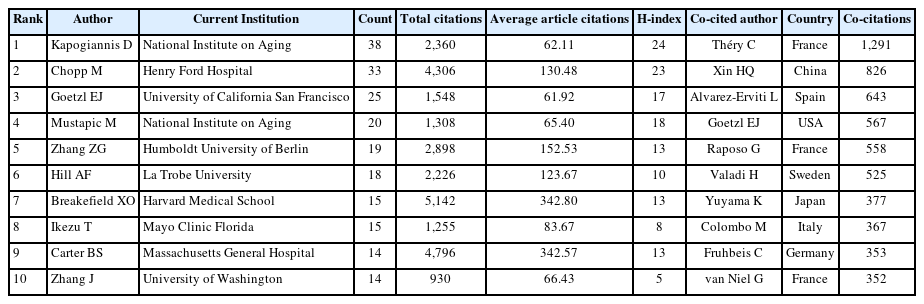
The top 10 most productive authors and co-cited authors were displayed in Table 4. Kapogiannis D from National Institute on Aging had the most significant number of published articles (n = 38) and the highest H-index (n = 24), followed by Chopp M (33 publications, H-index of 23) from Henry Ford Hospital. Besides, Breakefield XO from Harvard Medical School ranked first in terms of total citations (n = 5,142) and the average number of citations (342.80 per paper), followed by Carter BS (4,796 total citations, 342.57 citations per paper) from Massachusetts General Hospital. Co-cited authors are the relationship of 2 or more authors cited simultaneously. As illustrated in Fig. 4B, Théry C from France occupied the center of the co-cited author network map with the highest citation frequency of 1,291 times, followed by Xin HQ (826 times) from China and Alvarez-Erviti L (643 times) from Spain.
4. Journals and Co-cited Journals
In our study, there was a total of 762 academic journals related to exosomes-related CNS research, of which 60 journals had more than 10 publications. Based on this, we drew the network visualization map (Fig. 5A), which showed a close collaboration among major journals. Besides, we have listed the top 10 journals with the most articles published in this field (Table 5). Switzerland had 5, the United Kingdom had 3, Netherlands and the United States had one each. All 10 journals were distributed in the Q1 or Q2 region according to the JCR in 2021. International Journal of Molecular Sciences published the most articles (n = 102) with total citations of 2,521 times, followed by Scientific Reports (n = 54) with total citations of 2,696 times, and PLoS One (n = 44) with total citations of 2,380 times. Notably, Frontiers in Cellular Neuroscience (64.18) had the largest average number of citations and International Journal of Molecular Sciences (n = 27) had the highest value of H-index, Journal of Extracellular Vesicles (17.337) ranked first in terms of IF.

(A) The network visualization map of journals’ co-occurrence involved in exosomes-related central nervous system (CNS) research. (B) The network visualization map of journal co-citation analysis involved in exosomes-related CNS research. The size of the node represents the citation frequency, and the line between 2 nodes means that both were cited by 1 journal. (C) A dual-map overlay of journals involved in exosomes-related CNS research.
Co-citation frequency is also an important indicator for measuring a journal’s impact, reflecting its importance in a particular area of research. The journal co-citation analysis was displayed in Fig. 5B, 89 journals were co-cited over 500 times. The top 5 co-cited journals were Proceedings of the National Academy of Sciences of the United States of America (4,567 times), Journal of Biological Chemistry (4,522 times), PLoS One (4,500 times), Journal of Extracellular Vesicles (3,119 times), and Nature (3,015 times).
The dual-map overlay of journals reveals the distribution of relationships among journals, with the cited journals on the right and the citing journals on the left. The colored paths in the middle represent the citation relationships [17]. As illustrated in Fig. 5C, there was only one main citation path in this study. It implied that the publications of exosomes-related CNS research were mainly focused on Molecular/Biology/Immunology journals, while the most cited papers were published in Molecular/Biology/Genetics journals (z = 9.38905, f = 33,428).
5. References, Co-cited References, and References Burst
The top 10 most cited exosomes-related articles in the CNS field were listed in Table 6 [18-27]. Nature and its subjournals had a substantial scientific impact on this field, with 6 of the top 10 highly cited papers published in these journals. All the top 10 references were cited more than 700 times. The study published in Nature Cell Biology by Skog J et al. in 2008 was the most cited article (3,342 times) in this field up to now [18].
Co-citation analysis aims to measure the degree of association between references in a specific field of research. We created a network map of the co-cited references with a total of more than 100 times by VOSviewer (Fig. 6A). In addition, the top 10 most co-cited articles were summarized in Table 7 [28-32]. The most co-cited reference was entitled “Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells,” which was published in Nature Cell Biology with a co-citation number of 523 [28]. Overall, these studies mainly focused on the characteristics and biological functions of exosomes and their diagnostic and therapeutic role in CNS diseases.
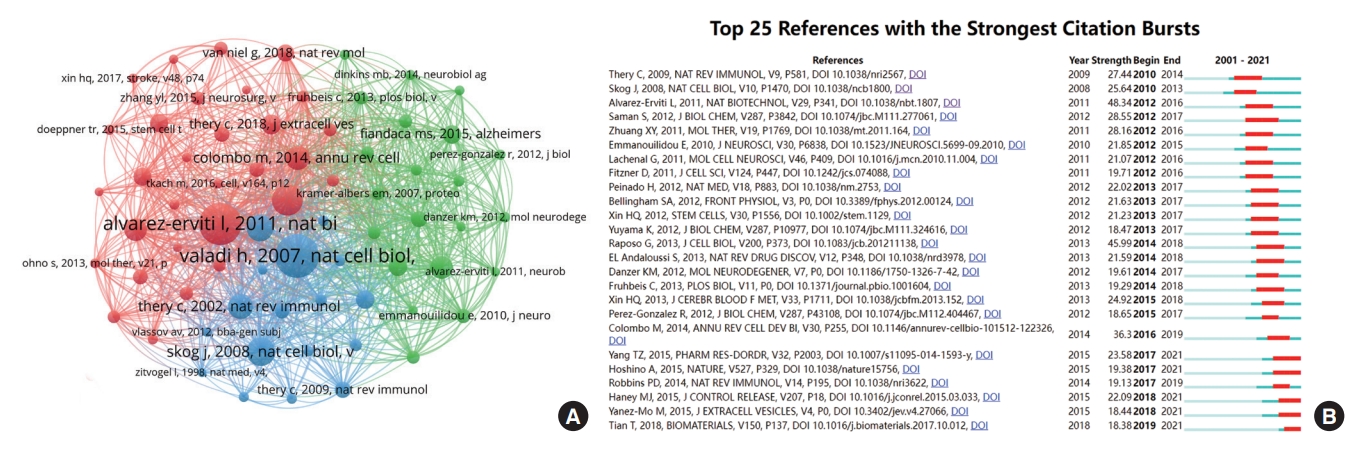
(A) The co-citation network map of references on exosomes-related central nervous system (CNS) research. The size of the node represents the citation frequency, and the line between 2 nodes means that both were cited by 1 paper. (B) The top 25 references with the strongest citation bursts in exosomes-related CNS research. The blue bars indicate the time interval, and the red bars indicate the active time.
The references with the strongest citation bursts were explored by CiteSpace software, and the top 25 references were displayed in Fig. 6B. References with citation bursts first appeared in 2010, and the burst was attributed to a publication in 2009 [33]. Most of the references had citation bursts between 2012 and 2017. Notably, the study entitled “Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes,” which was published in Nature Biotechnology [19], was the strongest burst reference (2012–2016, strength 48.34). The latest reference with a citation burst emerged in 2019 [34], and the burst is continuing.
6. Keywords Analysis of Research Hotspots
Through the analysis of keywords, including keywords cooccurrence and bursts, as well as keyword clustering and timeline view analysis, the research hotspots and scientific trends of exosomes-related studies in the CNS field have been revealed. As illustrated in Fig. 7A, after merging the keywords with similar meanings, a network visualization map of 90 keywords with occurrence more than 50 times was generated by VOSviewer. Further, the high-frequency keywords that occurred over 200 times have been summarized in Table 8.

(A) The network visualization map of keywords co-occurrence in exosomes-related central nervous system (CNS) research. The size of the node represents the occurrence times of keywords, the line between 2 nodes represents the co-occurrence of keywords. (B) The cluster network map of keywords in exosomes-related CNS research. (C) The timeline viewer of keywords involved in exosomes-related CNS research. (D) The top 25 keywords with the strongest citation bursts in exosomes-related CNS research.
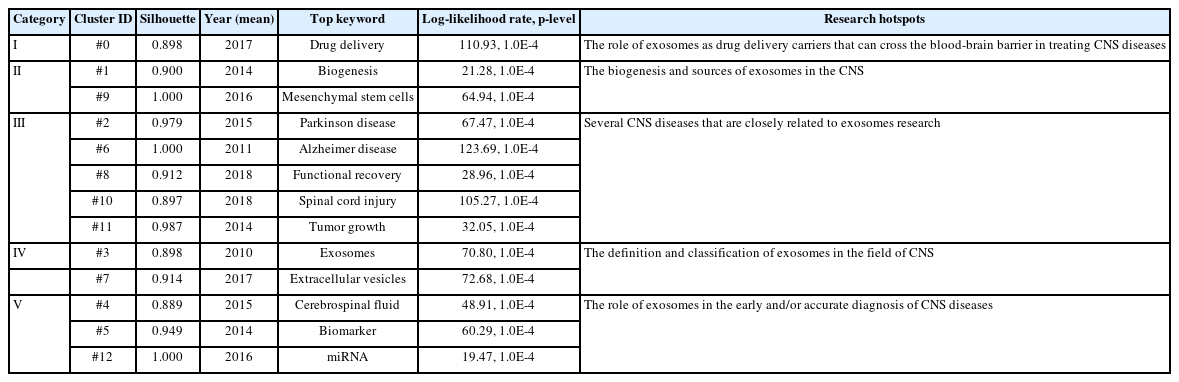
Cluster analysis of the included keywords can demonstrate the knowledge structure of a particular field [35]. The cluster network map of keywords was generated by CiteSpace software and was shown in Fig. 7B. There were 164 nodes and 540 links with a modularity Q of 0.8203 and a mean silhouette score of 0.9355. All the included keywords were classified into 13 clusters: Cluster #0 “Drug delivery” (LLR 110.93, 1.0E-4; Silhouette value 0.898), Cluster #1 “Biogenesis” (LLR 21.28, 1.0E-4; Silhouette value 0.900), Cluster #2 “Parkinson’s disease” (LLR 67.47, 1.0E-4; Silhouette value 0.979), Cluster #3 “Exosomes” (LLR 70.8, 1.0E-4; Silhouette value 0.898), Cluster #4 “Cerebrospinal fluid” (LLR 48.91, 1.0E-4; Silhouette value 0.889), Cluster #5 “Biomarker” (LLR 60.29, 1.0E-4; Silhouette value 0.949), Cluster #6 “Alzheimer’s disease” (LLR 123.69, 1.0E-4; Silhouette value 1.000), Cluster #7 “Extracellular vesicles” (LLR 72.68, 1.0E-4; Silhouette value 0.914), Cluster #8 “Functional recovery” (LLR 28.96, 1.0E-4; Silhouette value 0.912), Cluster #9 “Mesenchymal stem cells” (LLR 64.94, 1.0E-4; Silhouette value 1.000), Cluster #10 “Spinal cord injury” (LLR 105.27, 1.0E-4; Silhouette value 0.897), Cluster #11 “Tumor growth” (LLR 32.05, 1.0E-4; Silhouette value 0.987), Cluster #12 “miRNA” (LLR 19.47, 1.0E-4; Silhouette value 1.000). On this basis, in order to clarify the relationship between exosomes and CNS research, we grouped these clusters into 5 categories in accordance with the commonality of research hotspots, which were listed in Table 9. The timeline view, designed based on the mutations and interactions among keywords, can be used to explore the stage characteristics and evolutionary track in exosomes-related CNS research. As shown in Fig. 7C, from 2005 to 2007, CNS research related to exosomes was still in its infancy. From 2008 to 2016, research in this field significantly increased, and more attention was paid to the characteristics of exosomes, and their intracellular and extracellular functions, leading to a profound impact on later research. From 2017 to 2021, deep mechanism research accelerated, and the topic scope was broadened. The trends in research have gradually shifted from experimental studies to clinical treatments.
The keywords with the strongest citation bursts were displayed in Fig. 7D, aiming to reflect research frontiers and developing trends. The strongest burst keyword was “multivesicular body (2006–2016, strength 13.74)”. The citation bursts time of keywords “survival”, “contribute”, and “diagnosis” has continued from 2019 to 2021.
DISCUSSION
1. General Information
The presence of exosomes was proved by Johnstone et al. more than 4 decades ago [36]. However, exosomes were initially thought to be waste products excreted by cells [37] and did not receive much attention from researchers. In the field of CNS, Fauré et al. [38] first described the release of exosomes by cortical neurons in vitro. Recently, exosomes have been found to be a novel form of information exchange between cells [39,40]. Communication among cells in the CNS includes intercellular and extracellular interactions. Specifically, intracellular interactions act via ions [41,42], while extracellular interactions are mainly composed of wiring transmission and volume transmission [43]. Synapses are the primary vehicle for wiring transmission, while exosomes and transmitters appear to play a critical role in volume transmission [44]. Therefore, the contribution of exosomes under physiological or pathological conditions in the CNS has become a research hotspot and has shown great promise [45,46].
2. Detailed Information
Bibliometrics and visualization analysis can be employed to characterize the current situation and forecast future research trends in an area of research [47]. Our study revealed that there has been a marked increase in the number of publications and the quality of studies from the perspective of exosomes-related CNS research during the past 2 decades. Combining the results of Figs. 2 and 7C, the period between 2001 and 2007 can be characterized as the embryonic stage of exosomes-related CNS research due to its relatively small number of publications and citations, as well as low output of high-impact keywords; the period between 2008 and 2016 can be regarded as the steady growth stage, which produced a lot of high-quality research and laid the necessary groundwork for future directions; the period between 2017 and 2021 can be described as high growth stage, the number of relevant studies focusing on various hotspots from different perspectives increased significantly. Predictably, the topic of exosomes is likely to remain a research hotspot in the CNS field in the future.
From the results of Fig. 3A-D and Tables 1-3, European and American countries, as well as China, were the main participants, with many academic institutions, funding agencies, and scholars making essential contributions to exosomes-related CNS research. In more detail, the United States was undoubtedly the leader in this field, with the largest number of publications and citations, as well as the highest impact. This was in large part because the United States had the largest number of academic institutions involved and the most significant amount of funding in this field. Comparatively, China also had a vast number of publications and the second-highest ranking of participating institutions and funding agencies. However, the number of average article citations in China was the lowest among the top 10 productive countries. Besides, the centrality of both China and Chinese institutions was relatively below average. It is reasonable to suggest that the impact and quality of related research in China must be improved. Considering the late start of China, and the noninclusion of many Chinese studies not written in English, China has much more potential in this field of research to some extent. Besides, active international collaboration is particularly crucial for all countries and regions.
In our study, Harvard University was the top research institution in this field, with the highest value of H-index and centrality. Scholars interested in exosomes-related CNS research may cooperate with this institution. From the results of Fig. 4A-B, and Table 4, the top 4 most prolific authors were all from United States institutions, indicating that these scholars and their teams have been at the forefront of the research [48] and were most likely to achieve breakthrough outcomes. Théry was the only one to have been co-cited more than 1,000 times. The main contributions of Théry were listed as follows: (1) Describing different approaches for the isolation and purification of exosomes from various sources [30]; (2) Explaining the composition, biogenesis, and function of exosomes [31]; (3) Publishing a position statement of the International Society for Extracellular Vesicles and updating the MISEV2014 guidelines as a critical participant [32]. Notably, Goetzl EJ ranked in the top 4 in both the number of publications and co-citation frequency, suggesting that Goetzl et al. were leading in exosomes-related CNS research with significant quantity and quality. His main achievements focused on the role of exosomes derived from various sources in neurodegenerative disorders, especially Alzheimer disease [49-51].
The journal analysis in our study was shown in Fig. 5A-B, and Table 5. All the high-quality journals came from developed countries. It was evident that the journal with the most publications in this field is the International Journal of Molecular Sciences, while Proceedings of the National Academy of Sciences of the United States of America ranked first in co-citations. Thus, these journals had a considerable impact, and more attention should be paid to the research published on them. As shown in Fig. 5C, only one main citation path was available in this study, suggesting that multidisciplinary cooperation is badly needed in exosomes-related CNS research in the future.
The reference analysis can help identify core literature and thereby better understand the developmental history and scientific frontier in exosomes-related CNS research. From the results of Fig. 6A, and Tables 6-7, the most cited study in this field was published in Nature Cell Biology [18] and was also listed as the third most co-cited paper. It mainly introduced glioblastomaderived microvesicles that delivered genetic information (i.e., RNA) and proteins to recipient cells, promoting tumor growth and providing diagnostic biomarkers. This work not only provided strong evidence of the involvement of tumor cell-derived exosomes in disease pathology but also revealed that the cargo of exosomes could assist in the diagnosis. Besides, Alvarez-Erviti et al. [19], published in Nature Biotechnology in 2011, ranked second in terms of citation and co-citation frequency, demonstrating the therapeutic potential of exosomes-mediated siRNA delivery in the treatment of Alzheimer disease (AD). Similarly, Rajendran et al. [24], published in 2006, both ranked in the top 10 in terms of citation and co-citation frequency, which suggested the critical role of exosomes in the pathogenesis of AD. Remarkably, Théry contributed 3 of the top 10 most highly co-cited references, which was the main reason why he was listed as the most highly co-cited author.
References burst refers to research that is frequently cited over a certain time period. As seen in Fig. 6B, most of the references with citation bursts appeared between 2012 and 2017, which was generally in line with the steady growth stage of exosomes-related CNS research summarized above. The first burst reference was published in 2009 by Théry et al. [33], which focused on the role of exosomes in the communication between immune cells and tumor cells. It is worth noting that there are 5 references still in burstness, showing that this topic has gained sustained interest in recent years.
The keywords analysis can be used to identify the current direction of exosomes-related CNS research [52]. As displayed in Fig. 7B, 13 clusters were generated after cluster analysis of the included keywords. As listed in Table 9, these clusters were divided into 5 categories based on the commonality of research hotspots.
(I) Cluster #0 “Drug delivery” mainly reflected the role of exosomes as drug delivery carriers in treating CNS diseases. It is well-known that the BBB excludes almost 100% of large-molecule therapeutic drugs and at least 98% of small-molecule drugs from the brain [53]. However, exosomes can cross BBB with the advantage of nano-size, low immunogenicity, and homing capacity [9]. It has been found that exosomes can steadily transport their cargo to target cells due to the structure of membrane-enclosed vesicles for therapeutic purposes [54]. Yang et al. [55] have discovered that anticancer drugs delivered by brain cell-derived exosomes across the BBB significantly reduced the number of markers of tumor growth and transplanted cancer cells. Thus, exosomes have been considered natural carriers for the delivery of therapeutic molecules [56].
(II) Cluster #1 “Biogenesis” and Cluster #9 “Mesenchymal stem cells” mainly reflected the sources of exosomes. The nomenclature of mesenchymal stem cells (MSCs) was formalized by Caplan [57] in the 1990s. Nowadays, MSC-exosomes have gained great attention because of their regenerative and immunomodulatory functions [58]. In addition, exosomes can be released by almost all cells in the body, exosomes derived from dissimilar sources have different biological functions and characteristics depending on their origin [31]. For instance, Hoshino et al. [20] found that the lung, liver, and brain-tropic tumor cell-derived exosomes can fuse preferentially with resident cells at their predicted destination.
(III) Cluster #2 “Parkinson’s disease”, Cluster #6 “Alzheimer’s disease”, Cluster #8 “Functional recovery”, Cluster #10 “Spinal cord injury”, and Cluster #11 “Tumor growth” mainly represented several CNS diseases that were closely related to exosomes research. Evidence from the other studies also supported our findings, exosomes have played an essential role in the research of Parkinson disease [59], AD [60], functional recovery after stroke [61], spinal cord injury [62], and tumor growth [63].
(IV) Cluster #3 “Exosomes” and Cluster #7 “Extracellular vesicles” mainly discussed the definition and classification of exosomes. Currently, vesicular bodies with a bilayer membrane structure secreted from cells or separated from the cell membrane are collectively defined as extracellular vesicles (EVs) [6]. In fact, EVs can be categorized as microvesicles (200–1,000 nm) [8], exosomes (40–100 nm) [64], and apoptotic bodies (0.5–3.0 μm) [65] depending on their size. However, exosomes as a subtype of EVs are by far the most widely researched, and the term EVs is used to refer just to exosomes in some articles.
(V) Cluster #4 “Cerebrospinal fluid”, Cluster #5 “Biomarker”, and Cluster #12 “miRNA” mainly embodied the role of exosomes in the diagnosis of CNS diseases. Early diagnosis of CNS disorders has long been a challenge, it has been demonstrated that exosomes from CNS can be identified in peripheral body fluids and cerebrospinal fluid, and their cargo changes with disease [66]. As exosomes can cross the BBB in highly stable conditions, it has an attractive prospect to choose exosomes to monitor disease progression and enable early diagnosis of CNS diseases. Lai et al. [67] suggested miR-193b-3p was differentially expressed in the plasma exosomes of the patients with subarachnoid hemorrhage (SAH), then they found exosomes/miR-193b-3p treatment alleviated neurobehavioral impairments and neuroinflammation following SAH. Therefore, exosomal miRNAs are involved in the occurrence and development of various CNS diseases, playing a vital role in the early diagnosis and targeted therapy in this field [68].
The keywords burst analysis in Fig. 7D showed that “multivesicular body” was the keyword with the strongest citation bursts. Exosomes are produced within multivesicular bodies and released into the extracellular space by a merging of the multivesicular body with the plasma membrane [69]. Notably, the time period for the citation burst of “survival,” “contribute,” and “diagnosis” has lasted from 2019 to 2021, implying the diagnostic and therapeutic roles of exosomes in CNS disease have the potential to become significant research hotspots in the future.
3. Summary
Exosomes are vesicles released by multiple cells, and containing various biological materials such as lipids, metabolites, proteins, and nucleic acids. Exosomes are involved in a wide range of physiological and pathological processes in the CNS, including propagation of neuroinflammation, synaptic plasticity, neuroprotection, regulation of neuronal firing, and so on [70]. In summary, there are 3 main aspects of exosomes-related CNS research. The first aspect concerns the methodology for the isolation and identification of exosomes, and their biological characteristics in the CNS [71]. The second aspect concerns the potential of exosomes as diagnostic and prognostic biomarkers for CNS diseases, mainly including brain tumor [18], neurodegenerative disease [60], cerebrovascular disease [61], and spinal cord injury [62]. The last aspect is about the application of exosomes as drug delivery carriers loaded with specific agents in the CNS, among which the delivery of siRNA and miRNA has received more attention. The most common method of loading is electroporation [19,68].
In fact, the 3 aspects mentioned above interact with each other and deepen gradually, reflecting the shift in focus of exosomes-related CNS research from experimental studies to clinical application [72]. Nowadays, with the further development of experimental techniques, more attention has been paid to biological tissue-derived exosomes [73] and engineered exosomes [74]. Therefore, it is reasonable to assume that the clinical application of these techniques in CNS diseases will be hotspots in future research.
4. Strengths and Limitations
The literature on exosomes-related CNS research evaluated in our study was obtained from the WosCC database, which was the most utilized database in the bibliometric analysis and the most exhaustive collection of high-quality medical research [75]. As far as we know, it is the first-ever bibliometric study to provide a systematic analysis of exosomes-related CNS research combined with visualized mapping. Despite our efforts to ensure the objectivity and rigor of our data analysis, several limitations do exist. First, owing to the lack of English abstracts or references in most non-English articles [76], the included studies were limited to publications written in English from the WosCC database. Thus, some critical studies published in other languages or from other databases such as PubMed, Scopus, and Google Scholar might have been missed. Second, this bibliometric analysis mainly focused on global scientific research and was not adjusted for social reality, such as population size, economic conditions, and other factors, which may lead to differences between actual research conditions and bibliometric results. Third, as the data in 2022 is constantly being updated, the studies published in 2022 were excluded because of the incompletion. It may result in missing out on the latest research findings. However, we believe our study has covered the vast majority of publications in the field since 2001, and the conclusion would not be changed even with the additional small amount of updated data.
CONCLUSION
Since 2001, exosomes-related CNS research has continuously advanced qualitatively and quantitatively, it is currently in a stage of high growth. China has made significant progress, while the United States and Europe are dominating in this field. Global cooperation among countries and institutions is necessary and beneficial. Currently, exosomes-related CNS research mainly focuses on the sources and biological functions of exosomes and their promising role in diagnosing and treating CNS diseases. The topic of biogenesis, biomarker, and drug delivery will serve as hotspots in future research. Moreover, the clinical translation of the results from exosomes-related CNS research will be of great importance. These findings will assist scholars in keeping abreast of trends in exosomes-related CNS research.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This work was supported by the National Natural Science Foundation of China (No. 82274666); the Natural Science Foundation of Hunan Province (No. 2022JJ30036); The Key Scientific Research Project of Department of Education of Hunan Province (No. 20A363); The Integrative Medicine Open Fund Project of “Domestic First-Class Training Discipline” of Hunan Province (No. 2021ZXYJH15, No. 2020ZXYJH37); The Key Project of Postgraduate Scientific Research and Innovation of Hunan Province (No. CX20210682).
Author Contribution
Conceptualization: YZ, KA, HZ; Data curation: JP, JX, GZ, ZJ, RZ; Formal analysis: YZ, KH, BW; Funding acquisition: HZ, YZ; Methodology: YZ, KA, HZ; Project administration: HZ; Visualization: YZ, BW; Writing - original draft: YZ; Writing - review & editing: YZ.