Clinical and Radiological Outcomes of a New Cage for Direct Lateral Lumbar Interbody Fusion
Article information
Abstract
Objective
In Korea, direct lateral interbody fusion (DLIF) was started since 2011, using standard cage (6° lordotic angle, 18mm width). Recently, a new wider cage with higher lordotic angle (12°, 22mm) was introduced. The aim of our study is to compare the clinical and radiologic outcomes of the two cage types.
Methods
We selected patients underwent DLIF, 125 cases used standard cages (standard group) and 38 cases used new cages (wide group). We followed them up for more than 6 months, and their radiological and clinical outcomes were analyzed retrospectively. For radiologic outcomes, lumbar lordotic angle (LLA), segmental lordoic angle (SLA), disc angle (DA), foraminal height change (FH), subsidence and intraoperative endplate destruction (iED) were checked. Clinical outcomes were compared using visual analog scale (VAS) score, Oswestry disability index (ODI) score and complications.
Results
LLA and SLA showed no significant changes postoperatively in both groups. DA showed significant increase after surgery in the wide group (p<0.05), but not in the standard group. Subsidence was significantly lower in the wide group (p<0.05). There was no difference in clinical outcomes between the two groups. Additional posterior decompression was done more frequently in the wide group. Postoperative change of foraminal height was significantly lower in the wide group (p<0.05). The iED was observed more frequently in the wide group (p<0.05) especially at the anterior edge of cage.
Conclusion
The new type of cage seems to result in more DA and less subsidence. But indirect foraminal decompression seems to be less effective than standard cage. Intraoperative endplate destruction occurs more frequently due to a steeper lordotic angle of the new cage.
INTRODUCTION
Direct lateral interbody fusion (DLIF) is the method of minimal invasive lumbar interbody fusion where a trans-psoas approach is made using a tubular retractor system. It is also termed as lateral trans-psoas lumbar interbody fusion or extreme lateral lumbar interbody fusion. Currently, DLIF is usually performed with specialized instruments (Medtronic Sofamor Danek, Inc., Memphis, TN, USA) in Korea. Since first report by MeAfee PC et al in 199823), it has been widely performed for degenerative lumbar disease such as spinal stenosis, spondylolisthesis, scoliosis, and multi-level degenerative disc disease7,17). It was first introduced to Korea in May of 2011 and it has been actively used at several institutes. Due to its lateral approach method and structural characteristics of cage, DLIF has several advantages comparing to other approaches like posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF) or anterior lumbar interbody fusion (ALIF). First, various important anatomical structures including back muscles, anterior and posterior longitudinal ligaments and facet joint are preserved25,29). Second, a minimal invasive approach can reduce the intraoperative bleeding or surgical time4). As compared with the ALIF, the risks of injuries to peritoneum, bowels, and great vessels are relatively lower18). As compared with the TLIF, the DLIF cage, with higher profile and wider width, can effectively raise the disc height and decompress neural foramen indirectly25). DLIF cage is also known to be effective for restoration of coronal and sagittal balance in the patients with lumbar degenerative kyphosis (LDK) or scoliosis4,16,20,30). The standard type of DLIF cage has a 6° of lordotic angle and 18mm of width (Fig. 1). According to the papers using the standard type of cage, insufficient restoration of lumbar lordotic angle for regional or global sagittal alignment and subsidence were indicated constantly1,12,32). This arises from a lower lordotic angle (6°) and relatively narrow width of the standard type DLIF cage. A new DLIF cage with higher lordotic angle (12°) and wider width (22mm) seemed to overcome the limitations of the standard cage, which had been launched in Korea two years ago (Fig. 1). Although there had been several papers about the wide cage19,22), they seemed not to discuss pitfalls of the new cage thoroughly. Moreover, there was no report about the new cage in Korea yet. The current study was conducted to investigate the clinical and radiological outcomes of the new wide cage comparing to those of the standard cage.
MATERIALS AND METHODS
1. Patient Selection
The current study examined 163 patients who underwent DLIF between May 2011 and January 2014. The operation was performed by a single surgeon. We performed a retrospective analysis of the postoperative clinical and radiological outcomes.
2. Indications and Surgical Candidates
Surgery was performed for the levels from T12 and L5. Surgical indications were spinal stenosis, spondylolisthesis (≤grade 2), degenerative scoliosis, and infectious spondylitis. We excluded the patients with retroperitoneal adhesion due to prior surgery, severe spondylolisthesis (≥grade 3), severe rotational deformity, or active infection.
3. Surgical Technique
Under general endotracheal anesthesia, patients were placed in a true right lateral position. By bending the bed, the space between the rib and iliac crest was maximally extended. The disc space of index level should be confirmed with C-arm. Flexion of left hip and knee is helpful to relax psoas muscle of the approach side. After draping, the target level was confirmed using C-arm again. The dissection site was marked on the skin. Using a No. 10 blade, an approximately 3 cm transverse skin incision was made. Thereafter, the three layers of abdominal muscles were dissected one by one. When the retroperitoneal fat was exposed, the peritoneum and retroperitoneal fat were dissected away anteriorly from the posterior abdominal wall using an index finger. Then, the target disc under the psoas muscle was palpated. Tubular retractors were secured at the target disc space. Intraoperative EMG monitoring was done during the procedures. After discectomy, the endplate preparation was done to expose the bony endplates. Although autologous bone graft is the most ideal fusion material for fusion10), it was hard to harvest autologous bone during DLIF because of its minimal invasive approach. Currently, recombinant bone morphogenetic protein-2 (rh-BMP2) is widely used for bone fusion28,33) in many countries. But the material is not available in Korea due to the strict policy of Korean government. We used demineralized bone matrix (DBM) as a fusion material. Following the insertion of the cage, the wound was closed by layers.
4. Radiological Analysis
Whole spine standing X-ray studies were pre- (1 day before surgery) and post-operatively (6 days after surgery) to measure lumbar lordotic angle (LLA), segmental lordotic angle (SLA) and disc angle (DA) (Fig. 2). LLA was measured by the Cobb angle formed between the lower endplate of L1 and the upper endplate of S1. SLA was measured by the angle between the upper endplate of the upper vertebra and the lower endplate of the lower vertebra at the corresponding level. In addition, DA was measured by the angle between the two endplates at the operated disc level. Post-operative change of foraminal height (FH) was measured based on the mean height of bilateral foramens on lumbar spine oblique X-ray films. Subsidence was measured as the change in the distance between the upper endplate of upper vertebra and the lower endplate of lower vertebra of index level using lumbar spine lateral X-ray films, comparing the distances checked immediate postoperatively to those at 6 months postoperatively. Postoperative change in the distance more than 2mm was considered to be a meaningful subsidence14,24) (Fig. 3). Intraoperative endplate destruction (iED) was checked on the immediate postoperative simple X-ray films, usually around the anterior and posterior sharp edges of DLIF cage.

Lumbar lordotic angle (LLA), segmental lordotic angle (SLA), disc angle (DA) and foraminal height (FH). LLA is defined as a Cobb angle formed between the lower endplate of L1 and the upper endplate of S1. SLA is defined as an angle between the upper endplate of the upper vertebra and the lower endplate of the lower vertebra at the corresponding level. DA is defined as an angle between the upper endplate of the upper vertebra and the lower endplate of the lower vertebra at the corresponding level.
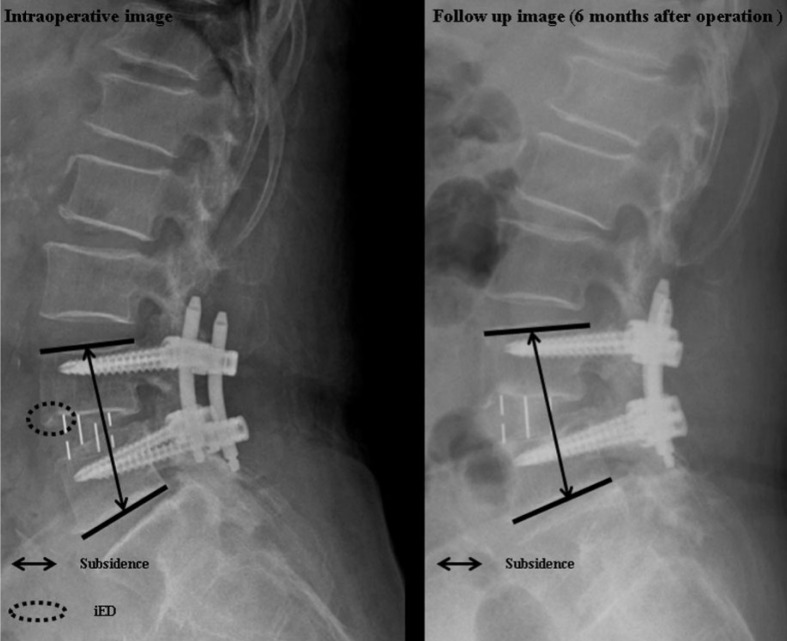
Subsidence and intraoperative endplate destruction (iED). Subsidence is measured as the change in the distance between the upper endplate of upper vertebra and the lower endplate of lower vertebra of index level using lumbar spine lateral X-ray films, comparing the distances checked immediate postoperatively to those at 6 months postoperatively. iED is checked on the immediate postoperative simple X-ray films, usually around the anterior and posterior sharp edges of DLIF cage.
5. Clinical Outcomes
We checked pre- and post-operative (6 months after surgery) visual analog scale (VAS) scores and Oswestry disability index (ODI). We also evaluated surgical complications.
6. Statistical Analysis
Student T-test, χ2 test, and multiple logistic regression analysis were used for statistical analysis. p<0.05 was considered to be a statistical significance.
RESULTS
1. Demographic Data
Of the 163 patients, 125 and 38 were assigned to the standard group and wide group, respectively. Mean age and male-to-female ratio were 60.51-14.51 years and 50:75 in the standard group, and 63.29-9.99 years and 12:26 in the wide group (p>0.05). T-scores of bone mineral density (BMD) were -0.82 and -0.88 in the standard and wide groups, respectively (p>0.05) (Table 1). Total numbers of fusion levels were 172 in the standard group and 57 in the wide group.
2. Surgical Data
In the standard group, there were 88 (70.4%) patients with single level, 28 (22.4%) patients with 2 levels, 8 (6.4%) patients with 3 levels and 1 (0.8%) patient with 4 levels fusion (Table 1). In the wide group, these values were 24 (63.2%), 10 (26.3%), 3 (7.9%) and 1 (2.6%) in the corresponding order (p>0.05). The mean operation time for 1 level of DLIF was 39.7-16.2 minutes in the standard group and 38.5-23.1 minutes in the wide group (p>0.05). The proportion of the patients who underwent additional posterior decompression at the time of surgery was 50.4%(63/125) in the standard group and 84.2%(32/38) in the wide group (p<0.001).
3. Radiological Outcomes
Pre- and postoperative mean LLA in the standard group were 39.5±18.3° and 44.5±18.8° (p>0.05) (Table 2). Preand postoperative mean LLA in the wide group were 40.6±9.8° and 46.2±9.6° (p>0.05). Pre- and postoperative SLA were 9.1±4.4° and 10.1±5.2° in the standard group, and 8.9±3.5° and 12.7±6.1° in the wide group (p>0.05). Preand postoperative DA was 6.5±4.5° and 8.1±5.1° in the standard group (p>0.05), and 6.9±3.9° and 10.1±7.8° in the wide group (p<0.05). There was no significant difference in the preoperative DA's between the two groups. But the postoperative DA was significantly higher in the wide group comparing to that of standard group (p<0.05) (Table 2). The postoperative change in the FH was significantly lower in the wide group (2.7±1.2mm) comparing to that of the standard group (4.3±2.8mm) (p<0.05). Subsidence was significantly less in the wide group (1.2±0.5mm) comparing to that of the standard group (4.4±2.5mm) (p<0.05) (Table 3). The number of the patients with iED was significantly higher in the wide group (16, 42.1%) comparing to that of standard group (31, 24.8%) (p<0.05) (Table 3). The iED was occurred more frequently at the anterior edge of cage in the wide group, whereas at the posterior edge of cage in the standard group (p<0.05) (Table 3).
4. Clinical Outcomes
VAS scores were significantly decreased postoperatively from 6.1±2.4 to 2.3±1.2 in the standard group (p<0.001), and from 5.9±1.3 to 2.1±0.9 in the wide group (p<0.001) (Table 4). ODI scores were also improved postoperatively from 41.4±15.0% to 12.5±4.4% in the standard group (p<0.001), and from 48.0±15.1% to 14.5±7.6% in the wide group (p<0.001) (Table 4). But there was no statistical significance in the changes of VAS and ODI scores between the groups. There were 17 cases of psoas muscle symptom (13.6%), 5 genitofemoral nerve injury (4.0%), 3 lateral femoral cutaneous nerve symptom(2.4%), 1 bowel perforation (0.8%), and 1 infection (0.8%) in the standard group. In the wide group, there were 6 cases of psoas muscle symptom(15.8%), 3 genitofemoral nerve injury (7.9%), and 2 lateral femoral cutaneous nerve symptom(5.3%). There was no significant difference in the postoperative complications between the two groups (Table 4).
DISCUSSION
DLIF can use a large interbody cage less invasively as compared with other types of lumbar interbody fusion techniques2). Indirect increase of foraminal height, less subsidence, and considerable restoration of lumbar lordotic angle were reported to be important advantages of DLIF procedure5,25). However, some papers reported that there was still considerable rate of subsidence, and the effect of DLIF on making lumbar lordotic angle seemed to be insufficient for correction of sagittal imbalance12). To solve the problems, a new type of cage (wide cage) had been developed with higher lordotic angle (12°) and wider width (22mm) comparing to those of standard cage (12° and 8mm) (Fig. 1). We recently have experienced a series of patients underwent DLIF surgery using the new cage. In this paper, we tried to compare radiological and clinical outcomes between the standard and wide cages.
There were several papers reporting the effect of DLIF for correction of coronal alignment without detailed investigation for the effect of DLIF on lumbar sagittal angle3,13,31,32). Therefore, we checked sagittal angles including LLA, SLA and DA. According to our data, DLIF did not seem to have significant effect on LLA. This finding seems to be related with short segment fixation in most of our cases. This is similar to the reports of other papers1,31,32), where they pointed the insufficient effect of short segment DLIF on LLA. But significant increase of DA by the new cage seemed to suggest the possible usefulness of DLIF with the high angle cage in the surgery of sagittal imbalance which is still in controversy. Despite of significant increase of DA by the new cage, SLA did not show significant change. The discrepancy between SLA and DA seems to arise from the anatomic characteristics of vertebral body. The downward angle of the upper endplate of upper vertebral body, making anterior wedging shape, looks like the cause of less SLA comparing to the increased DA. So, we think the DA can reflect the actual change of lumbar sagittal angle by insertion of interbody cage.
Kepler CK et al and Oliveira L et al15,25) measured the area of foraminal height, and reported effective indirect decompression of neural foramen by DLIF. FH was also reported to be increased effectively by DLIF more than ALIF or TLIF11). However, the neural foraminal area can be decreased during rod compression to make more lordotic angle. There was a significantly less increase of FH by the wide cage as compared with those by the standard cage, suggesting the wide cage has a lower degree of indirect foraminal decompression. This might be due to the structural differences between the two cages. The standard cage has 6° lordotic angle resulting in a 2mm height difference between the anterior and posterior parts of the cage (Fig. 1). But, the posterior height of the wide cage is 4mm less than the anterior height because of its high angle, 12° (Fig. 1). As a result, posterior disc height and foraminal height can be smaller by using the new cage than the standard cage. To compensate the disadvantages, we tried to use the wide cage with higher profile or to perform an additional posterior decompression. It was the reason of the increased use of higher profile cage and more frequent posterior decompression in the group using the new cage. However, the use of higher profile cage in the wide group seems to be related with the increase intraoperative endplate destruction. The intraoperative endplate destruction occurs more frequently at the anterior edge of the new cage, whereas the standard cage tends to destruct endplate more frequently at its posterior edge.
Our data showed that subsidence can be decreased significantly by using wide cage. This might be due to a larger footprint of the wide cage, which can cover more marginal area of vertebral body providing stronger mechanical support and more resistance to subsidence21). The thicker wall of the wide cage seems to be another reason of higher mechanical resistance to load (Fig. 1).
There was no significant difference in the VAS and ODI scores between the standard and wide groups, which is similar to other reports8,9,31). The long term clinical outcome of DLIF was not significantly different from that of TLIF26). Complication rates were also similar in both groups like as other reports6,27,32).
The limitations of the current study are the relatively small number of patients and short follow-up period with the wide group. Most of our cases were operated in a short segment which is insufficient to interpret the effect of the new cage for deformity correction especially in sagittal imbalance. It seems to be needed a long term study with more number of patients and long level fusion cases in the future.
CONCLUSION
The new DLIF cage has a higher lordotic angle and a larger width comparing to those of the pre-existing standard cage. The characteristic shape of the new cage has advantages for making more lordotic angle at the index disc level and reducing subsidence. But, at the same time, it seems to have some weak points such as an increased risk of intraoperative endplate destruction and a possible risk of neural foraminal stenosis due to its steep angle.




