Detection of Glioma-Related Hotspot Mutations Through Sequencing of Cerebrospinal Fluid (CSF)-Derived Circulating Tumor DNA: A Pilot Study on CSF-Based Liquid Biopsy for Primary Spinal Cord Astrocytoma
Article information
Abstract
Objective
Although cerebrospinal fluid (CSF)-based liquid biopsy was proved to be practical in molecular analysis of intracranial gliomas, liquid biopsy of primary intramedullary astrocytoma was rarely reported. Given the distinct genomic profiles between primary intramedullary glioma and intracranial astrocytoma, whether the feasibility of CSF-based molecular analysis of intracranial gliomas can be replicated in primary spinal cord astrocytoma needs to be investigated. The aim of this pilot study is to evaluate the feasibility of molecular analysis of primary intramedullary astrocytoma through sequencing CSF-derived circulating tumor DNA (ctDNA).
Methods
Two grade IV diffuse midline gliomas, 1 grade II, and 1 grade I astrocytoma were included. Intraoperative collection of peripheral blood and CSF samples was conducted, along with postoperative collection of matched tumor tissues. A panel covering the 1,021 most common driver genes of solid tumors was used for targeted DNA sequencing.
Results
CSF-derived ctDNA was detected in 3 CSF samples (2 grade IV diffuse midline gliomas and 1 grade I astrocytoma), 5 mutations were found in both tumor tissues and CSF samples, while 11 mutations and 20 mutations were detected exclusively in tumor tissues and CSF samples, respectively. Importantly, hotspot genetic alterations, including H3F3A K28M, TP53, and ATRX, were identified in CSF and the average mutant allele frequency was often higher in CSF than in tumor tissues.
Conclusion
CSF-based liquid biopsy showed potential feasibility for molecular analysis of primary intramedullary astrocytoma through sequencing of ctDNA. This approach may assist in diagnosis and prognostic evaluation of this rare spinal cord tumor.
INTRODUCTION
Liquid biopsy via biofluid sampling, such as blood, ascites, hydrothorax, cerebrospinal fluid (CSF) etc., was increasingly used to analyze molecular profile of tumors [1-4]. Various tumor-derived molecules, including proteins, nucleic acids, and extracellular vesicles etc., can serve as biomarker platform for liquid biopsy. Among these, circulating tumor DNA (ctDNA), which is shed by necrotic or apoptotic tumor cells into body fluid, has gain traction in recent years. Sequencing ctDNA can describe the mutational landscape of tumor. Importantly, mutation analysis by ctDNA hold several advantages over that by traditional tissue biopsy, including mini-invasion, dynamic monitoring and capturing global tumor genome [3,5]. In central nervous system tumor, CSF is considered the optimal carrier of ctDNA shed by brain tumor compared to blood due to blood-brain barrier. As expected, CSF-derived ctDNA has been successfully and increasingly used to assess molecular profile of brain gliomas [6,7]. However, the utility of CSF-derived ctDNA in mutation analysis of primary spinal cord astrocytoma, the second common intramedullary glioma, has been seldomly reported. Given that the clinical characteristics and molecular profile of primary spinal cord astrocytoma were reported to be different from its intracranial counterpart [8,9], it was suspected that the feasibility of mutation analysis through sequencing CSF-derived ctDNA in intracranial glioma could be reproduced in primary spinal cord astrocytoma.
Here, we report results from a pilot study in patients with primary spinal cord astrocytoma aiming to evaluate the practicality of molecular analysis through sequencing of CSF-derived ctDNA.
MATERIALS AND METHODS
1. Patients
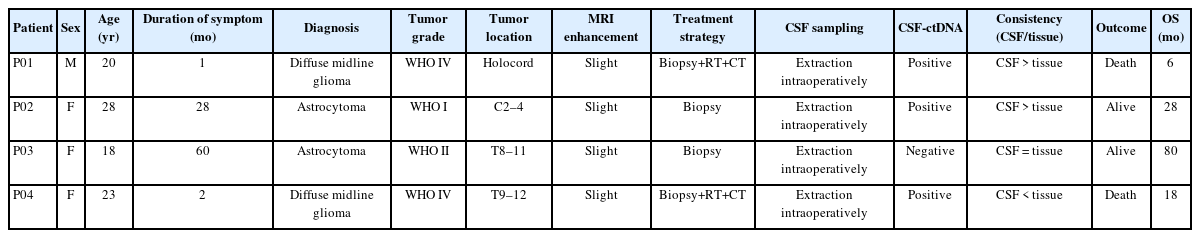
Four patients with primary intramedullary tumor underwent surgical treatment in Xuanwu Hospital, Capital Medical University and postoperative pathology confirmed astrocytoma. Tumor grade was classified according to 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System by 2 independent neuropathologists. Formalin-fixed, paraffin-embedded tumor samples were obtained postoperatively for genomic DNA extraction. Matched peripheral blood (PB) samples were obtained at the time of anesthetic induction and CSF was collected upon opening dura and prior to tumor resection (Fig. 1). Demographic data and clinicoradiological features were collected. Follow-up data until June, 2020 were available. Overall survival was defined as the duration from the date of the diagnosis to the date of final follow-up or death. This study was approved by the Clinical Research Ethics Committee of Xuanwu Hospital (2022020). All participants gave written informed consent.
2. Sample Preparation: Sample Processing and DNA Extraction
More than 10 mL of PB and 7–10 mL CSF were collected from each patient using cell-free DNA (cfDNA) collection tubes (Streck, Omaha, NE, USA) at room temperature before receiving any treatment. PB samples and CSF samples were processed within 3 days after gathering, and centrifuged at 2,500 g for 10 minutes, then moved to microcentrifuge tubes and centrifuged at 16,000 g for 10 minutes to remove remaining cellular debris. Peripheral blood lymphocytes (PBLs) generated from the first centrifugation were gathered as germline control samples. Plasma, CSF supernatant and PBLs were stored at -80°C. The DNA of PBL was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). Circulating cfDNA was isolated from 1.6–2.8 mL plasma and 7–10 mL CSF using QIAamp Circulating Nucleic Acid Kit (Qiagen). DNA concentration was measured with the Qubit fluorometer using dsDNA HS kit (Life Technologies, Carlsbad, CA, USA), and the size of cfDNA fragments were assessed using the Agilent 2100 BioAnalyzer and DNA HS kit (Agilent Technologies, Santa Clara, CA, USA).
3. Targeted Sequencing: Sequencing Library Construction and Hybridization Capture-Based Sequencing
Before library construction, genomic DNA were sheared into 200–250 bp fragments with a Covaris S2 instrument (Covaris, LLC, Woburn, MA, USA). Indexed Illumina NGS libraries were prepared from PBL germline and circulating DNA using the KAPA Library Preparation Kit (Kapa Biosystems, Wilmington, MA, USA). For cfDNA, after end repairing and A-tailing, well-designed adapters with unique identifiers were ligated to both ends of the double-stranded cfDNA fragments. The SeqCap EZ Library system (Roche NimbleGen, Madison, WI, USA) was used for target enrichment. All libraries were hybridized to custom-designed biotinylated oligonucleotide probes (IDT, Coralville, IA, USA) covering 1.6 Mbp of the genome. The captured genomic regions included the most common driver genes of solid tumors, including glioma. We chose their entire exome regions to construct the basic panel. Next, genomic regions relevant to the effects of chemotherapy, targeted drugs, and immunotherapy per available clinical and preclinical research were added to the panel. Finally, high-frequently mutant regions recorded in the Catalogue of Somatic Mutations in Cancer (COSMIC, http://cancer.sanger.ac.uk/cosmic) and The Cancer Genome Atlas (TCGA, https://cancergenome.nih.gov/) were involved. All included 1,021 genes are shown in Supplementary Table 1 . Captured DNA fragments were amplified after hybrid selection and then pooled into several multiplexed libraries. Sequencing was performed using the MGIseq-2000 sequencing system (BGI, Shenzhen, China) according to the manufacturer’s guideline.
4. Raw Data Processing
After removal of terminal adaptor sequences and low-quality reads (> 50% N rate, > 50% bases with Q< 5), remaining reads were mapped to the reference human genome (hg19) and aligned using Burrows-Wheel Aligner (version 0.7.12-r1039, http://biobwa.sourceforge.net/) with default parameters, followed by duplicate reads identification using Picard’s Mark Duplicates tool (https://software.broadinstitute.org/gatk/documentation/tooldocs/4.0.3.0/picard_sam_markduplicates_MarkDuplicates.php). Base quality recalibration and local realignment were conducted by the Gene Analysis Toolkit (GATK, https://www.broadinstitute.org/gatk/).
5. Mutation Identification
Somatic single-nucleotide variations and insertions or deletions of small fragments (Indels) were called by MuTect algorithm (https://software.broadinstitute.org/gatk/documentation/tooldocs/3.8-0/org_broadinstitute_ gatk_tools_walkers_cancer_m2_MuTect2.php). PBL sequencing data were used to filter germline mutations. All reliable alterations were supported by ≥ 5 high-quality sequencing reads (mapQthres > 30, baseQthres > 30). Multiple single-nucleotide polymorphism databases (dbsnp, https://www.ncbi.nlm.nih.gov/projects/SNP/;1000G, https://www.1000genomes.org/; ESP6500, https://evs.gs.washington.edu/; ExAC, http://exac.broadinstitute.org/; self-built SNP database) were used to ensure the accuracy of somatic detection.
RESULTS
1. Clinicoradiological Features
All the 4 patients underwent biopsy only. Postoperative histological morphology confirmed astrocytoma, and integrating with molecular pathology, final pathological diagnosis revealed 2 diffuse midline glioma (DMG), H3 K27M-mutant (WHO IV), 1 grade II diffuse astrocytoma, 1 grade I diffuse astrocytoma. Mean age was 22.3 years (range, 18–28 years). The duration of symptom was 1 month and 2 months for the 2 patients with high-grade astrocytoma, respectively, while the duration of symptom was 10 months and 60 months for patient with grade I and grade II astrocytoma, respectively. Of the 4 spinal cord astrocytomas, 2 confined to the thoracic region, 1 in cervical region, and 1 involved holocord. The 2 patients with DMG received postoperative radiotherapy and chemotherapy with Temozolomide, while the remaining 2 patients did not receive postoperative adjuvant treatment. At the last follow-up, 2 patients (DMG) died with an average survival time of 12 months, while the remaining 2 patients were alive with an average survival time of 42 months. Clinicoradiological features were showed in Table 1 and preoperative MRI were showed in Fig. 2.
2. Tumor-Specific Mutations Detected in CSF-Derived ctDNA
CSF-derived tumor DNA was identified in 3 CSF samples (2 DMGs and 1 grade I astrocytoma) and was sequenced to an average depth of 1,039 (range, 100–2,041). The median mutant allele frequency (MAF) was 3.65% (range, 0.74%–84.84%). At least 1 tumor mutation was identified in these 3 CSF samples. The most frequently mutated genes were TP53 (3/4), H3F3A K27M (2/4), ATRX (2/4), APC (1/4), ZFHX3 (1/4), SETD2 (1/4), NOTCH2 (1/4), BLM (1/4), NCOR1 (1/4), PIK3R1 (1/4), CD5L (1/4), KRAS (1/4), EGFR (1/4), BRAF (1/4), MLL3 (1/4), PTEN (1/4) and CDKN1A (1/4). The average MAF of these mutated genes were TP53 53.83%, H3F3A K27M 36.53%, ATRX 21.75%, APC 4.30%, ZFHX3 (4.36%), SETD2 (4.70%), NOTCH2 (7.11%), BLM (5.80%), NCOR1 (5.95%), PIK3R1 (1.49%), CD5L (0.74%), KRAS (7.02%), EGFR (3.38%), BRAF (5.34%), MLL3 (1.85%), PTEN (1.46%) and CDKN1A (21.05%), respectively (Supplementary Table 2).
3. Concordance of Molecular Profile Between Paired Tissues and CSF Samples
Using the targeted sequencing approach, a total of 16 somatic mutations were identified in the 2 DMGs tissues, while no mutation was determined in grade I and grade II tumor tissues, respectively, average sequencing depth was 340 (range, 186–452) and the average MAF was 22.36% (Fig. 3A). In 3 CSF samples with detectable ctDNA, 28 somatic mutations were identified, the average MAF was 13.84%; of those, 5 of 28 mutations (17.86%) were shared between tumor tissues and CSF (Fig. 3B, C); the average MAF of the 5 variants detected in CSF was 52.9% (range, 30.0%–84.8%) compared to that of 36.4% (range, 16.1%–91.0%) in tumor tissues (Fig. 3D–F). Twenty-three mutations and 11 mutations were detected exclusively in CSF and tumor tissues, respectively (Fig. 3C). No tumor mutation was identified in matched blood.
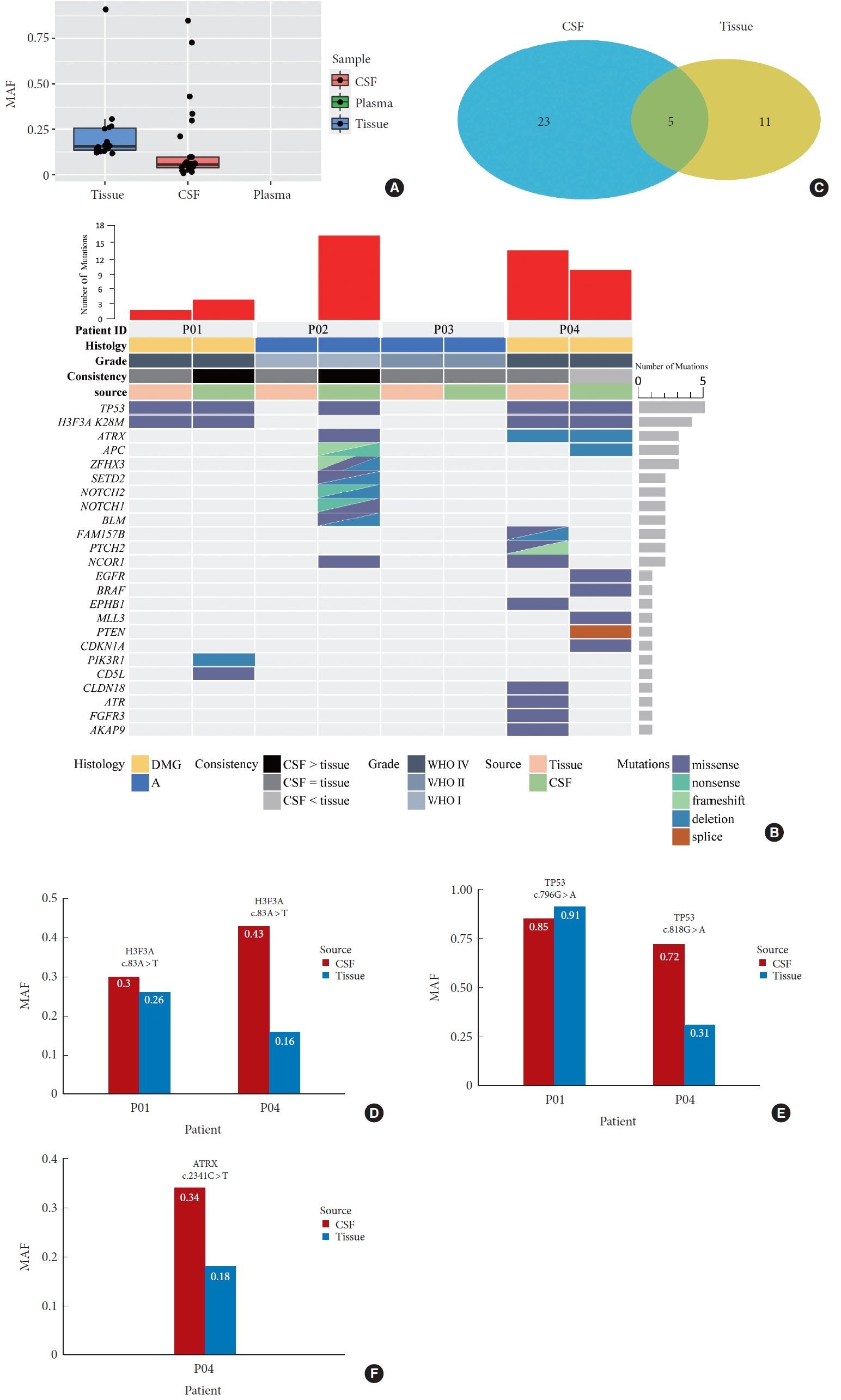
(A) Average mutant allele fraction of mutations detected in tumor tissues, cerebrospinal fluid (CSF) and blood, respectively. (B) Mutational characteristics and concordance between CSF and tumor tissues, molecular biomarkers associated with survival and diagnosis, such as H3F3A K27M, TP53, and ATRX, were detected in CSF. (C) Five mutations were shared in CSF and tumor tissues, number of mutations detected in CSF were more than that in tumor tissue. (D–F) The mutant allele frequency (MAF) of the 5 mutations shared in CSF and tumor tissues was frequently higher in CSF than that in tumor tissues. DMG, diffuse midline glioma; WHO, World Health Organization.
DISCUSSION
Primary spinal cord astrocytoma is a rare disease. Different from its intracranial counterpart, intramedullary astrocytoma rarely receives gross total resection without neurological deficit, due to poorly defined margins between the tumor and normal spinal cord, as well as the highly dense nerve fiber tracts in the spinal cord. Therefore, less-invasive diagnostic methodology and postoperative longitudinal monitoring of residual tumor in molecular level have clinically practical value and may help clinicians evaluate treatment response. Blood-based liquid biopsy has been used in clinical practice for assessing several noncentral nervous system tumors, including lung cancer, colorectal cancer, pancreatic cancer, melanoma and others [10-15]. As to central nervous system tumor, molecular analysis via CSF-derived ctDNA was emergingly confirmed to be feasible in brain glioma in proof-of-principle studies [7,16-19]; which may pave the way for the application of CSF-derived ctDNA to assess the molecular characteristics of primary spinal cord astrocytoma. However, given the distinction in genetic profiles between intramedullary glioma and cranial glioma, especially in terms of MAF, the MAF in spinal cord astrocytoma is likely to be low due to the fact that most spinal astrocytomas occur in children and young-aged populations [9]. Therefore, further investigation is warranted to determine the feasibility of applying CSF-derived ctDNA to evaluate the genetic profile of primary spinal cord astrocytoma.
In this pilot study, concordance of genetic alteration detected in paired tissues and CSF samples was evaluated. The results showed that 5 mutations shared in tumor tissues and CSF samples, but 11 mutations detected in tumors tissues were not identified in CSF, additionally, 23 mutations were exclusively detected in CSF. The concordance rate was lower than that in brain/brainstem gliomas [7,17]. Of note, in spite of the low concordance rate, genetic mutations in H3F3A, TP53, and ATRX, which were hotspot mutations found in spinal cord astrocytoma and were diagnostic and prognostic markers of glioma [20-22], could be detected in CSF-derived ctDNA using next-generation sequencing approach and showed favorable concordance with tissuebased testing. Particularly, H3F3A K27M, the representative diagnostic marker of DMG, was identified in CSF samples of the 2 patients with DMG, suggesting that CSF-based H3F3A K27M testing has the potential to serve as a noninvasive method for the diagnosis of DMG. Although H3F3A K27M does not exclusively occur in DMG, other gliomas, such as glioblastoma, can also harbor this mutation [23-25], the clinicoradiological features of primary spinal cord DMG and glioblastoma are distinct. Therefore, noninvasive-multimodal diagnostic modality integrating CSF-based liquid biopsy of H3F3A K27M with clinicoradiological parameters, rather than CSF-based H3F3A K27M testing alone, hold promise for the differential diagnosis of DMG in lieu of traditional biopsy and real-time monitoring of tumor relapse and response [26].
Additionally, of the mutations shared in tumor tissues and CSF, the MAF in CSF was frequently higher than that in tumor tissues, which may be attributed to intratumoral heterogeneity or sampling bias via biopsy. Consequently, mutation analysis based on tumor tissues obtained via a biopsy could not provide comprehensive mutation assessment of all tumor sites [5,27], while ctDNA has been proven to be a reliable source for intratumoral heterogeneity analysis, due to ctDNA is shed from the various heterogeneous tumor clones [28]. Additionally, molecular analysis using biopsy-obtained tumor tissues with a low proportion of tumor cells might be prone to error. That is why several mutations were exclusively detected in CSF.
Several factors likely contribute to the detectability of ctDNA in CSF. Tumor grade and location were reported to be highly associated with the detectability of ctDNA in CSF [7,18]. From a technological standpoint, the quantity of ctDNA released into CSF by high-grade glioma and tumor that abut a CSF reservoir is more likely to be sufficient for detection. In our study, one patient with grade I astrocytoma showed positive ctDNA in CSF, that patient had a 28-month history of primary spinal cord lesion and MRI showed the tumor almost involved totally cross-sectional spinal cord and adjacent leptomeningeal enhancement, thus, complete exposure to CSF reservoir likely contributes to positive detection of ctDNA in CSF. Of note, in our study, all CSF samples were collected intraoperatively from the site of tumor rather than through lumbar puncture. CSF circulates in spinal canal, therefore, it’s hypothesized that the anatomical location of CSF collection has no impact on the detectability of ctDNA. Consistently, the study by Miller et al. [17] revealed the genomic profiles of CSF samples collected in different locations were highly concordant. Thus, molecular analysis in CSF collected via lumbar puncture is clinically feasible.
To our knowledge, this is the first study exclusively focused on the feasibility of CSF-derived ctDNA in evaluating the molecular profile of primary spinal cord astrocytoma to date. ctDNA could be detected in CSF from primary spinal cord astrocytoma. However, given that only 4 patients were included, the concordance of molecular profiles between paired tissues and CSF samples needs further investigation in large sample-sized study. Favorably, hotspot genetic alterations that could assist molecular diagnosis or prognostic evaluation for gliomas, such as H3F3A K27M, TP53, ATRX, etc. could be identified in CSF, indicating that extraction and sequencing of CSF-derived ctDNA hold promising potential in assisting differential diagnosis or prognostic assessment of spinal cord lesions.
CONCLUSION
CSF-derived ctDNA could be detected in patients with primary spinal cord astrocytoma. Molecular analysis through sequencing of CSF-derived ctDNA hold promise in assisting with the diagnosis and prognostic evaluation for primary spinal cord astrocytoma.
Supplementary Materials
Supplementary Tables 1-2 and Fig. 1 can be found via https://doi.org/10.3345/cep.2022.00766.
1021 cancer related genes on the 1021-panel
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: FJ; Data curation: WD, JG, KW, ZL, XW, ZW, HW, ZC; Formal analysis: LC; Funding acquisition: FJ; Methodology: LC; Project administration: LC; Writing - original draft: LC; Writing - review & editing: FJ.
Acknowledgements
We thank our patients and their families for the information and DNA samples provided for our study. The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: HRA003965) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa.