The Role of Exercise in the Alleviation of Neuropathic Pain Following Traumatic Spinal Cord Injuries: A Systematic Review and Meta-analysis
Article information
Abstract
Objective
The objective of this systematic review and meta-analysis was to assess the efficacy of exercise in neuropathic pain following traumatic spinal cord injuries.
Methods
The search was conducted in MEDLINE, Embase, Scopus, and Web of Science by the end of 2022. Two independent researchers included the articles based on the inclusion and exclusion criteria. A standardized mean difference was calculated for each data and they were pooled to calculate an overall effect size. To assess the heterogeneity between studies, I2 and chi-square tests were utilized. In the case of heterogeneity, meta-regression was performed to identify the potential source.
Results
Fifteen preclinical studies were included. Meta-analysis demonstrated that exercise significantly improves mechanical allodynia (standardized mean difference [SMD], -1.59; 95% confidence interval [CI], -2.16 to -1.02; p < 0.001; I2 = 90.37%), thermal hyperalgesia (SMD, 1.95; 95% CI, 0.96–2.94; p < 0.001), and cold allodynia (SMD, -2.92; 95% CI, -4.4 to -1.43; p < 0.001). The improvement in mechanical allodynia is significantly more in animals with a compression model of SCI (meta-regression coefficient, -1.33; 95% CI, -1.84 to -0.57; p < 0.001) and in mild SCI (p < 0.001). Additionally, the improvement was more prominent if the training was started 7 to 8 days postinjury (coefficient, -2.54; 95% CI, -3.85 to -1.23; p < 0.001) and was continued every day (coefficient, -1.99; 95% CI, -3.07 to -0.9; p < 0.001). Likewise, voluntary exercise demonstrated a significantly more effect size (coefficient, -1.45; 95% CI, -2.67 to -0.23; p = 0.02).
Conclusion
Exercise is effective in the amelioration of neuropathic pain. This effect in mechanical allodynia is more prominent if voluntary, continuous training is initiated in the subacute phase of mild SCI.
INTRODUCTION
Injuries or functional disorders of the nervous system can lead to neuropathic pain, causing a persistent hypersensitivity to innocuous stimuli (allodynia) or an exaggerated response to nociceptive stimuli (hyperalgesia). Depending on the site of injury, neuropathic pain can have a central or a peripheral origin [1]. Spinal cord injury (SCI) is a pathological condition in which cascades of inflammatory and immunologic responses lead to improper neuroregeneration in the central nervous system (CNS) and cause subsequent sensorimotor deficits [2]. Patients with SCI commonly suffer from debilitating chronic pain that can range in severity. The overall prevalence of chronic pain is estimated to be around 68% in SCI patients, immensely affecting the patient’s quality of life and psychological well-being [3]. Chronic pain is hard to treat, and management is generally limited to pharmacological treatments and lifestyle modifications for temporary relief [4,5].
Long-term analgesics use is common in patients with chronic pain, resulting in dependence and tolerance over time [6]. Accordingly, researchers have focused on alternative treatment strategies that could be more effective in this setting. For instance, novel molecular therapies such as gene therapy and the use of viral vectors for the exclusive delivery of biological analgesic molecules have been developed recently [7,8]. Recent studies have shown that nonpharmacological approaches such as dermal skin stimulation, intracranial magnetic stimulation, acupuncture, and exercise therapy are also reasonably effective in the management of neuropathic pain [9-11].
Physical exercise is an essential part of a healthy lifestyle and is known to have a multitude of benefits for the body. Exercise improves cardiovascular health, enhances muscle strength and endurance, and reduces the risk of various chronic diseases [12-14]. Additionally, recent studies have shown that exercise can also benefit individuals who suffer from neuropathic pain. Exercise can help alleviate pain by increasing the blood flow and oxygen supply to the injured tissues, reducing inflammation and hence improving neuronal function [15,16]. Moreover, the release of endorphins during physical exercise, reduces anxiety, improves patients’ mood, and ultimately contributes to the amelioration of pain [17-19]. Studies have demonstrated a variety of exercise techniques that can be used to alleviate neuropathic pain, including aerobic exercises such as walking, jogging, cycling, or swimming, strength training, and stretching exercises [20,21].
Clinical studies have shown that even light exercise can alleviate pain in conditions such as cancer, musculoskeletal disorders, diabetes, and SCI [22-25]. Despite these findings, there is still a lack of a comprehensive consensus on the role of different exercise protocols in the treatment of neuropathic pain following SCI. Therefore, this systematic review and meta-analysis were conducted to evaluate the efficacy of active exercise in the amelioration of neuropathic pain following traumatic SCI.
MATERIALS AND METHODS
1. Study Design
The objective of this systematic review and meta-analysis was to assess the efficacy of exercise as a therapeutic intervention for neuropathic pain following traumatic SCI. The present study employed 3 strategies for selecting keywords, including the use of MeSH (in the MEDLINE database) and Emtree (in the Embase database) to find related entries, consultation with experts in the field, and a review of the related articles. Based on the selected keywords, an exhaustive search was conducted in the electronic databases of MEDLINE, Embase, Scopus, and Web of Science by the end of 2022 to identify relevant articles. Search strategies were based on keywords related to exercise, SCI, and pain (Supplementary Material 1).
2. Inclusion Criteria
The definition of PICO was as follows: The population (P) of the included articles were humans or animals (rats or mice) with compression, transection, hemisection, or contusion models of SCI. The intervention (I) was the use of any active exercise technique to alleviate neuropathic pain. The comparison (C) was made with a control group that did not receive the intervention or received standard treatment. The outcomes (O) were the reported rating scales of pain perception in humans and allodynia and hyperalgesia in animals. Accordingly, we excluded studies that did not execute an active exercise program as the intervention, incorporated only assisted or combinative exercise programs, case reports, case series, studies without a control group, human studies that were not designed as randomized clinical trials, pre- posttest studies, studies without an SCI model, studies on transgenic animals, protocols, studies not reporting a desired outcome or lacking the sufficient data, studies that evaluated nonneuropathic pain, follow-up studies, and reviews.
3. Data Gathering
The results of the systematic search in electronic databases were collected in the 20th version of the Endnote program. In the initial screening process, 2 independent researchers assessed the titles and abstracts of the obtained articles and selected the potentially relevant studies. Then, the full text of the selected articles was reviewed, the inclusion and exclusion criteria were applied, and articles meeting the criteria were included. A search in the grey literature (Google, Google Scholar, and the thesis section of the ProQuest database) was conducted to avoid any missing articles. Data were summarized in a checklist based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [26]. The obtained data included information regarding the study design, the sample size and characteristics, the SCI injury model and severity, the interval from SCI to the initiation of exercise, the exercise protocol, the interval to outcome assessment, and the outcome measurement test. If multiple articles were based on the same data, we included the article with the largest sample size or the longest follow-up interval. For data that were not presented in the article, we contacted the corresponding author. For articles in languages other than English, the data were extracted with the help of a translator fluent in both languages. Any disagreements were resolved through discussions with the third reviewer.
4. Quality Control and Certainty of Evidence
The risk of bias in animal studies was evaluated using SYRCLE’s risk of bias assessment tool [27] and a traffic light plot with a summary figure was created using the Robvis visualization tool [28] (Supplementary Material 2). The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was used to evaluate the certainty of evidence [29] (Supplementary Material 3). In the case of a disagreement, the conflict was resolved through discussions with a third researcher.
5. Statistical Analyses
The statistical analyses were conducted using Stata 17.0 (StataCorp LLC, College Station, TX, USA). The included studies were classified and summarized according to the classification of the reported neuropathic pain. A standardized mean difference (SMD) with a 95% confidence interval (95% CI) was calculated for each sample and they were pooled to calculate an overall effect size. It should be noted that meta-analysis was only performed if data were reported by at least 3 separate analyses. If a study used a scale in which a higher efficacy was observed with a lower score on the index scale, the absolute SMD value was inserted into the analysis. In this study, a random or fixed effect model was chosen based on the presence or absence of heterogeneity. To assess the heterogeneity between studies, I2 and chi-square tests were utilized. In the case of heterogeneity, subgroup analyses, and meta-regression were performed to identify the potential source. Subgroup analyses were performed on different animal species, levels of SCI, models of SCI, severities of SCI, SCI to exercise timing, the duration of exercise protocol, number of days in the week that the animals were trained, and whether exercise was conducted voluntarily. Sensitivity analyses were performed to evaluate the robustness of the findings. Additionally, publication bias was reported with a funnel plot using the modified Egger’s test proposed by Doleman et al. [30].
6. Ethic’s approval
This research has been approved by Tehran University of Medical Sciences and Health Services and Iran Ministry of Health and Medical Education.
RESULTS
1. Preclinical Studies
1) Characteristics of the included studies
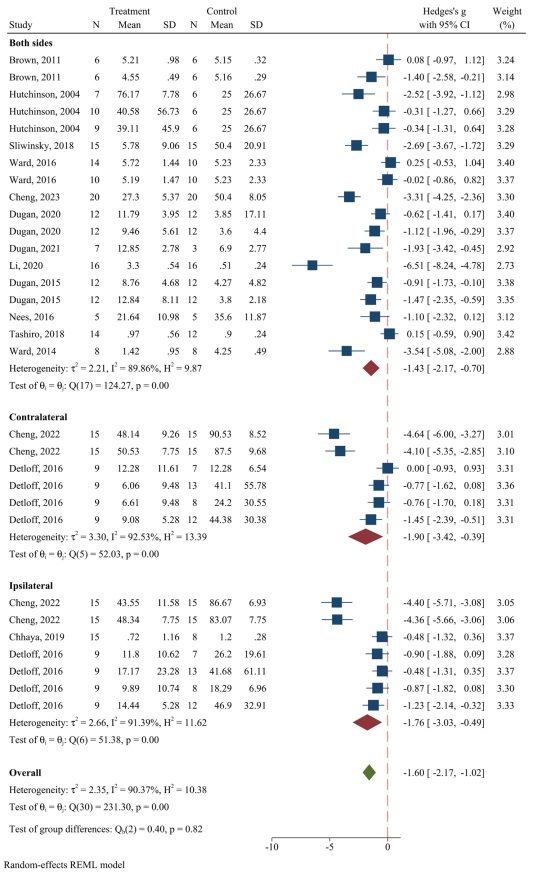
The systematic search resulted in 2,656 nonduplicate records, of which the full text of 167 was reviewed in detail. Finally, 15 animal interventional studies met the inclusion criteria (Fig. 1) [31-45]. Notably, the search in human studies resulted in no eligible articles. In the included preclinical studies, 11 wielded strains of rats and 4 wielded mice. Eleven studies used the contusion model of SCI (9 moderate and 2 severe) and 4 used the compression model (1 mild and 3 moderate). The time interval between the induction of SCI and the initiation of exercise varied from 1 to 42 days postinjury; in 2 studies exercise was initiated in the first 5 days postinjury, 4 initiated the exercise protocol in 7 to 8 days postinjury, and in 6 studies exercise was initiated after the first 14 days. In 3 articles the interval from SCI to training was a combination of the aforementioned timelines. Twelve studies used a quadrupedal training program (running wheel, treadmill, exercise ball), 1 study used a bipedal gait training exercise and 2 studies used both quadrupedal and bipedal training. Apart from 1 study that continued the intervention for 2 years, the duration of exercise varied between 2 to 12 weeks. All exercise protocols were carried out 5–7 days per week. In 3 studies, animals were allowed to move freely during the exercise protocol, while in others each session lasted between 20 to 58 minutes. The last follow-up was immediately postintervention in all articles, except for one, that reported the outcome in 1 week and 2 weeks postintervention. All articles evaluated mechanical allodynia, 10 evaluated thermal hyperalgesia, and 4 evaluated cold allodynia. The results were separately reported for the contralateral or ipsilateral side of the injury in 3 articles (Table 1).
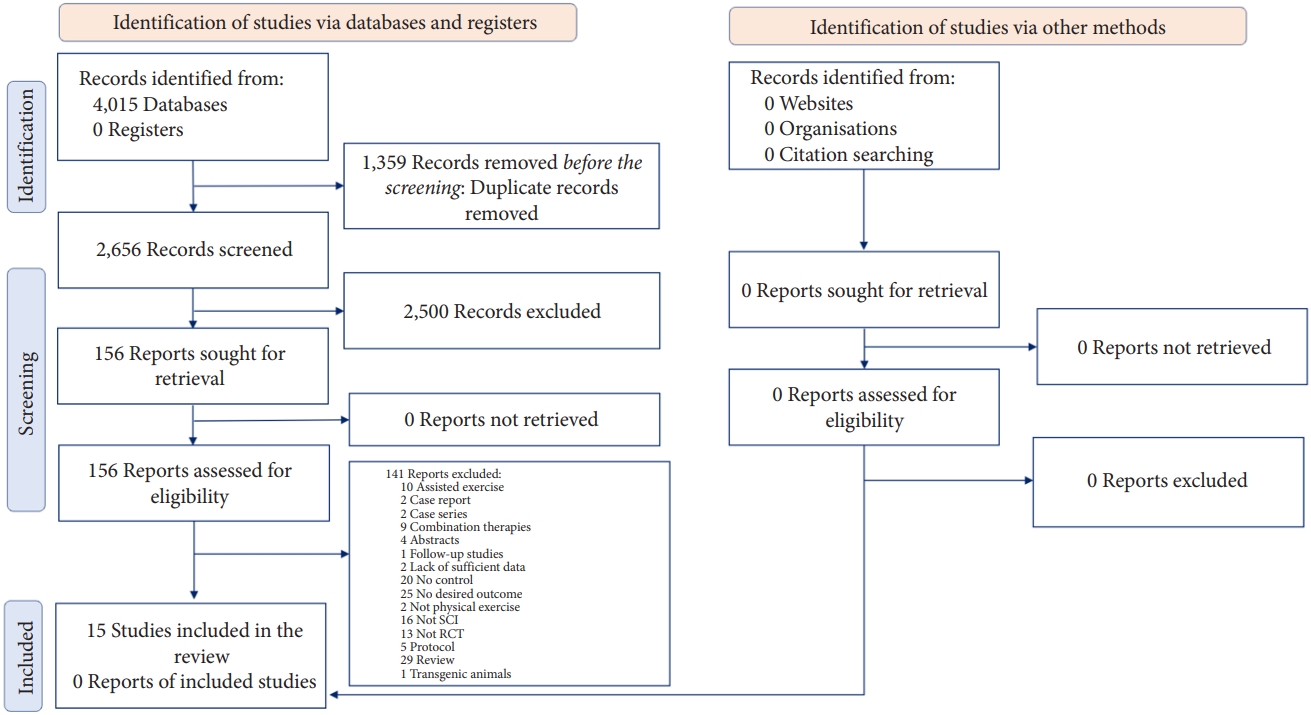
PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow diagram of the screening process.
2. Meta-analysis
1) The effect of exercise on mechanical allodynia
Categorizing the effect of exercise on mechanical allodynia into ipsilateral, contralateral, and bilateral mechanical allodynia, 31 separate analyses were included in this meta-analysis. Pooled data analysis demonstrated that exercise significantly improves bilateral mechanical allodynia (SMD, -1.43; 95% CI, -2.17 to -0.7; p<0.001; I2=89.86%). Moreover, pooled data analyses in the contralateral side exhibited meaningful improvement in mechanical allodynia following exercise (SMD, -1.9; 95% CI, -3.42 to -0.39; p=0.014; I2=92.53%). The results were similar on the ipsilateral side (SMD, -1.76; 95% CI, -3.03 to -0.49; p=0.007; I2=91.39%). In total, exercise therapy significantly improves mechanical allodynia in animals (SMD, -1.59; 95% CI, -2.16 to -1.02; p<0.001; I2=90.37%) (Fig. 2).
2) Subgroup analyses for mechanical allodynia
Subgroup analyses and meta-regressions were performed to detect sources of heterogeneity. The improvement in mechanical allodynia was significant in almost all subgroups, except for animals with thoracolumbar SCI, which was investigated only in 3 distinct experiments. Performing a subgroup analysis was not methodologically feasible due to the low number of included experiments for animals that were trained 6 days per week. In the next step, meta-regressions demonstrated that the extent of improvement is significantly more in animals with a compression model of SCI (meta-regression coefficient, -1.33; 95% CI, -1.84 to -0.57; p<0.001), while less improvement was evident in both moderate SCI (meta-regression coefficient, 2.95; 95% CI, 1.61–4.29; p<0.001) and severe SCI (meta-regression coefficient, 3.67; 95% CI, 2.24–5.11; p<0.001). Moreover, meta-regression showed a meaningful relationship between the effect size of improvement in mechanical allodynia and the SCI to training interval of 7 to 8 days (coefficient, -2.54; 95% CI, -3.85 to -1.23; p<0.001) and also with animals that were trained every day (coefficient, -1.99; 95% CI, -3.07 to -0.9; p<0.001). The improvement in mechanical allodynia was significantly more in animals that were in a voluntary exercise setting (meta-regression coefficient, -1.45; 95% CI, -2.67 to -0.23; p=0.02) (Table 2). Determining the level of evidence in the pain-ameliorating effects of exercise on mechanical allodynia revealed a moderate level of evidence using the GRADE framework (Supplementary Material 3).
3) The effect of exercise on thermal hyperalgesia
In thermal hyperalgesia, 17 separate analyses were included. Pooled data analysis showed a significant improvement of thermal hyperalgesia reported on both sides following exercise (SMD, 1.95; 95% CI, 0.96–2.94; p<0.001; I2=91.37%). Meta-analysis also demonstrated a meaningful improvement of thermal hyperalgesia on the contralateral (SMD, 3.03; 95% CI, 2.3–3.77; p<0.001; I2=0%) and ipsilateral side (SMD, 2.38; 95% CI, 1.73–3.03; p<0.001; I2=0%). In total, exercise therapy had a meaningful effect on the amelioration of thermal hyperalgesia in animals (SMD, 2.38; 95% CI, 1.73–3.03; p < 0.001; I2=88.72%) (Fig. 3).
4) Subgroup analyses for thermal hyperalgesia
Subgroup analyses demonstrated that exercise improved thermal hyperalgesia in rats, different levels of SCI, models of SCI, severities of SCI, intervals between SCI to exercise, and different groups of training durations in a week. However, meta-regressions showed that there were no significant differences in the effect sizes of different subgroups (Table 2). The level of evidence was demonstrated moderate using the GRADE framework (Supplementary Material 3).
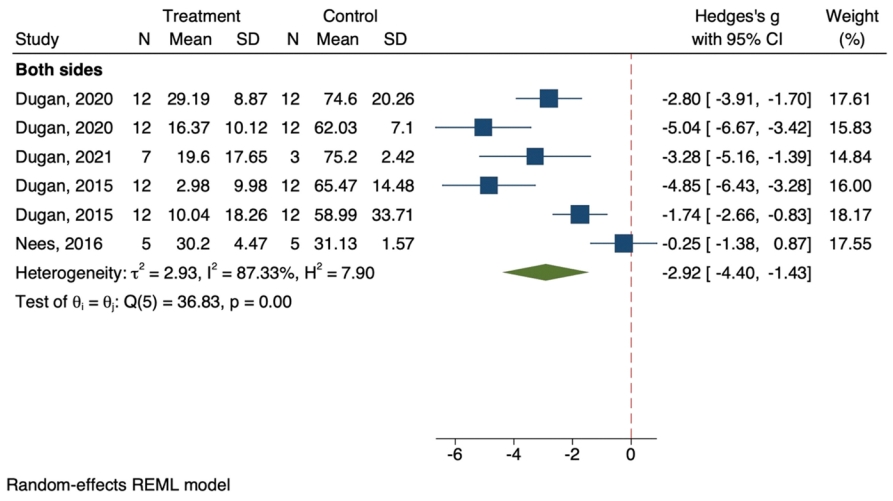
5) The effect of exercise on cold allodynia
Cold allodynia was reported bilaterally in all included articles. Pooled data analysis on 6 separate analyses from 4 articles revealed that exercise significantly affected the improvement of cold allodynia (SMD, -2.92; 95% CI, -4.4 to -1.43; p < 0.001; I2=87.3%) (Fig. 4). The level of evidence was determined as moderate in the pain-ameliorating effect of exercise on cold allodynia (Supplementary Material 3).
6) Sensitivity analyses
We performed sensitivity analyses for mechanical allodynia and thermal hyperalgesia due to the sufficient number of included experiments to evaluate the robustness of our results. The findings of these analyses demonstrated that the efficacy of exercise in the amelioration of mechanical allodynia and thermal hyperalgesia is evident in all different sets of experiments (Supplementary Material 4).
3. Clinical Studies
1) Characteristics of the included studies
Even though our search resulted in no eligible studies on the human population for the aforementioned outcome, we summarized the obtained randomized controlled trials (RCTs) that evaluated the amelioration of chronic pain in SCI patients following active exercise protocols [21,46-49]. In this regard, shoulder pain and generally perceived pain were the 2 reported outcomes and were subsequently included in separate meta-analyses. Two articles evaluated the WUSPI (wheelchair user shoulder pain index), 2 reported the 36-item Short-Form survey (SF-36), and 1 reported both. All articles included chronic SCI patients. Three studies conducted a home-based exercise program whereas 2 articles conducted the exercise protocol in rehabilitation centers. All exercise protocols were for the upper extremities except for 1 study that exercised all limbs. The exercise duration ranged between 6 to 36 weeks, 2 to 4 days a week. Except for 1 article that only included resistance training, others included aerobic, stretching, and resistance training in their exercise protocol (Table 3).
4. Meta-analysis
1) The effect of active exercise on chronic pain following traumatic SCI
Pooled data analysis on 3 different samples demonstrated that exercise therapy significantly improved the general perception of pain in chronic SCI patients (SMD, -0.76; 95% CI, -1.14 to -0.37; p<0.001; I2=0%). Conversely, the meta-analysis exhibited no significant improvement in shoulder pain following exercise (SMD, -0.43; 95% CI, -1.04 to 0.17; p=0.159) (Fig. 5).
2) Quality control
In the included preclinical studies, all studies adjusted the experiment and the control groups at baseline for confounders. Randomization and its proper concealment were adequately applied. The risk of bias was unclear in the domain of random housing in all included articles. Drop-out animals were not adequately mentioned and imported in analyses of 4 studies (Supplementary Material 2).
In RCTs, the allocation concealment and the blinding of the participants were not adequately addressed in any of the included articles. The blinding of the study team and the outcome assessor were unclear in 3 articles. One article had incomplete outcome data. In total, the risk of bias in all RCTs was considered high (Supplementary Material 5).
3) Publication bias
No publication bias was observed in the articles reporting mechanical allodynia (p=0.288), thermal hyperalgesia (p=0.163), and cold allodynia (p=0.686) (Supplementary Material 6). Due to the low number of included RCTs, publication bias assessment ws not feasible in terms of methodology.
DISCUSSION
The purpose of the present systematic review and meta-analysis was to gather current literature regarding the efficacy of exercise in the amelioration of neuropathic pain following traumatic SCIs. We demonstrated that exercise significantly improves mechanical allodynia, thermal hyperalgesia, and cold allodynia in rodent models of SCI. Meta-regressions showed a significantly more effect size for the amelioration of mechanical allodynia in compression models of SCI and mild SCIs. Likewise, the extent of this improvement was substantially more if the exercise protocol began in the subacute phase [50] of SCI, continued every day, and was voluntary.
The development of neuropathic pain is a consequence of nerve injury, either in the periphery or the CNS [51]. Even though a multitude of factors have been suggested to provoke the pathological pathways that lead to neuropathic pain, the distinct molecular and cellular alterations involved in the process remain understudied [52,53]. The underlying mechanisms of neuropathic pain can be categorized into the alterations of pain threshold in the primary afferent nociceptive neurons, activation of nonnociceptive receptors, changes in neurotransmitter transduction, and rewiring of neurons in the pain perception pathways of the CNS [54,55].
As the resident immune cells in the CNS and the main modulators of neuroinflammation, microglia play an important role in both central and peripheral mechanisms of mechanical allodynia [56]. Following an injury to the nervous system, microglia undergo activation and morphologic changes, exerting both beneficial and detrimental effects on the tissue healing process. One way the activation of these cells could contribute to the development of neuropathic pain is the activation of the ionotropic adenosine triphosphate receptor P2X4 which eventually leads to the release of brain-derived neurotrophic factor (BDNF), altering the pattern of excitability in the sensory and dorsal horn neurons [57]. Physical activity has been shown to exert an anti-inflammatory effect that suppresses microglia activation and thus improves neuro-inflammation [58]. With the role of BNDF being controversial in the pain perception pathways, the relationship between exercise and subsequent alterations in BDNF levels should be addressed in future studies [59-62].
Furthermore, recent literature argues that high-intensity and interval training programs could promote oxidative stress and neuroinflammation, whereas low- to moderate-intensity and continuous exercises would exert more anti-inflammatory and neuroprotective properties [63]. In rodent models, it was previously reported that forced treadmill exercise contributed more to the production of proinflammatory molecules in comparison with voluntary wheel running [64]. Considering that different exercise programs influence the neuroplasticity of distinctive brain regions [65-67], the present systematic review along with previous studies exhibited that continuous and voluntary exercise protocols are more beneficial in the amelioration of neuropathic pain.
We demonstrated that exercise—regardless of the timing of commencement after SCI—is effective for the amelioration of mechanical allodynia. However, the extent of this improvement is more if exercise is started in the subacute phase, and similar for both acute and chronic phases. We could explain this by increased neural plasticity involved in the acute and subacute phases of SCI. As reported previously, early therapeutic approaches in the management of SCI lead to better outcomes and fewer postoperative complications, both in clinical and preclinical evidence [68-71].
Even though we aimed at summarizing the evidence from both animal and human populations, our search resulted in no clinical research that would meet our inclusion criteria. The chronic shoulder pain and generally perceived pain that was evaluated in previous RCTs could have etiologies other than neuropathic pain, and their inclusion is considered an ancillary analysis in this meta-analysis. Evidence with a high risk of bias showed a significant improvement in generally perceived pain in chronic SCI patients following exercise, but no such effect was observed in the amelioration of chronic shoulder pain (Supplementary Material 5).
We considered the level of evidence for our included preclinical articles to be moderate, due to serious risks of bias. Conversely, it is recently argued that the current guidelines for the evaluation of the risk of bias in preclinical studies are not in compliance with the guidelines on how to conduct them; therefore, the risk of bias could be overestimated due to the lack of reporting in some domains of risk of bias assessment, simply because the authors did not document them in their manuscripts [72]. Since our results demonstrated an acceptable efficacy for exercise in the management of neuropathic pain, we prominently recommend further clinical research with robust methodologies in this field. In order to have more reliable findings, it should be emphasized that the blinding of participants and caregivers in studies that involve active physical activity is an important cause for bias; active exercise requires the patient’s cooperation with the assessor, and the lack of blinding is inevitable. Therefore, we recommend more attention to proper blinding in future studies.
In addition, our analyses for the detection of publication bias demonstrated no bias. However, in order to address the asymmetry in our funnel plots, it is noteworthy that according to the study by Egger et al. [73], asymmetry in funnel plots could be due to different reasons: publication bias, selective outcome reporting, poor methodological quality, the presence of substantial heterogeneity, sampling variations, and by chance. Therefore, the observed asymmetry could be due to the aforementioned reasons.
As an inevitable limitation of review articles, it is important to draw attention to the low number of included experiments for some subgroups, and the importance of cautious interpretation of findings. For instance, even though we found a significantly more effect size in compression models of SCIs and in mild SCIs, only 1 article with 4 experiments was included in mild SCI, and 4 articles out of the included 15 executed a compression model of SCI. Notably, all compression SCIs were mild to moderate in severity, and contusion SCIs were all moderate to severe. Nevertheless, sensitivity analyses demonstrated a similar level of difference in the effect sizes of different subgroups, omitting the possible role of the observed heterogeneity in our findings.
At last, we would like to acknowledge that the protocol of this systematic review was not registered on an open-access database. The design and methodology of the present study validate the robustness of our results, but it is important to mention that protocol registration contributes to a better evaluation of outcomes and prevents duplicate efforts.
CONCLUSION
Continuous voluntary exercise initiated in the subacute phase of moderate to severe SCI is strongly associated with the amelioration of neuropathic pain in rodents. Although physical activity improves the general perception of pain in chronic SCI patients, its efficacy in the amelioration of shoulder pain should be further evaluated in further studies.
Supplementary Material
Supplementary Materials 1-6 can be found via https://doi.org/10.14245/ns.2346588.294.
Search strategies
Risk of bias assessment of the included preclinical studies according to the SYRCLE’s risk of bias assessment tool.
The certainty of evidence
Sensitivity analyses on mechanical allodynia and thermal hyperalgesia
Risk of bias assessment for the randomized clinical trials
Publication bias for mechanical allodynia (A), thermal hyperalgesia (B), and cold allodynia (C). s.e., standard error.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This research has been supported by Tehran University of Medical Sciences and Health Services and Iran Ministry of Health and Medical Education (Grant number: 96-04-159-36946).
Author Contribution
Conceptualization: HAR, ARV, MH, MY, VRM; Data curation: AT, HAR, PG, ARV, HZ, MH, MY, VRM; Formal analysis: AT, ARV, MH, MY, VRM; Funding acquisition: VRM; Methodology: AT, HAR, PG, ARV, HZ, MH, MY, VRM; Project administration: ARV, MH, MY, VRM; Visualization: MH; Writing - original draft: AT, HAR, PG, ARV, HZ, MH, MY, VRM; Writing - review & editing: AT, HAR, PG, ARV, HZ, MH, MY, VRM.