Management of Cerebrospinal Fluid Leakage by Pump-Regulated Volumetric Continuous Lumbar Drainage Following Anterior Cervical Decompression and Fusion for Ossification of the Posterior Longitudinal Ligament
Article information
Abstract
Objective
Cerebrospinal fluid (CSF) leakage is a major concern related to anterior cervical decompression and fusion for ossification of the posterior longitudinal ligament (OPLL). We propose a management algorithm for CSF leakage following anterior cervical decompression and fusion for OPLL involving the use of pump-regulated volumetric continuous lumbar drainage.
Methods
We retrospectively reviewed patients who underwent anterior cervical decompression and fusion for OPLL and were managed with the proposed algorithm between March 2018 and July 2022. The proposed management algorithm for CSF leakage by pump-regulated volumetric continuous lumbar drainage was as follows. On exposure of the arachnoid membrane with or without CSF leakage, a dural sealant patch was applied to manage the dural defect. In case of persistent CSF leakage despite application of the dural sealant patch, patients underwent pump-regulated volumetric continuous lumbar drainage.
Results
Fifty-one patients were included in the study. CSF leakage occurred in 14 patients. Of these 14 patients, 9 patients underwent lumbar drain insertion according to the proposed management algorithm. Successful resolution of CSF leakage was observed in 8 of the 9 patients who underwent lumbar drainage. All patients were encouraged to ambulate without concern of CSF overdrainage due to gravity, because it could be avoided with pump-regulated volumetric continuous CSF drainage. Therefore, complications associated with absolute bed rest or CSF overdrainage were not observed.
Conclusion
The proposed management algorithm with pump-regulated volumetric continuous lumbar drainage showed safety and efficacy for management of CSF leakage following anterior decompression and fusion for OPLL.
INTRODUCTION
Ossification of the posterior longitudinal ligament (OPLL) is characterized by abnormal calcification of the ligament. OPLL most commonly occurs at the cervical vertebrae [1]. Cervical OPLL induces spinal cord compression, leading to myelopathy and radiculopathy [2,3]. Primary treatment for OPLL involves surgical decompression, which can be performed with an anterior, posterior, or combined approach. Posterior surgeries, such as laminoplasty, laminectomy alone, or laminectomy and instrumented fusion help indirectly decompress the spinal cord by enlarging the spinal canal [4-8]. The advantage of posterior surgery is it does not require manipulation of the spinal cord and OPLL. Therefore, posterior surgery is associated with a low incidence of cerebrospinal fluid (CSF) leakage due to dural tear.
Anterior cervical decompression and fusion is another surgical option that can help decompress the spinal cord, and nerve roots through direct resection of the OPLL [9-15]. However, in contrast to posterior surgery, anterior surgery involves direct manipulation of the OPLL and dura mater and therefore increases the risk of CSF leakage due to dural tear. A systematic management algorithm for CSF leakage following anterior surgery for OPLL is therefore essential. Previous literature presents various intraoperative and postoperative methods to manage CSF leakage [16-18]. Continuous lumbar CSF drainage is a conventional postoperative technique of managing CSF leakage. However, this technique requires absolute bed rest (ABR) during lumbar drainage, which can cause complications associated with ABR, such as atelectasis, pulmonary thromboembolism, and deep vein thromboembolism [19]. Furthermore, owing to the gravity-dependent nature of lumbar drainage, abrupt large-volume CSF drainage and subsequent intracranial hypotension-related complications may occur [19,20]. Therefore, pump-regulated volumetric continuous lumbar CSF drainage has been introduced to prevent these complications [21,22]. We aimed to evaluate the efficacy of pump-regulated volumetric continuous lumbar CSF drainage and propose a management algorithm for CSF leakage following anterior surgery for OPLL.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB No. 2022-1633), ensuring compliance with ethical standards. Given the retrospective nature of the study, the requirement for informed consent was waived by the IRB.
1. Patient Population
We retrospectively reviewed the electronic medical records, operative records, and radiographic images of all patients who underwent surgery for OPLL between March 2018 and July 2022 at Asan Medical Center. The surgeries were performed by a single surgeon. We included patients who underwent anterior cervical diskectomy and fusion (ACDF), vertebral body sliding osteotomy (VBSO), or anterior cervical partial corpectomy and fusion owing to a limited working space that made primary closure of the dural tear unfeasible. Patients who underwent anterior cervical corpectomy and fusion or revisional ACDF were excluded.
2. Radiologic Evaluation
All patients underwent preoperative plain radiograph, computed tomography (CT), and magnetic resonance imaging to localize the OPLL and identify any coexisting pathologies, such as spinal stenosis and ossification of the ligamentum flavum. In patients with OPLL, the dura mater may be calcified and fused with the posterior longitudinal ligament, which is known as dural ossification (DO); radiographically, it appears as the “double-layer sign” [23,24]. Preoperative axial CT images showed the double-layer sign, which is characterized by anterior and posterior rims of hyperdense ossification separated by a central hypodense mass of variable size. We classified the double-layer sign into 3 types based on the radiographic morphology of DO: type A, crescent shape (anterior rim > posterior rim); type B, short-straight shape (anterior rim < posterior rim, less than half the width of the spinal canal); and type C, long-straight shape (anterior rim < posterior rim, more than half the width of the spinal canal) (Fig. 1) [24]. OPLL exhibited 4 distinct morphological types on preoperative sagittal CT images: continuous, segmental, mixed, and circumscribed [2]. The number of vertebrae across the craniocaudal extent of OPLL was also counted on preoperative sagittal CT images.
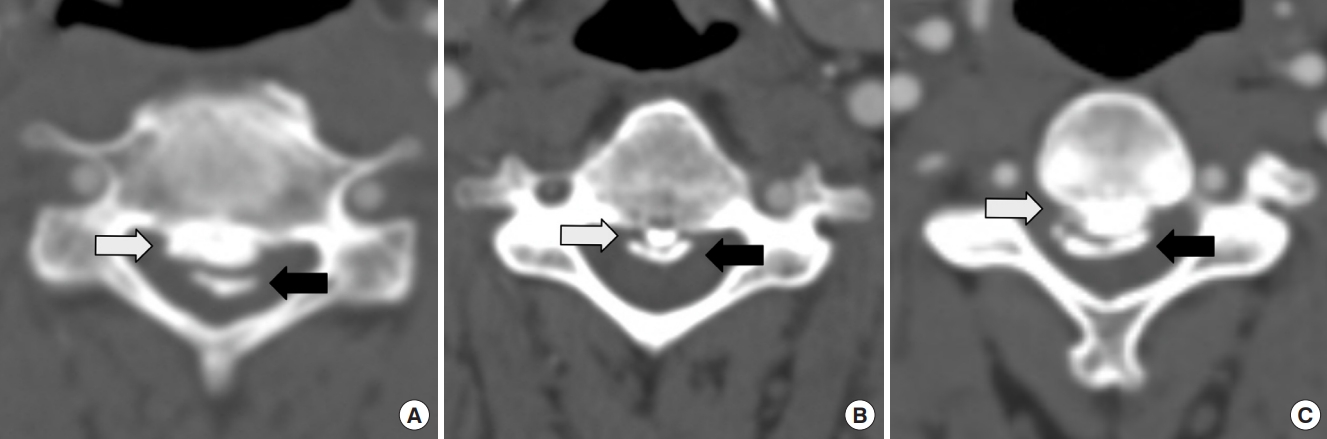
The double-layer sign is classified into 3 distinct categories. (A) Type A is characterized by a crescent shape with the anterior rim (white arrow) exceeding the posterior rim (black arrow). (B) Type B is characterized by a short-straight shape with the anterior rim (white arrow) being lower than the posterior rim (black arrow) and occupying less than half the width of the spinal canal. (C) Type C is characterized by a long-straight shape with the anterior rim (white arrow) lower than the posterior rim (black arrow) and occupying more than half the width of the spinal canal.
3. Management Algorithm for CSF Leakage by Pump-Regulated Volumetric Continuous Lumbar Drainage
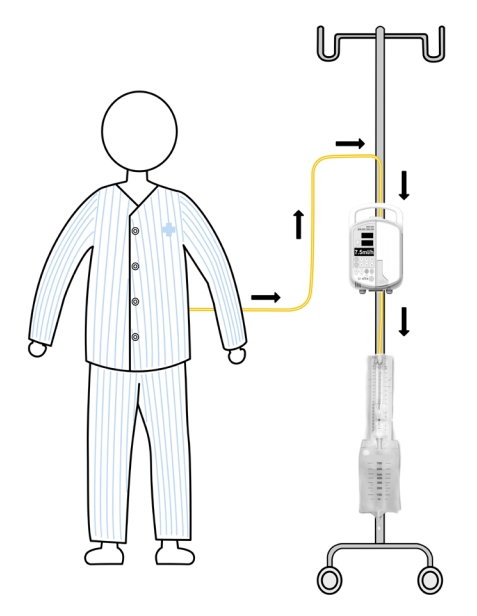
The intraoperative and postoperative management algorithm for CSF leakage is presented in Fig. 2. In cases where the arachnoid membrane remained intact upon exposure with no CSF leakage, a dural sealant patch (TachoSil, Takeda Austria GmbH, Linz, Austria) was applied to protect the exposed arachnoid membrane [25]. Moreover, when a defect was created in the arachnoid membrane, leading to CSF leakage, a dural sealant patch was placed to cover the defect. In case of persistent intraoperative microscopic CSF leakage despite application of a dural sealant patch, a lumbar drain was inserted immediately after completing the operation. In the absence of CSF leakage following placement of the dural sealant patch to repair the dural defect, although the anesthesiologist performed the Valsalva maneuver, the operation was completed without the need for lumbar drain insertion. If anterior neck swelling was observed postoperatively due to delayed CSF leakage and CSF drained into the Jackson-Pratt drainage, a lumbar drain was inserted in the general ward. The lumbar drain was connected to the volumetric infusion pump (Flo-Gard Infusion pump, Baxter, Toronto, ON, Canada). The infusion pump was set to withdraw CSF at a rate of 7.5–10 mL/hr from the lumbar drain into the CSF collection bag (Fig. 3). Patients were not restricted to ABR and were encouraged to ambulate. First-generation cephalosporins were prophylactically administered for the duration of lumbar drainage. The lumbar drain was maintained for 7–14 days. In the absence of clinical signs of CSF leakage after clamping for 24 hours, the lumbar drain was removed. If CSF leakage persisted beyond 14 days of lumbar drainage, revision surgery was performed to repair the dural defect.
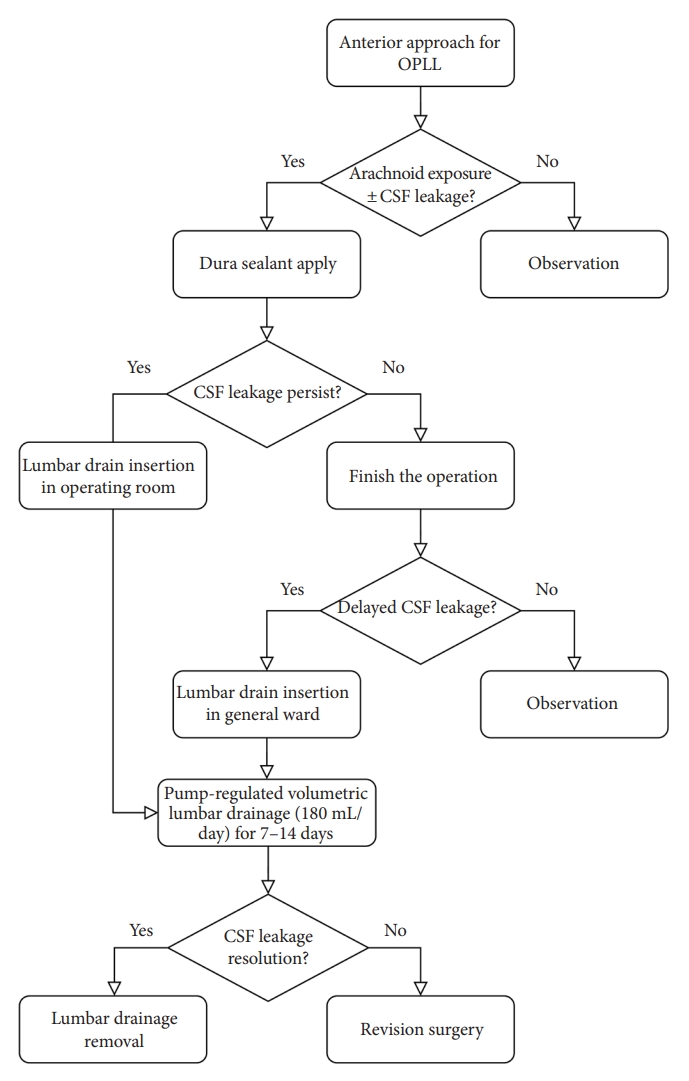
Management algorithm for CSF following anterior cervical decompression and fusion for OPLL. OPLL, ossification of the posterior longitudinal ligament; CSF, cerebrospinal fluid leakage.
4. Statistical Analysis
All statistical analyses were performed using R software (ver. 4.1.1, R Foundation for Statistical Computing, Vienna, Austria). Statistical significance (p < 0.05) was determined using a 2-tailed test. Continuous variables (age and extent of OPLL) are presented as mean ± standard deviation or median (range). Categorical variables (sex, presence of arachnoid exposure and CSF leakage, type of the double-layer sign, and OPLL pattern) are presented as a number (%). Fisher exact test was performed to compare categorical variables.
RESULTS
1. Demographics
A total of 53 patients had undergone anterior cervical decompression and fusion for OPLL. Of them, 2 patients were excluded because one had undergone corpectomy and the other had undergone revisional ACDF. Among the 51 patients, the mean patient age was 58.1 ± 11.7 years. Thirty-eight patients (74.5%) were male, and 13 (25.5%) were female. They underwent surgical treatment for the following OPLL types: continuous type, 5 (9.8%); segmental type, 18 (35.3%); mixed type, 26 (51.0%); circumscribed type, 2 (3.9%). Twenty-eight, 18, and 3 patients underwent ACDF, VBSO, and half corpectomy, respectively. Two patients underwent combined ACDF and VBSO. Arachnoid membrane was exposed in 20 patients (39.2%), and CSF leakage occurred in 14 patients (27.5%). Patient demographics and the OPLL characteristics are summarized in Table 1.
2. Double-Layer Sign
The double-layer sign was observed on the preoperative axial CT images of 40 patients (78.4%). The type B double-layer sign was the most common (N = 23, 57.5%) and type A was the least common (N = 5, 12.5%) (Table 1).
3. Arachnoid Membrane Exposure According to Double-Layer Sign Classification
Of the 51 patients, the arachnoid membrane was exposed in 20. CSF leakage occurred in 14 of these patients. The incidence of arachnoid membrane exposure was the least in patients without DO (N = 2 of 20, 10.0%) and highest in patients with the type C double-layer sign (N = 8 of 20, 40.0%). However, statistical analysis did not reveal a significant difference (p = 0.166) in the incidence of arachnoid membrane exposure between the groups with or without DO. Furthermore, statistically remarkable difference could not be observed (p = 0.114) in the incidence of arachnoid membrane exposure according to the double-layer sign classification (Table 2).
4. CSF Leakage According to Double-Layer Sign Classification
Of the 51 patients, CSF leakage occurred in 14. CSF leakage did not occur in patients without DO, and its incidence was most common in patients with the type C double-layer sign (N = 7 of 14, 50.0%). Statistical analysis revealed a significant difference (p = 0.023) in the incidence of CSF leakage between the groups with or without DO. In addition, there was a significant difference (p = 0.013) in the incidence of CSF leakage according to the double-layer sign classification (Table 3).
5. Clinical Outcomes of CSF Leakage on Applying the Management Algorithm
The arachnoid membrane was exposed in 20 of 51 patients. Six of them had no intraoperative CSF leakage, and the operation was completed after applying a dural sealant patch. In the remaining 14 patients, CSF leakage occurred despite application of the dural sealant. In 7 of these patients, a lumbar drain was inserted in the operating room following the operation. In contrast, the other 7 patients did not require lumbar drain insertion as the CSF leakage successfully halted with the application of the dural sealant. However, delayed CSF leakage occurred in 2 patients. Subsequently, a lumbar drain was inserted for delayed CSF leakage in the ward. Pump-regulated volumetric continuous CSF drainage was performed using an infusion pump. The infusion pump was applied to the lumbar drain to withdraw CSF at a rate of 7.5–10 mL/hr, which is equivalent to 180–240 mL/day. Patients with lumbar drainage were encouraged to ambulate. The lumbar drain was maintained for 7–14 days. If there was no sign of neck swelling or bulging at the operation site, the lumbar drain was removed after 24 hours of clamping if no complications were observed during clamping. CSF leakage was successfully resolved in 8 of 9 patients who underwent lumbar drainage insertion. However, one patient underwent revision surgery due to persistent CSF leakage. In this patient, CSF did not drain continuously despite pump-regulation owing to intermittent occlusion of the lumbar drain. For the revision surgery, a fat graft was obtained from the abdominal wall and grafted into the disc space, and no further complications occurred after the surgery.
6. Complications
No complication associated with the insertion of lumbar drainage, including meningitis or spondylitis occurred. Moreover, the occurrence of pulmonary embolism or deep venous thrombosis due to ABR was avoided as the patients were encouraged to ambulate [26]. Additionally, the consistent volumetric drainage of CSF through the infusion pump prevented the occurrence of intracranial hypotension and its associated symptoms such as nausea and vomiting due to excessive CSF drainage.
DISCUSSION
We proposed a management algorithm using pump-regulated volumetric continuous lumbar drainage for CSF leakage due to anterior decompression and fusion for OPLL. This algorithm demonstrated safety and efficacy in managing CSF leakage. In this study, the arachnoid membrane was exposed in 20 patients, and 14 of them had CSF leakage. The preoperative CT images of all 14 patients exhibited the double-layer sign. The patients were managed using the management algorithm proposed in this study, and successful resolution of CSF leakage was observed without any notable complications.
In the management of OPLL, indirect decompression surgeries, such as laminoplasty, laminectomy alone, and laminectomy with fusion, have been performed through a posterior approach without directly manipulating the OPLL [4-8]. Nonetheless, in some cases, an anterior approach is more advantageous than the posterior approach to treat OPLL. The anterior approach allows direct decompression through resection of the OPLL. Consequently, when the OPLL mass is sizeable (occupying ratio of OPLL ≥ 60%) or when kyphosis is present, indicated by a negative K-line, the anterior approach is more beneficial [9-15]. Furthermore, C5 palsy, a postoperative complication following cervical spine surgery, occurs more frequently in patients treated with the posterior approach than those treated with the anterior approach [27,28]. Nevertheless, the most important concern in using the anterior approach to manage OPLL is the occurrence of CSF leakage. Following anterior cervical surgery for OPLL, CSF leakage frequently occurs due to dural injury or absence of dura, with an incidence rate of 4.3%–32% [23,24,29-40]. CSF leakage could result in secondary complications, including meningitis, wound dehiscence, airway obstruction, cutaneous CSF fistula, and pseudomeningocele [31,38,41-43]. Consequently, effective management of CSF leakage is crucial in anterior surgery for OPLL. Several strategies have been proposed for managing CSF leakage, such as primary dural repair, application of a collagen matrix onlay graft, and insertion of lumbar drainage [38]. Although primary dural repair is a potential treatment option, the narrow surgical field associated with the anterior approach makes suturing of dura defects challenging. In addition, CSF leakage may persist despite collagen matrix onlay grafting. Lumbar drainage is a widely used method for managing CSF leakage due to dural injury during spinal surgery [44,45]. Previous studies have also reported its utility in managing CSF leakage subsequent to anterior surgery for OPLL [31,34,36]. Nevertheless, the use of a lumbar drain has limitations, such as ABR, which can cause patient discomfort and lead to complications, such as pulmonary embolism, deep vein thrombosis, or cardiac problems [26]. In addition, this gravity-dependent drainage system poses a risk of sudden large-volume CSF drainage [21]. Swanson et al. [22] first introduced flow-regulated continuous spinal drainage to manage CSF rhinorrhea due to skull base fracture following motor vehicle accident. Subsequently, Houle et al. [21] described the use of pump-regulated lumbar drainage to manage CSF leakage resulting from dura injury following lumbar decompression in 10 patients. Their findings demonstrated the safety and efficacy of pump-regulated lumbar drainage as a therapeutic option for CSF leakage. However, there are no published reports detailing the use of pump-regulated lumbar drainage as a treatment option for CSF leakage following anterior surgery in patients with OPLL. To the best of our knowledge, this is the first study to propose a management algorithm for CSF leakage through pump-regulated volumetric continuous lumbar drainage following anterior cervical decompression and fusion for OPLL.
We demonstrate the benefits of pump-regulated volumetric continuous lumbar drainage in terms of allowing ambulation and preventing complication associated with ABR. This is because patients were strongly advised to ambulate as CSF was being continuously drained irrespective of their posture or ambulation, owing to the use of an infusion pump. In addition, the gravity-dependent lumbar drainage of CSF is known to pose a risk of sudden overdrainage, particularly when the patient changes position (sitting or standing) or ambulates without clamping of the lumbar drain. Headache, vomiting, pneumocephalus, and temporary blindness have been reported as complications of CSF overdrainage [19,20]. In this study, no such complications were observed because volumetric continuous drainage of CSF via an infusion pump prevented overdrainage.
Moreover, volumetric pump-regulated CSF drainage facilitates upright positioning of patients, which may contribute to decreasing the CSF pressure at the cervical level. A decrease in the CSF pressure at the cervical level may enhance healing of the dural defects and promote resolution of CSF leakage. Carlson et al. [46] reported the results of animal experiments on the change in CSF pressure according to body position. They observed that cervical CSF pressure decreased significantly (p = 0.035) from an inclination of 0° to 90°; the CSF pressure was 10.0 ± 1.5 cmH2O at 0°, 9.5 ± 1.3 cmH2O at 30°, 7.5 ± 1.2 cmH2O at 45°, and 7.1 ± 1.8 cmH2O at 90°. This may be attributed to the hydrostatic pressure gradient between the cervical and lumbar levels due to gravity on upright positioning.
The reported incidence of CSF leakage following anterior surgery for OPLL ranges from 4.3%–32% [23,24,29-39]. In our study, 27.5% (14 out of 51) of the patients experienced CSF leakage following anterior cervical surgery for OPLL. The presence of the doublelayer sign was identified in all patients who experienced CSF leakage. Statistical analysis revealed that the double-layer sign was a significant contributing factor (p = 0.023) for the occurrence of CSF leakage in patients with OPLL undergoing anterior cervical surgery. Yang et al. [24] reported that all patients with the type C double-layer sign experienced CSF leakage, and the occurrence of CSF leakage was significantly higher in patients with the type C sign than in those with other types (p < 0.01). Consistent with this finding, the occurrence of CSF leakage was significantly higher (p = 0.013) in patients with the type C double-layer sign than in those with types A and B. Therefore, surgeons should be cautious regarding potential intraoperative CSF leakage, especially in patients with OPLL exhibiting a double-layer sign, particularly type C.
This study had several limitations. Volumetric continuous lumbar drainage using an infusion pump was performed for 9 patients, which may be a small sample size to evaluate the safety and efficacy of this intervention. Moreover, there was no control group who used the gravity-dependent lumbar drainage for CSF leakage management. It would be necessary to conduct the study comparing patients who underwent pump-regulated lumbar drainage with a larger sample size to those who underwent gravity-dependent lumbar drainage in the future. In addition, this was a retrospective study. Therefore, prospective studies are required to evaluate the effectiveness of the proposed management algorithm for CSF leakage following anterior surgery for OPLL. Additionally, patient-reported outcome measures were not assessed following surgery as the focus of this study was on the safety and efficacy of the proposed management algorithm for controlling CSF leakage rather than on the overall safety and efficacy of anterior cervical surgery for OPLL. Furthermore, the study did not assess the discomfort experienced by patients on insertion of lumbar drains. Although patients were encouraged to ambulate with lumbar drains in place, some patients may have experienced discomfort due to the lumbar drain itself.
CONCLUSION
The proposed management algorithm based on volumetric continuous lumbar drainage using an infusion pump showed safety and efficacy in managing CSF leakage following anterior decompression and fusion for OPLL. Twenty of 51 patients, who experienced CSF leakage following anterior cervical decompression and fusion, were managed with the proposed management algorithm. Of them, 9 were managed through volumetric continuous lumbar drainage using an infusion pump. Volumetric continuous lumbar drainage using an infusion pump had advantages in terms of preventing medical complications associated with ABR and CSF overdrainage.
CSF leakage is a key concern when determining a suitable surgical approach for the management of OPLL. Therefore, despite the benefits of anterior decompression and fusion in cases of a large OPLL mass or kyphosis, surgeons may choose the posterior approach owing to a high probability of CSF leakage based on the double-layer sign. However, the proposed management algorithm could alleviate surgeons’ concerns regarding CSF leakage and help them select an optimal surgical approach regardless of the possibility of CSF leakage.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: JHP; Formal Analysis: SWJ, SHL; Investigation: SWJ, SHL; Methodology: SWJ, SHL, JHP; Project Administration: JHP; Writing – Original Draft: SWJ, SHL; Writing – Review & Editing: SWJ, SHL, JHP, HKS, SRJ, SWR.
Acknowledgements
This material was presented as an oral abstract at the 15th Korean Cervical Research Society Regular Academic Conference held at the Sejong Convention Center on June 18, 2022.