Does H3K27M Mutation Impact Survival Outcome of High-Grade Spinal Cord Astrocytoma?
Article information
Abstract
Objective
To evaluate the impact of H3K27M mutation in the prognosis of histological high-grade intramedullary astrocytoma.
Methods
A total of 78 patients who were diagnosed with high-grade spinal cord astrocytoma were included. Clinical data consisting demographic, radiological, molecular features and treatment data were recorded. Univariate and multivariate Cox analysis were performed to investigate variables associated with survival outcome of histological high-grade spinal cord astrocytoma.
Results
Median survival time was 21 months. Overall survival (OS) at 1 and 3 years was 65.7% and 40.7%, respectively. Sex, location, and tumor span did not present significant association with OS. Patients with H3K27M mutation showed significant shorter duration of symptom than patients with H3K27 wild-type. As respect to adjuvant treatment, adjuvant radiotherapy and chemotherapy were associated with favorable OS (both p = 0.01). Younger patients (age ≤ 18 years) had shorter OS (p = 0.008) than adult patients (age > 18 years). Of note, H3K27M mutation did not show significant impact on the survival outcome, regardless of histology grade 3 or grade 4 (p = 0.3).
Conclusion
Histological high-grade spinal cord astrocytoma has dismal prognosis. Our study demonstrated that H3K27M mutation did not show significant impact on survival outcome of histological high-grade spinal cord astrocytoma.
INTRODUCTION
Primary spinal cord astrocytoma is a rare disease with dismal functional and survival prognosis. In particular, patients with high-grade astrocytoma presented adverse overall survival (OS) time [1,2]. Unlike its intracranial counterpart, high-grade intramedullary astrocytoma (grade, 3–4) lacks distinct boundaries with normal spinal cord tissue and does not have a unified treatment guideline [3-6]. Furthermore, due to its rarity, prognostic factors associated with high-grade intramedullary astrocytoma and the role of H3K27M mutation in high-grade high-grade intramedullary astrocytoma have not been established [7-11]. In this study, we aimed to evaluate the prognostic factors associated with high-grade intramedullary astrocytoma and, especially, the impact of H3K27M mutation on survival outcome of histological high-grade intramedullary astrocytoma in a large sample-size cohort.
MATERIALS AND METHODS
1. Patient Population
After reviewing medical records systems, a total of 66 patients with primary spinal cord astrocytoma between 2016 and 2021 at Beijing Sanbo Brain Hospital were retrieved. Among them, 13 cases and 5 cases were histologically classified as grade 1and grade 2 spinal cord astrocytoma, respectively and were excluded. The remainning 48 patients who underwent surgery and were histologically diagnosed with high-grade spinal cord astrocytoma and included in our analysis. Clinical data, including age, sex, histological type, tumor location, preoperative modified McCormick Scale (MMS) [3] (Supplementary Fig. 1), postoperative modified MMS, last follow-up modified MMS, extent of resection, and radiotherapy, are recorded. OS was calculated as the time from date of diagnosis to date of death or last followup time, while duration of symptoms was defined as the time from date of related-symptom onset to date of diagnosis.
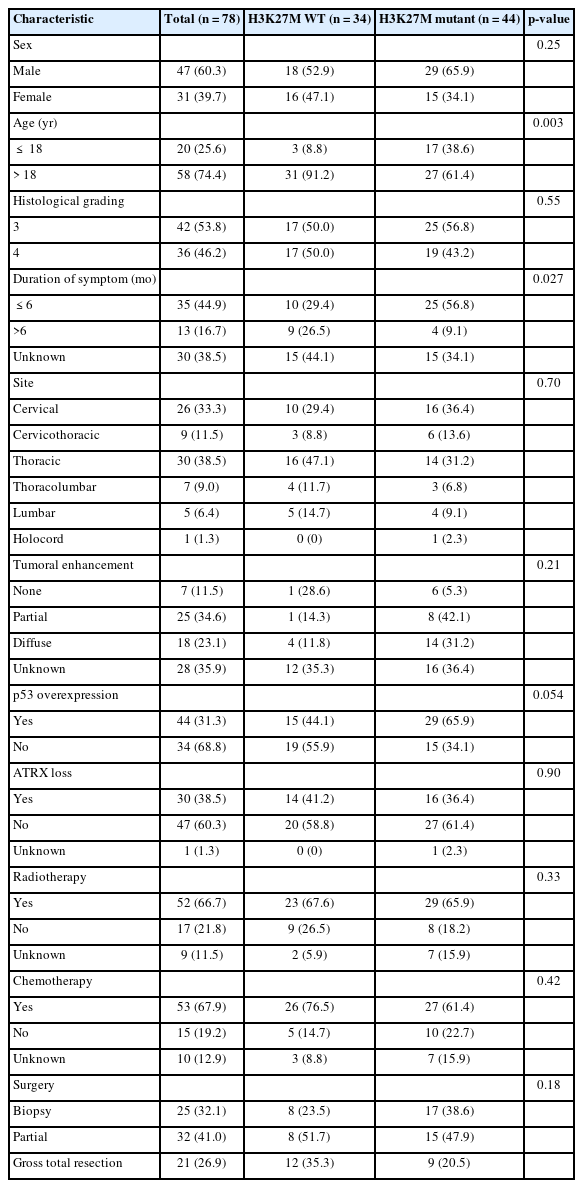
Considering sample-size limitations, data from the literatures were also retrieved and information, including age, sex, the presence of H3K27M mutation, ATRX loss, high p53 expression, and Ki67 expression, were extracted (Table 1) [12-14]. Age groups were divided into pediatric (≤ 18 years) and adult group (> 18 years) [13,15,16].
Our study is a investigator initiated trial, and the protocol of our study was approved by the Institutional Review Board of Capital Medical University (SBNK-YJ-2020-006-03). The informed written consent of the patient has been obtained.
2. Histological Morphology and Molecular Pathology
Firstly, histological morphology and grading were determined by 2 neuropathologists according to the 2021 World Health Organization (WHO) Classification [17-19]. Tumors with anaplasia and mitotic activity would be defined as histological grade 3, while diffuse astrocytoma with microvascular proliferation and/or necrosis was designated as glioblastoma, histological grade 4. Secondly, status of H3K27M was determined using immunohistochemistry (IHC) method (positive H3K27M protein and loss of histone tri-methylation). Tumors with H3K27M mutation would be designated as diffuse midline glioma, WHO grade 4. Besides, ATRX loss, IDH1-R132H mutation, and p53 overexpression Ki-67 were also detected using IHC method. MGMT promotor methylation were detected by the Streptomyces antibiotic protein- peroxidase ligation method [20].
3. Treatment
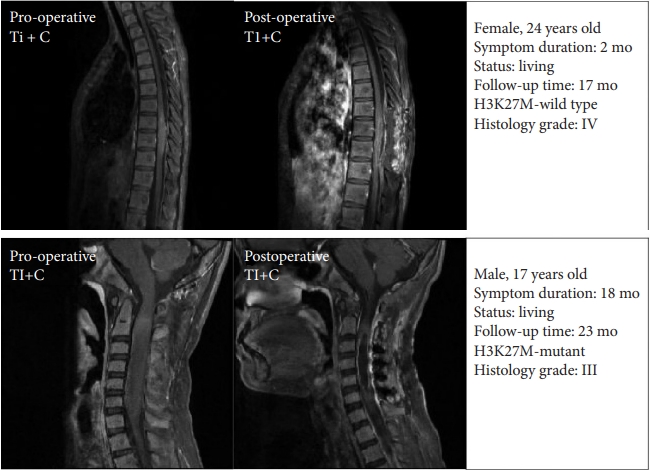
All patients received surgery under intraoperative neuroelectrophysiological monitoring. The extent of resection was divided into gross total resection (GTR) (≥ 90%), subtotal resection (SR) (≥ 50% and < 90%), partial resection (PR) (< 50%) and biopsy. Extent of resection was determined through operative magnetic resonance imaging (MRI) image (contrast-enhanced T1 weighted image) or determined by reviewing operation note and operative video. Illustrative cases were presented in Fig. 1. Postoperative adjuvant therapy was prescribed according to STUPP protocol [21]. In detail, patients were gaven radiotherapy (1.8 Gy per daily fraction (Monday to Friday) over 6 weeks, total 45–50 Gy) plus concomitant temozolomide (75 mg/m2, 7 days per week) and adjuvant temozolomide (6 cycles, 150 mg/m2 for 5 days, 28 days cycle).
4. Statistical Analysis
The statistical analysis was performed using R ver. 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were presented as mean± standard deviations or median and interquartile ranges, while categorical variables were expressed as frequencies and proportions. Pearson chi-square test or Fisher exact test was used to compare categorical variables. Kaplan-Meier curves was constructed for survival by stratified variables and compared using log-rank test. Unvariable and multivariable Cox proportional hazards regression analysis was used to determine risk factors associated with survival outcome. A p-value of < 0.05 was considered significant.
RESULTS
1. Clinical Data
A total of 78 patients who were histologically diagnosed as high-grade (grades 3–4) primary spinal cord astrocytoma based on tissue morphology were included. Out of these, 44 patients had H3K27M mutation and consequently, integrated diagnosis of the 44 patients was defined as diffuse midline glioma, H3K27M, WHO grade 4. Mean age of patients with and without H3K27M mutation was 29.4 and 42.2 years, respectively, and H3K27 wild-type (WT) frequently occurred in adult patients (p = 0.003). Forty-seven patients were male. The mean duration of symptom was 6.7 months, patients with H3K27M mutation exhibited shorter symptom duration than patients with H3K27 WT astrocytoma (p = 0.027) (Table 2).
2. Tumor Characteristics
For the location, the tumors were most frequently located in the thoracic cord (38.5%), followed by the cervical (33.3%), cervicothoracic (11.5%), and thoracolumbar (9.0%). The average length of tumor span was 4 segments. On contrast-enhanced MRI, partial enhancement was observed in 25 patients (34.6%), diffuse enhancement in 18 (23.1%), and no enhancement in 7 (11.5%). Image data for the remaining 28 patients were not available.
In terms of molecular features, none of the patients had IDH1-R132H mutation, while p53 overexpression occurred in 44 patients and ATRX loss occurred in 47 cases.
3. Treatment Strategy
Twenty-one patients underwent GTR (≥ 90%), while biopsy and PR (< 50%) were performed in 25 and 32 patients, respectively. A total of 57 patients received postoperative adjuvant treatment, out of which 48 patients received both chemotherapy and radiotherapy, while 4 patients received radiation therapy only and 5 patients received chemotherapy only, 10 patients did not receive adjuvant treatment and the postoperative treatment modality of the remaining 11 patients were unknown.
4. Survival Analysis
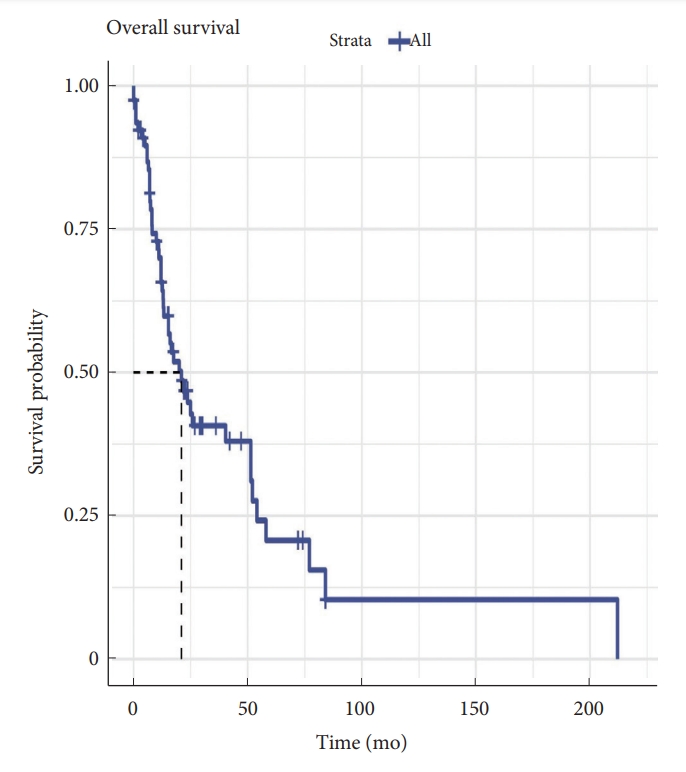
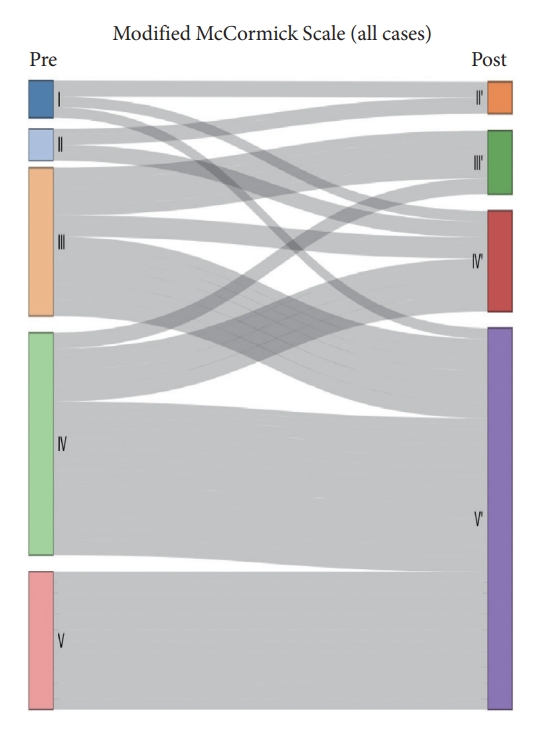
Median survival time was 21 months. OS at 1 and 3 years was 65.7% and 40.7%, respectively (Fig. 2). The median OS time of patients with and without H3K27M mutation was 15.3 months and 52.1 months respectively. We performed Kaplan-Meier curves and univariate and multivariate Cox analysis of clinicopathological features in 78 cases (Table 3). Kaplan-Meier curves showed that sex, location and tumoral enhancement did not present significant association with OS (Fig. 3A, D, E). Patients with H3K27M mutation showed significant shorter duration of symptom than patients with H3K27 WT (Fig. 3C). As respect to adjuvant treatment, adjuvant radiotherapy and chemotherapy was associated with favorable OS (both p = 0.01) (Fig. 4A, B). Younger patients (age≤18 years) had shorter OS (p = 0.008) than adult patients (age>18 years) (Fig. 3B). Of note, patients with H3K27M mutation did not show worse survival outcome than patients with H3K27 WT (p = 0.3), regardless of histology grade 3 or grade 4 (p = 0.9, p = 0.2) (Fig. 5A–C). In terms of ATRX loss, our analysis did not reveal ATRX loss had a significant impact on survival outcome (p = 0.07) (Fig. 5D). Conversely, patients harboring p53 overexpression demonstrated a favorable OS compared to those normal p53 expression (p = 0.009) (Fig. 5E). In the context of neurological function, data were collected from 48 patients who underwent surgical treatment at Sanbo Brain Hospital. Among these, 6 patients underwent biopsy, 23 underwent PR, and 19 underwent gross total resection. Notably, the majority of patients (19 cases, 24.3%) exhibited MMS IV at the time of presentation. In contrast, 12 cases (15.3%) displayed modified MMS III, and 11 cases (14.1%) were classified as MMS V. A smaller subset of patients (3 cases, 3.8%) presented with mild neurological deficits (MMS I, MMS II), respectively. The median preoperative modified MMS score was 4 (interquartile range [IQR], 3–4), while the postoperative MMS score was 5 (IQR, 4–5) (Fig. 6). Overall, no significant difference was observed between pre and postoperative MMS scores among different surgery groups (p = 0.638). In biopsy group, 4 cases exhibited postoperative worsening in their neurological status, 1 case showed improvement, and 1 case displayed no change. In PR group, 12 cases experienced postoperative neurological deterioration, while 11 cases showed no change. In gross total resection group, 10 cases experienced postoperative functional decline, while 9 cases displayed no change. Furthermore, multivariable Cox analysis showed age>18 (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.14–0.76; p = 0.009), PR (HR, 0.28; 95% CI, 0.09–0.82; p = 0.02), and biopsy (HR, 0.16; 95% CI, 0.04–0.6; p = 0.007) were associated with favorable survival of high-grade spinal cord astrocytoma.
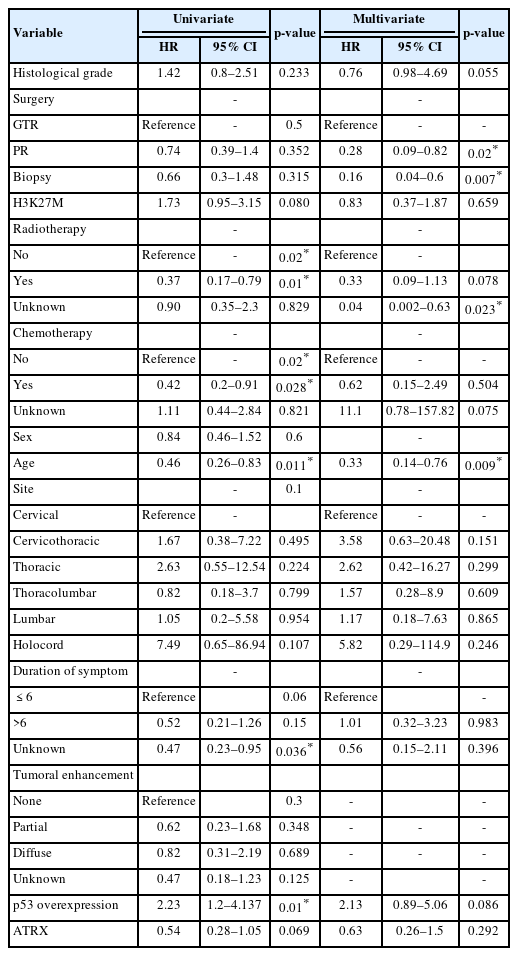
Univariate and multivariate Cox analysis of clinicopathological features in spinal cord astrocytomas
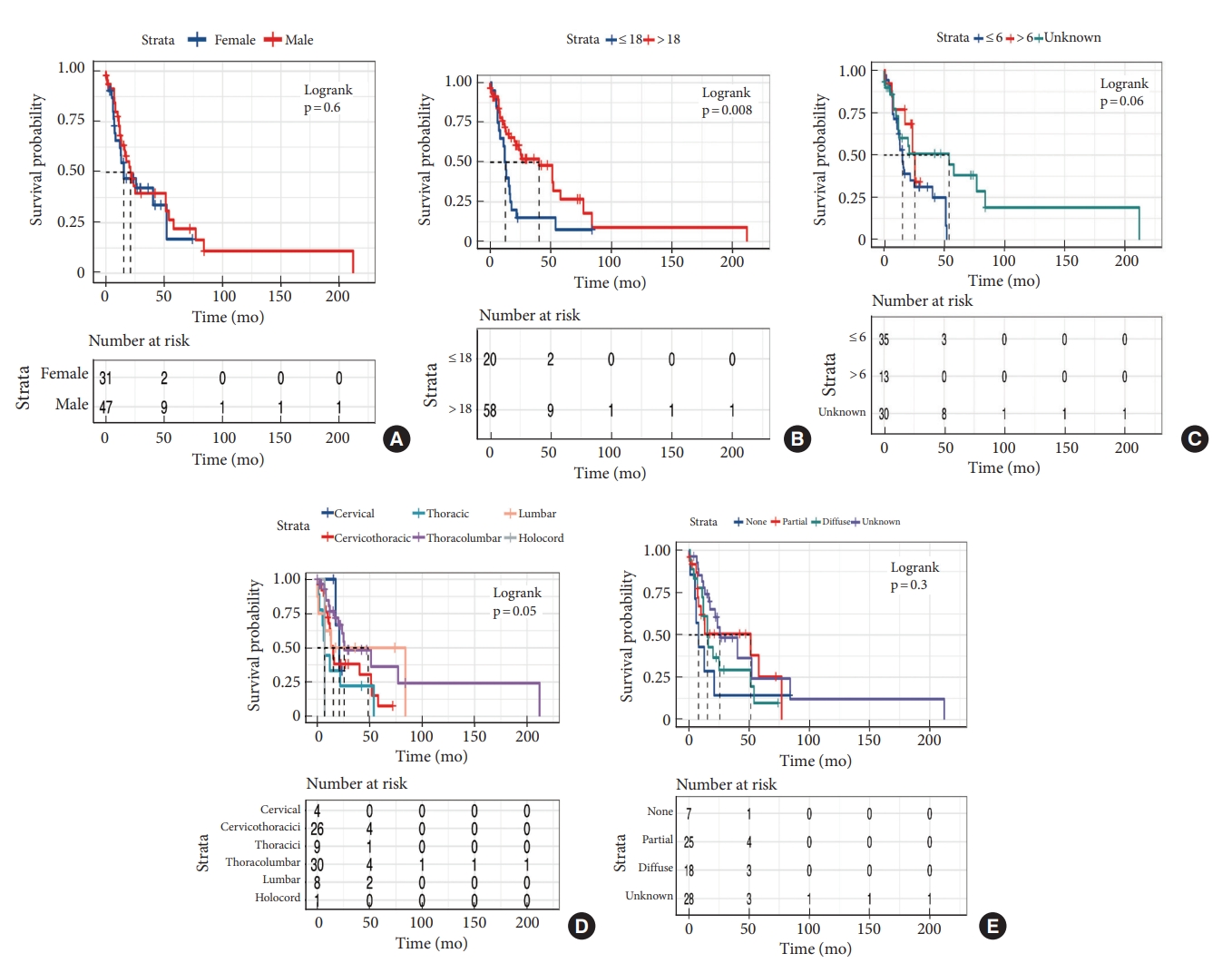
Kaplan-Meier survival curves of clinical features. (A) Sex. (B) Age. (C) Duration of symptom. (D) Tuumor location. (E) Tumoral enhancement.
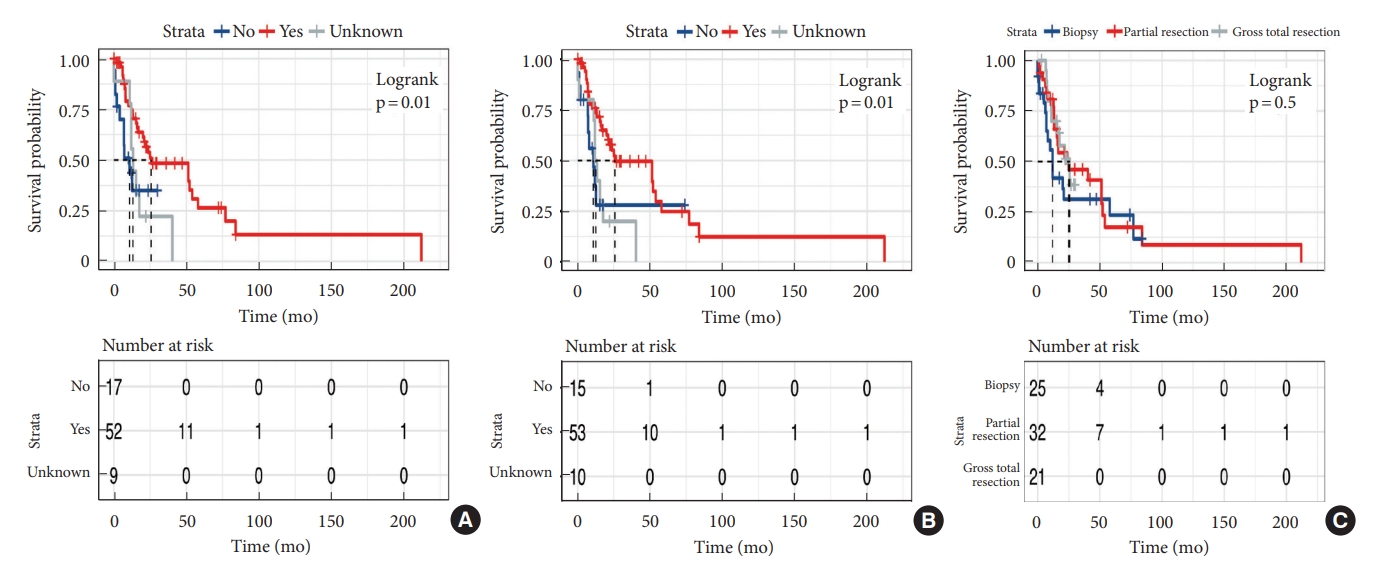
The effect of treatment on survival outcome for patients with high-grade. (A) Radiotherapy. (B) Chemotherapy. (C) Surgery.
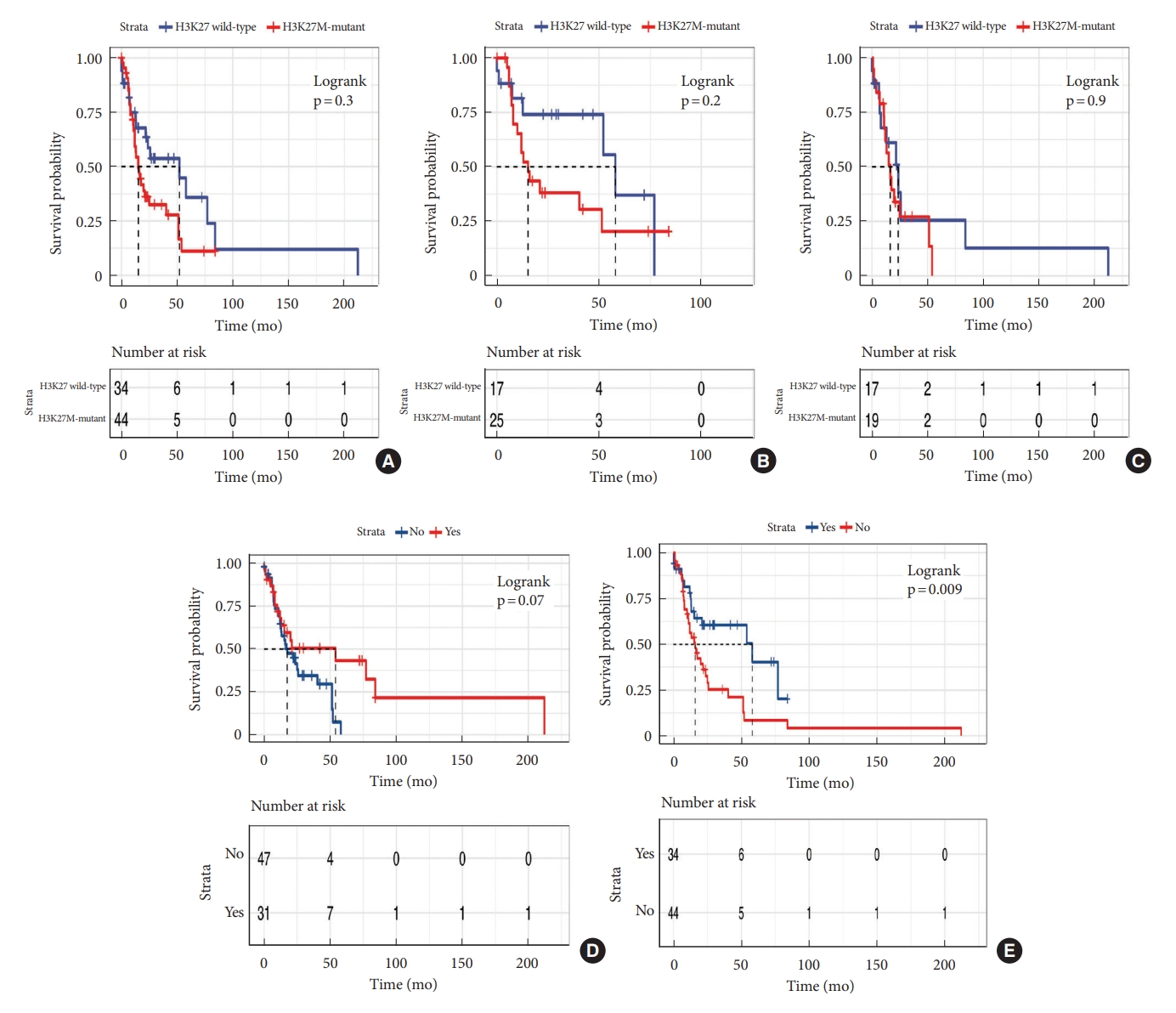
Kaplan-Meier survival curves of molecular prognosis biomarker. (A) H3K27M mutation in high-grade spinal cord glioma. (B) H3K27M mutation in histological 3. (C) H3K27M mutation in histological 4. (D) ATRX. (E) p53.
DISCUSSION
Primary spinal cord glioma represents 2% to 4% of all primary gliomas of the central nervous system, among which astrocytoma accounts for 2.4%–3% [1,22-24]. H3K27M mutation was confirmed to be prognostic and diagnostic molecular biomarker of diffuse midline glioma, H3K27M-altered [13,20,24,25]. Based on previous reports, the presence of H3K27M mutation in grade 1 spinal cord astrocytoma has been seldom documented. The prevalence of the K27M mutation in grades 2, 3, and 4 varies across different studies. Some investigations indicate no significant susceptibility in the occurrence rate of the H3K27M mutation among grades 2, 3, and 4 tumors. For instance, Wang et al. [26] reported mutation rates of 36.1%, 39.3%, and 24.6% in histological grades 2, 3, and 4, respectively. Chai et al. [27] noted a mutation rate of 40% in both grades 2 and 3 tumors, contrasting with 20% in grade 4. Cheng et al. [28] documented mutation rates of 32.1% in grades 2 and 4, and 35.7% in grade 3, other studies have revealed a frequent occurrence of the H3K27M mutation in high-grade spinal cord astrocytoma [29]. Consistently, in our cohort, 40% (2 of 5), 59.5% (25 of 42), and 52.7% (19 of 36) in grade 2, 3, and 4 primary spinal cord astrocytomas harbored H3K27M mutation, respectively. Diffuse astrocytoma with H3K27M mutation was reported to have dismal survival outcome (median OS time, 20.7 months) [27]. Of note, regardless of molecular biomarker, histological grading was always an essential prognostic factors and, similar to diffuse astrocytoma with H3K27M mutation, histological high-grade astrocytoma also showed poor prognosis [9,22,30,31]. The impact of H3K27M mutation on histological high-grade spinal cord astrocytoma is unclear, does H3K27M mutation worsen poor prognosis of histological high-grade spinal cord astrocytoma? The study by Yi et al. [7] revealed that patients with H3K27M mutant histological grade 4 astrocytoma had longer OS than patients with H3K27 WT histological grade 4 astrocytoma. However, Chai et al. [27] and Cheng et al. [28] found that there was no significant difference in prognosis between H3-WT and H3K27M-mutant histological grades 3–4 gliomas. Consistently, in our study, H3K27M mutation did not show association with survival outcome of histological high-grade spinal cord astrocytoma, regardless of histological grade 3 and grade 4 tumors. This finding likely suggested that histological grading was still the most important prognostic factor of primary spinal cord astrocytoma. However, small sample size of our study might limit the power to find tiny prognostic difference potentially existed between histological high-grade astrocytoma with and without H3K27M mutation. A large sample-sized study is warranted to investigate the role of H3K27M mutation in histological high-grade intramedullary astrocytoma.
In term of treatment, the study by Wang et al. demonstrated that extent of resection and adjuvant treatment were not prognostic factors of OS in patients with primary spinal cord glioma [8,25,32]. In a large cohort study, Chalif et al. [30] found that GTR and adjuvant therapy independently improve survival for highgrade spinal cord glioma. Our study which just focused on histological high-grade spinal cord glioma showed that GTR did not provide additional survival benefit, while patients could benefit from adjuvant radiotherapy or chemotherapy. GTR without function compromise for primary spinal cord glioma is a great challenge due to no definite margin existing between normal spinal cord and tumor. Consequently, adjuvant treatment was routinely prescribed to patients with high-grade spinal cord glioma in our center. Limitations of this study include the relatively small sample size, owing to the rarity of this disease. As a result, a detailed multivariate Cox regression analysis was no performed. Limited by the unavailability of sufficient samples for comprehensive analysis, only the fundamental molecular characteristics of the tumors were examined. Additionally, selection bias in treatment modality, which is one nature of retrospective study, might cofound the final conclusion, hence, large sample-size prospective study is warranted to investigate the role of adjuvant treatment in primary spinal cord glioma.
CONCLUSION
Histological high-grade spinal cord astrocytoma has dismal prognosis and H3K27M mutation did not show significant impact on survival outcome of histological high-grade spinal cord astrocytoma.
Supplementary Material
Supplementary Material: Supplementary Fig. 1 can be found via https://doi.org/10.14245/ns.2346650.325.
Modified McCormick scale.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: LC, KW, DZ, TF; Data curation: FZ, YW; Formal analysis: DZ, XZ; Funding acquisition: CL, TF; Methodology: FZ, LC, SW, ZZ, KW, DZ, TF; Project administration: FZ, DZ, TF; Visualization: FZ, CL; Writing - original draft: FZ, LC, ZD, SW, ZZ, KW, YW; Writing - review & editing: XZ, CL, DZ, TF.
Acknowledgements
We are particularly grateful to Jian Qiang and Cheng Jiahui for technical support.