A Modified Laminotomy for Interlaminar Endoscopic Lumbar Discectomy: Technical Report and Preliminary Results
Article information
Abstract
Objective
To introduce a technique of laminotomy using a common trephine to enlarge the interlaminar space at L4/5 segment for interlaminar endoscopic lumbar discectomy (IELD) and report the anatomical basis of this procedure, technical details, as well as primary clinical outcomes of a consecutive patient cohort with L4/5 lumbar disc herniation (LDH).
Methods
On anteroposterior fluoroscopy, the intersection of the medial edge of the inferior articular process and the inferior endplate of L4 vertebra was taken as the target. Using a common trephine, laminotomy was performed to remove a big portion of the posterior wall of the canal under the guidance of endoscopy. From June 2018 to December 2021, the consecutive patients who underwent L4/5 IELD were prospectively studied. Clinical outcomes were assessed at the day before surgery, 1 day, 1 month, 3 months, 12 months after surgery, and the last follow-up. Numerical Rating Scale, Roland-Morris Disability Questionnaire (RMDQ), and MacNab criteria were used to evaluate back and leg pain, the quality of life, and clinical efficacy, respectively.
Results
There were 64 men and 44 women, with an age of 50.3 ± 14.9 years. The operating time was 74.54 ± 17.42 minutes. The mean follow-up time was 32.7 ± 18.6 months (range, 12–64 months). The complications of IELD included numbness, neck pain, and recurrence. Both leg pain (6.2 ± 1.9 vs. 1.8 ± 0.8, p < 0.001) and back pain (3.1 ± 2.3 vs. 1.7 ± 0.9, p < 0.001) quickly improved after this procedure and maintained (1.1 ± 1.5, 1.1 ± 1.3) at final follow-up. Physical disability due to back pain, as assessed using RMDQ, was improved remarkably after surgery (15.0 ± 5.8 vs. 2.9 ± 4.1, p < 0.001). In addition, MacNab outcome grade was evaluated as good-to-excellent in 96 cases (88.9%).
Conclusion
A convenient technique of laminotomy using a common trephine was proposed for the L4/5 IELD. It can efficiently enlarge the interlaminar entry to perform endoscopic discectomy. This procedure is particularly suitable for treating LDH with concomitant lumbar spinal stenosis and migrated herniated disc.
INTRODUCTION
Lumbar disc herniation (LDH) is a common degenerative lumbar disorder which may lead to back pain, radiculopathy, or both. Although most symptomatic LDH patients can be treated with conservative therapies successfully, there are a considerable percentage of patients who failed and had to undergo surgical treatment such as percutaneous endoscopic lumbar discectomy (PELD). As a minimally invasive procedure, PELD is as efficacious as the traditional open discectomy [1,2], yet has a number of advantages over the open procedure, including less paravertebral muscle injury, shorter hospital stay, and quicker function recovery [3-5].
PELD can be performed via transforaminal or interlaminar entry. While most PELD used transforaminal approach, this technique requires accurate puncture to the Kambin triangle and typically needs multiple fluoroscopies. Moreover, it is difficult to perform transforaminal endoscopy for the L5/S1 segment, particularly when there is high iliac crest, large facet joint, or narrowed lumbosacral foramen [6-8]. In addition, it is sometimes challenging to address the lateral recess compression resulting from facet joint hypertrophy and migrated disc tissues, especially when the herniated disc tissues caudally migrated to the pedicle level or below [9].
First reported in 2006, interlaminar endoscopic lumbar discectomy (IELD) is mainly used for the L5/S1 segment where the interlaminar space is relatively large [10,11]. Spine surgeons are familiar with the surgical route and usually one or 2 fluoroscopies is adequate to locate the interlaminar entry [12,13]. Unlike transforaminal endoscopic lumbar discectomy (TELD), it is easy to deal with concomitant canal pathologies via the interlaminar approach, such as lateral recess narrowing and ligament flavum hypertrophy. For the L4/5 segment, however, IELD is barely used, since the interlaminar space is usually small and narrow. A surgical technique which can quickly enlarge the L4/5 interlaminar space thus may extend clinical application of interlaminar endoscopy.
In this study, we introduced a novel surgical technique of laminotomy to efficiently enlarge the interlaminar space using a common trephine. The anatomical basis of this procedure, technical details, as well as primary clinical outcomes of a consecutive LDH cohort, are reported.
MATERIALS AND METHODS
1. Anatomy of a Relative Safety Zone in the Canal
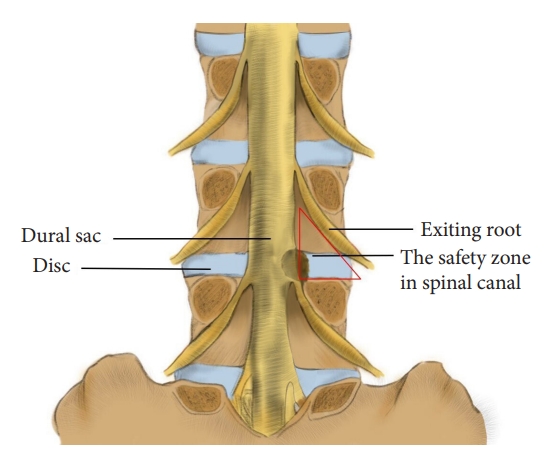
This technique is designed for the L4/5 segment and involves a quick laminotomy using a trephine. The modified laminotomy is based on a relative safe zone in the lumbar spine canal. While the Kambin triangle is defined on the lateral side of the lumbar spine, this safe zone virtually is a posterior view of the Kambin triangle. It is surrounded by the exiting root (lateral edge), dural sac (medial edge), and pedicle (caudal edge), with the disc as the bottom (Fig. 1) and the inferior articular process of the superior vertebra and underlying ligamentum flavum as the posterior wall. The aim of laminotomy is to efficiently remove the posterior wall and “open” this safe zone to expose the travelling root. Through this window, the surgeon can remove hypertrophied ligament flavum and perform discectomy.
2. The Target Point of Percutaneous Puncture
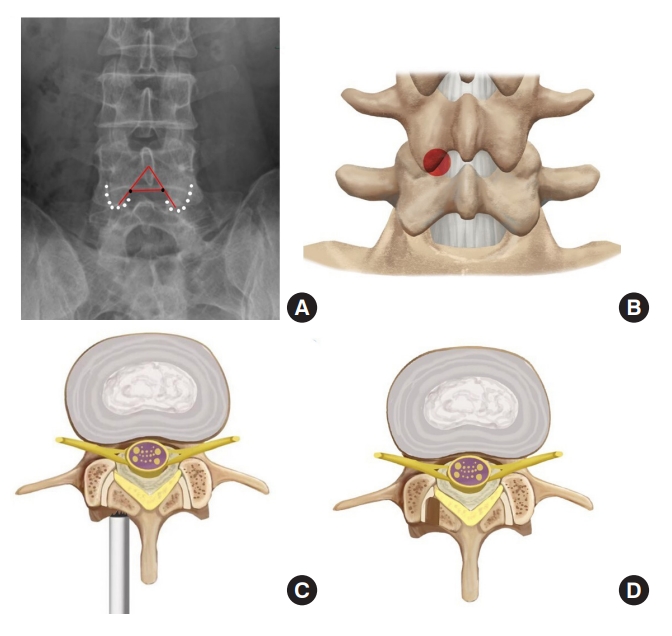
On the anteroposterior radiograph, the intersection of the medial edge of the inferior articular process and the inferior endplate of L4 vertebra is taken as a puncture target (Fig. 2A), which is at the junctional zone of the inferior articular process and lamina (Fig. 2B). Taking this point as the center of a round laminotomy can safely remove a big portion of the posterior wall of the safe zone (Fig. 2C, D) and thus, enlarge the interlaminar entry.
3. Surgical Procedures
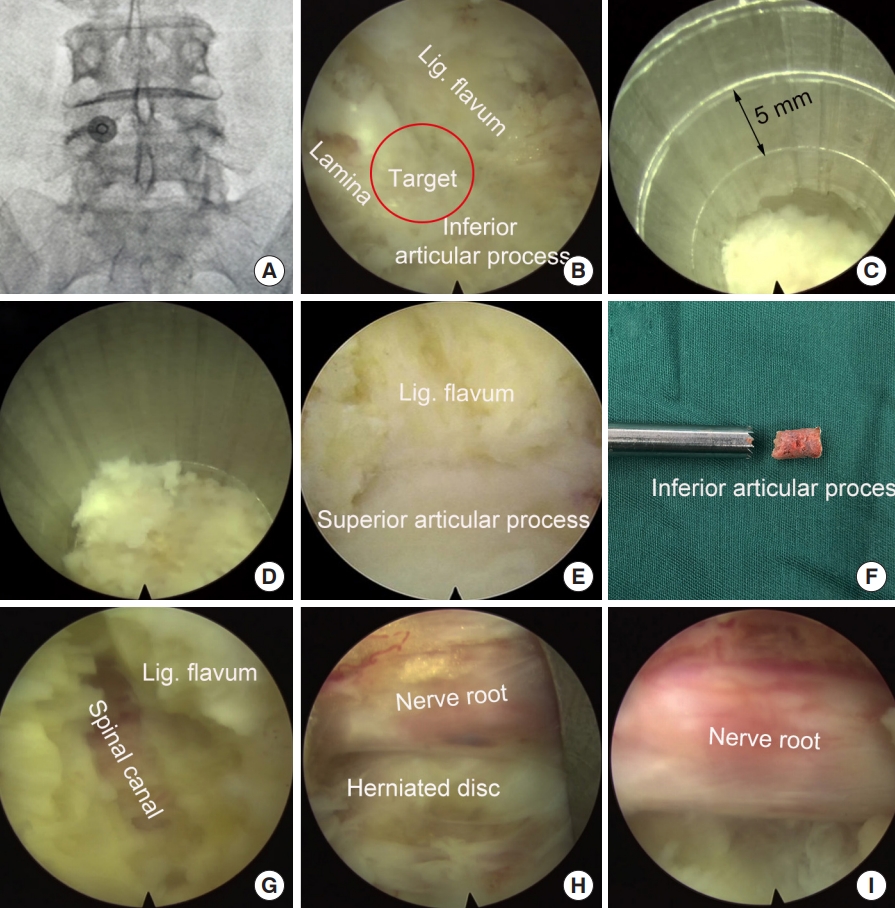
Under general anesthesia, the patient was placed at a prone position. A Spinedos endoscopic system (Spinedos GmbH, Munich, Germany) was used to perform PELD. The diameter of the high-resolution endoscope is 7.0 mm, with a 4.3-mm intraendoscopic working channel. The working sheath has a beveled opening, with a diameter of 8.5 mm and a vision of 30° angle. The trephine used for laminotomy was in a diameter of 7.5 mm and thus, can be put between the endoscope and working sheath to perform laminotomy under endoscopic supervision (Fig. 3).

Diagram of the tools used for endoscopic laminotomy. (A) Endoscope, trephine and working sheath. (B) The trephine was between the endoscope and working sheath.
1) Laminotomy using a trephine under the guidance of endoscopy
Intraoperative x-ray was taken to locate the surgical segment and the puncture target point (Fig. 4A). A longitudinal incision (10 mm) was made and the endoscopy system was introduced through a working channel. Important anatomic landmarks, including the lamina, inferior articular process, and the ligament flavum in the interlaminar entry were exposed using a high-voltage bipolar probe (Ellman Innovations, New York, NY, USA) (Fig. 4B). The junction of the lamina and the inferior articular process is the target of laminotomy.
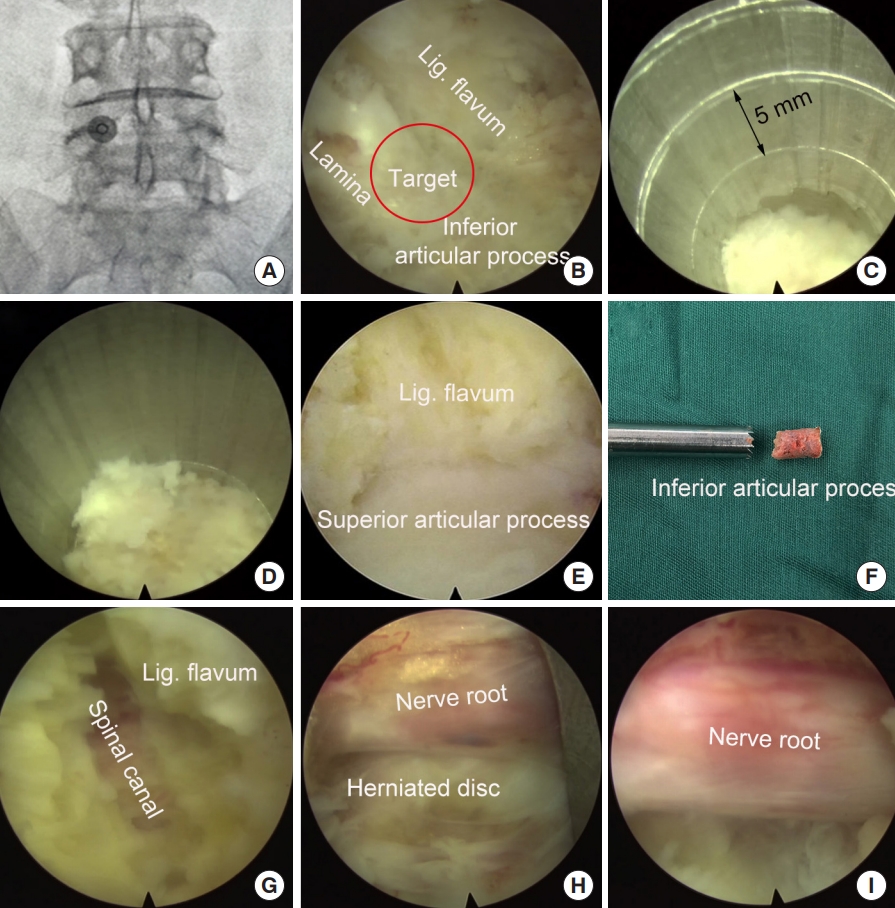
Using a trephine, laminotomy was performed to efficiently establish a large entry for interlaminar endoscopic lumbar discectomy for the L4/5 segment. (A) Locating the puncture target point. (B) Expose the area of laminotomy. (C) A trephine was anchored on the bony structure to perform laminotomy. (D) The trephine went down for approximately 15 mm. (E) Further expose the ligamentum (Lig.) flavum and superior articular process. (F) Part of the lamina and inferior articular process was removed. (G, H) Resect ligamentum flavum and superior articular process. (I) Exposed nerve root after decompression.
The trephine was anchored to the bony structure of the target, and an oblique ending of working channel was placed on the medial part of the laminar to protect the canal. Fluoroscopy can be used, if necessary, to confirm the appropriate positioning of the trephine. Laminotomy was performed by gently rotating the trephine back and forth under the guidance of endoscopy (Fig. 4C). When the trephine went down for 10–15 mm, the surgeon can obtain a sudden emptiness and the bony structure turned along with the trephine, which indicated the bony structure was completely saw off (Fig. 4D). At this time, the trephine typically reached the articular surface of the facet joint and the spinal canal was protected by ligament flavum (Fig. 4E). The trephine was then pull out, and often part of the bony lamina and inferior articular process has been cut off (Fig. 4F). Sometime the laminotomy bone remained in situ, one can pull it out using a schlesinger forceps. In addition, the channel can be further enlarged using endoscopic chisel, rongeur, or drill, as appropriate. After laminotomy, the original interlaminar entry and laminotomy window usually are connected as one, and is large enough to introduce the endoscope for further decompression and discectomy.
2) Decompress the spinal canal
The junction of the ligamentum flavum and upper articular process, which may be the main structures leading to neurological compression, was used as a second landmark. The ligamentum flavum was removed using a scissors to expose the transversing nerve root and dura sac (Fig. 4G, H). The hypertrophic superior articular process was then partially removed using a rongeur or endoscopic drill to perform lateral decompression.
3) Discectomy
After posterior and lateral decompression, the nerve root was retracted medially and routine interlaminar endoscopic discectomy was performed to remove the herniated disc tissues (Fig. 4I).
The overall operation procedure is presented as a Supplementary videoclip 1.
4. Subjects
During June 2018 to December 2021, the consecutive patients with LDH and were treated with L4/5 laminotomy and IELD were prospectively studied. The present study was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University (No. 20181103), and written consent was obtained for each patient.
Inclusion criteria were: (1) complaints of neurogenic claudication or radiculopathy, and conservatively treated for 6 weeks or more but failed; (2) LDH (with or without concomitant spinal stenosis) on computed tomography (CT) or magnetic resonance (MR) images explains clinical symptoms and findings. Exclusion criteria were: (1) cauda equina syndrome; (2) severe central spinal canal stenosis; (3) segmental instability in dynamic radiographs; (4) a history of lumbar spine surgery; (5) concurrent disease of other spinal pathologies that may lead to back pain, including spondylolisthesis, spinal infection, spinal tumor, spinal deformity.
The lumbar spinal stenosis was evaluated using Lee’s classification [14]. Patients with grade 1 and 2 spinal stenosis were included, and those with grade 3 were recommended for unilateral biportal endoscopy or traditional open laminectomy.
5. Clinical Measurements
Each patient was assessed at the day before surgery, and 1 day, 1 month, 3 months, 12 months after surgery, and the last follow-up. Numerical Rating Scale (NRS) and Roland-Morris Disability Questionnaire (RMDQ) were used to evaluate back pain, leg pain and quality of life [15]. Clinical efficacy was rated using the MacNab criteria [16]. The preliminary evaluation was conducted by a research nurse, and follow-ups were performed through telephone interview. Concomitant diseases, average surgery time, and related surgical complications were also evaluated.
6. Statistical Analysis
Data are presented as mean ± standard deviation. T-tests were used to examine the changes in back and leg pain after surgery. Chi-square tests were used to compare the incidence rates of various findings. Statistical analyses were performed using STATA 12 (StataCorp LLC, College Station, TX, USA).
RESULTS
1. Study Subjects
A total of 108 patients who underwent L4/5 endoscopic discectomy via the interlaminar approach were studied. There are 64 men and 44 women, with an age of 50.3 ± 14.9 years (range, 19–81 years old) (Table 1). Among them, 65 patients were diagnosed as LDH with concomitant radiological lumbar spinal stenosis, 41 were LDH with a migrated disc, and the remaining 2 were with facet joint cyst. Endoscopy surgery was successful for all patients in this cohort and none was converted to open surgery. In this study, additional decompression procedure of spinal canal was performed for 65 patients (60.2%) who had concomitant grade 1 and 2 spinal stenosis (Lee’s classification). The operating time was 74.54 ± 17.42 minutes (range, 60–180 minutes). The patients were followed up for at least 12 months and the mean follow-up time was 32.7 ± 18.6 months (range, 12–64 months). Demographics and baseline characteristics of subjects are presented in Table 1.
2. Clinical Outcomes
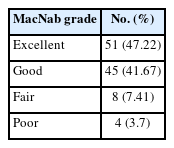
The patients’ leg pain significantly improved after the endoscopic surgery, and the NRS for leg pain decreased from 6.2 ± 1.9 to 1.1 ± 1.5 at the last follow-up (Fig. 5). Back pain also significantly improved at the final follow-up (3.1 ± 2.3 vs. 1.1 ± 1.3, Fig. 5). Physical disability due to back pain, as assessed using RMDQ, was remarkably improved after surgery (Fig. 6). In addition, MacNab outcome grade was rated as good-to-excellent in 96 cases (88.9%, Table 2).
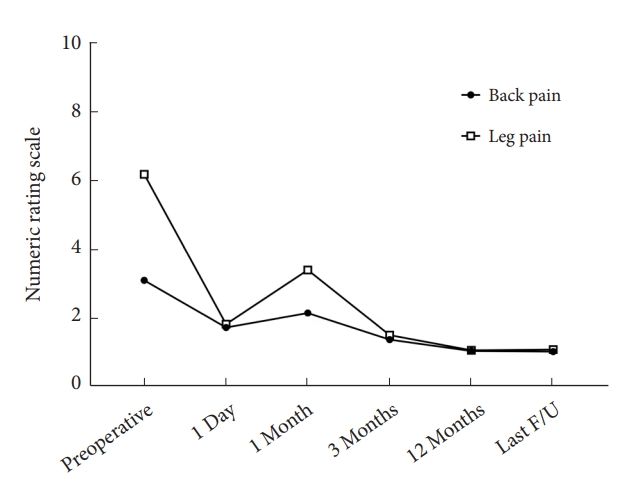
Changes of Numeric Rating Scales (NRS) of back pain and leg pain after interlaminar endoscopic lumbar discectomy. F/U, follow-up.
Changes of the Roland-Morris Disability Questionnaire scores after interlaminar endoscopic lumbar discectomy procedure. F/U, follow-up.
3. Representative Cases
1) Case 1
A 57-year-old man complained of claudication for 3 months. He had right leg pain and numbness after walking for 1,000 m. MR images demonstrated L4/5 disc herniation, concomitant lumbar spinal stenosis, and nerve root redundancy sign (Fig. 7A–C). He was treated with IELD. In endoscopy, the nerve root was found to be compressed by the upper articular process and hypertrophic ligamentum flavum. The nerve root was decompressed by laminotomy using a trephine and then the herniated disc was exposed and removed. Postoperative MR images showed a complete decompression of the nerve root and disappearance of the redundancy sign (Fig. 7D–F). On CT images, approximate one third of the lower articular process was removed and most of the articular surface was preserved (Fig. 7G–I). He recovered well 1 month after the surgery, without sign of claudication. He did not have back and leg pain at 2-year follow-up.
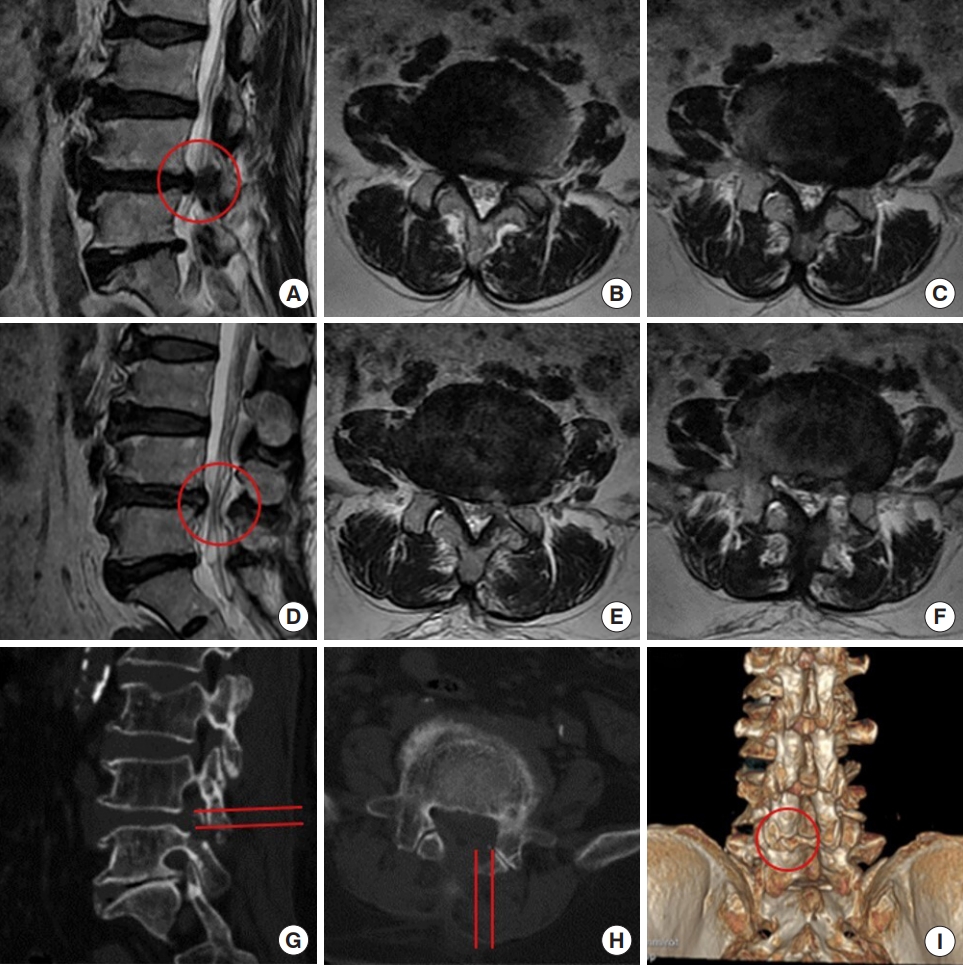
Decompression of the L4/5 spinal canal using interlaminar endoscopic lumbar discectomy with the aid of a trephine. (A–C) Preoperative magnetic resonance (MR) images. (D–F) Postoperative MR images. (G–I) Postoperative computed tomography images. There is a slight difference of in the levels of panels A and D. The red circle indicates the canal before (A) and after (D) the operation. (G, H) The red lines indicate the area received laminotomy. (I) The red circle indicates the bony defect after the laminotomy.
2) Case 2
A 62-year-old woman complained of left leg pain in walking or standing for 3 months. The pain occurred when she kept walking or standing for 10 minutes, but disappeared as long as she laid down. On MR images, there was a herniated disc at the L4/5 segment, with a concomitant facet joint cyst (Fig. 8A, C). In endoscopy, the joint cyst, which was in yellow, was found to compress the nerve root (Fig. 8E). When the cyst was removed, the nerve root was well decompressed (Fig. 8F). There was no specific finding on postoperative MR images (Fig. 8B, D) and she did not have leg pain any more in walking or standing.
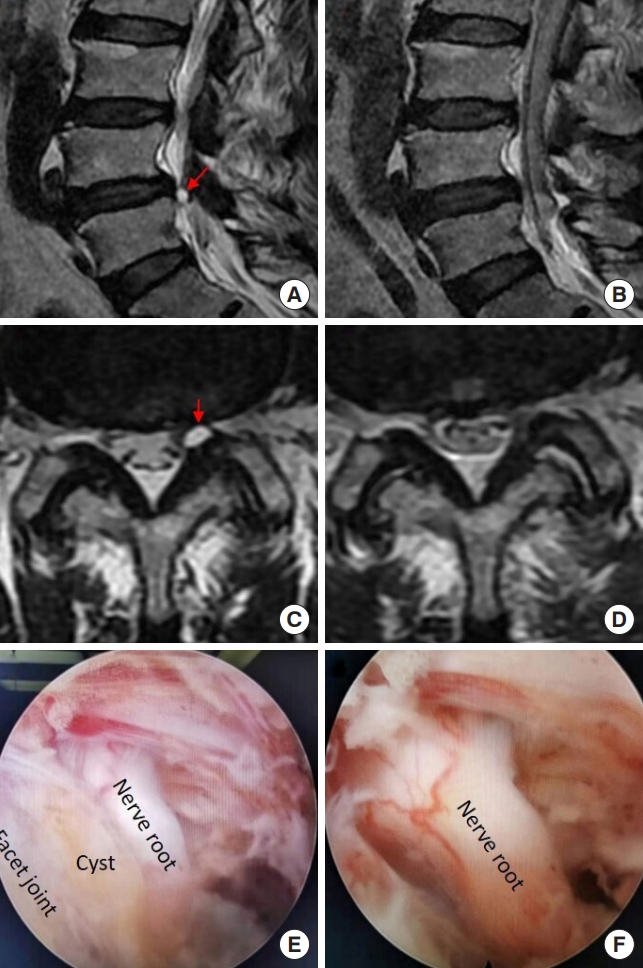
Removing a facet joint cyst at the L4/5 segment using interlaminar endoscopic lumbar discectomy with the aid of a trephine. (A, C) Preoperative magnetic resonance (MR) images. (B, D) The MR images postoperatively. (E, F) Intraoperative images under endoscopy. There is a slight difference of in the levels of panels A and B. The red arrow indicates the location of facet joint cyst before (A, C) the operation.
3) Case 3
A 61-year-old man complained of back pain with radiating pain in the left lower extremity for 3 months. He failed multiple conservative treatments. On MR images, there was a caudally migrated disc at the L4/5 segment (Fig. 9A, C). He underwent L4/5 IELD and we observed that a down-migrated disc compressing the nerve root (Fig. 9E). After the herniated disc was removed, the nerve root and inferior vertebral body were exposed (Fig. 9F). His leg pain relieved significantly soon after the surgery, and follow-up MR images at 1-month showed the nerve roots were without compression (Fig. 9B, D).
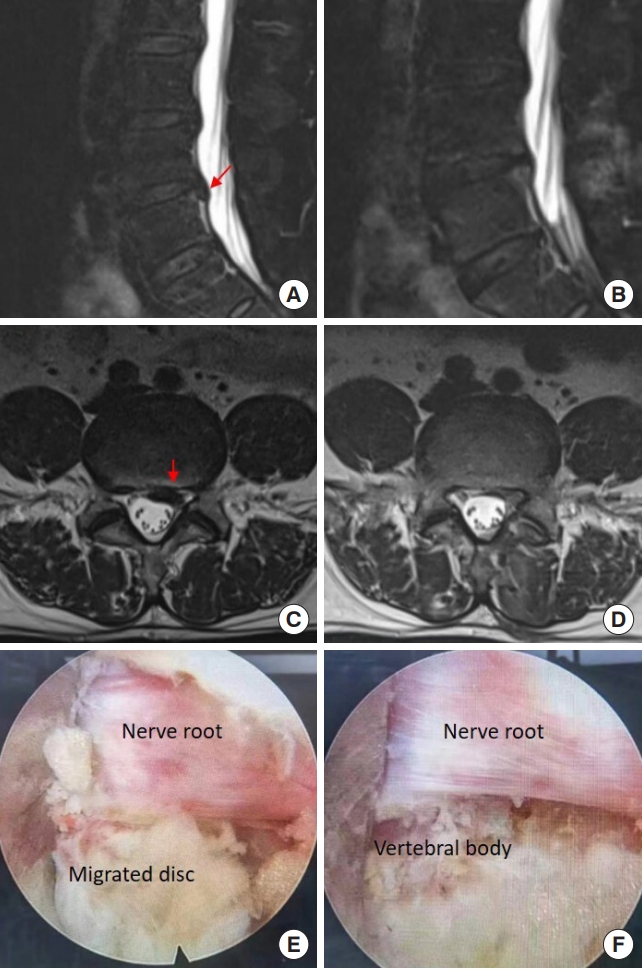
Removing a migrated disc at the L4/5 segment using interlaminar endoscopic lumbar discectomy with the aid of a trephine. (A, C) Preoperative magnetic resonance (MR) images. (B, D) The MR images postoperatively. (E, F) Intraoperative images under endoscopy. The red arrow indicated the location of disc herniation before (A, C) the operation.
4. Complications
No nerve root injury was observed in the trephine laminotomy and endoscopic discectomy. There are 2 patients who complained that the numbness at the lower extremities aggravated at postoperative day 2, though leg pain improved immediately after the surgery. At 3-month follow-up, the numbness at the lower extremities disappeared and both patients achieved full recovery. In addition, 2 patients had neck pain and headache after the surgery but soon relieved by resting. Recurrence of disc herniation occurred in 3 cases at the last follow-up. Two of them were treated with IELD again and the other one received open surgery. No epidural hematomas and infections were observed.
DISCUSSION
Here we reported a convenient technique of laminotomy using a trephine for L4/5 IELD. With this procedure, the L4/5 interlaminar space can be efficiently enlarged to perform endoscopic discectomy. While this technique was designed for treating LDH, it is particularly suitable for LDH patients who are with concomitant spinal stenosis. In this study, the patients’ back and leg pain significantly alleviated soon after IELD and RMDQ score substantially improved. Most patients (88.9%) obtained good-to-excellent outcomes, and the therapeutic efficacy maintained in the middle-long-term follow-up.
Unlike TELD which typically requires multiple fluoroscopies, IELD merely needs 1 or 2 fluoroscopies to localize the entry point [17,18]. Yet, IELD is suitable for the L5/S1 segment where the interlaminar space is large. For the L4/5 segment, however, the natural interlaminar space usually is too narrow for endoscopic discectomy. One has to remove the medial portion of the facet joint to enlarge the interlaminar space before a safe access to the disc can be established. This usually is time-consuming, and some special tools, such as endoscopic drill, may be needed. Modified from the traditional laminectomy for canal stenosis decompression, the developed laminotomy using a trephine is simple and safe in sophisticated hands. Trephine laminotomy usually can be done in a few minutes and can remove a large piece of the lower articular process, which is much quicker than using an endoscopic rongeur.
LDH in elderly patients are often complicated by canal stenosis resulting from the hypertrophy of the facet joints and ligamentum flavum [19-21]. Under such circumstances, these pathologies may be the main culprit responsible for sciatica or claudication and thus, discectomy alone may not be promising in relieving neurological symptoms. Instead of discectomy, decompression of the narrowed spinal canal should be the focus of the surgery [22]. For such cases, interlaminar approach is a better choice than the transforaminal approach, as it can conveniently address lateral and posterior canal stenosis [23-28]. This technique is primarily designed to promote the efficiency of interlaminar endoscopic discectomy, with additional unilateral and limited decompression. While severe spinal stenosis (grade 3) is characterized by hypertrophy of ligament flavum and facet joints on both sides, it is challenging and time-consuming for endoscopic laminotomy to achieve complete decompression of the whole spinal canal. For severe spinal stenosis, we can choose to use UBE or traditional open laminectomy, which are more efficient. There is, however, a report used unilateral laminotomy for bilateral recess decompression [29]. Wu et al. [30] reported posterior endoscopic decompression technique were good or excellent in 96% of patient who had lateral recess stenosis. Another study reported 70.8% of patients had complete pain relief and 86.5% of patients with central stenosis had subjective satisfaction after interlaminar endoscopic decompression [31].
It is challenging to remove a downward migrated disc fragment via the transforaminal approach, and reportedly the rate of residual disc fragment was rather high due to the block of the pedicle [32,33]. Nevertheless, the modified IELD procedure is suitable for LDH patients with highly-migrated discs, as laminotomy enlarged the cranial-caudal length of the interlaminar space, which facilitates the tilt of the endoscope for a full access of the migrated disc tissues. For these patients, the operation time and number of fluoroscopies in IELD were far lesser than those using the transforaminal approach [34-36]. In this study, the mean operation time was 74.5 minutes, and most patients used fluoroscopy for once.
One may fear that the trephine may go too deep and injure the dural sac or transversing nerve root. While the design of this approach is based on a safe zone, the procedure of laminotomy was performed under the endoscopic supervision in a stepby-step manner. While it should be caution of exiting nerve root injury when accessing the Kambin triangle in TELD [37]. In laminotomy and IELD, one should pay more attention to the transversing nerve root, as we are accessing the Kambin triangle from the back. The trephine should be carefully driven at the correct orientation to avoid damage to the dural sac or nerve root. The cutting depth should not exceed 1.5 cm (Fig. 4C), and a sudden emptiness highly indicates the arrival of the trephine in the spinal canal. If there is uncertainty, a lateral radiography may help to identify the depth of the trephine. Overall, this technique is safe since the medial portion of the L5 superior articular process and ligamentum flavum retain in situ, which protects the transversing nerve root [29]. For these reasons, we did not observe nerve root injury and significant neurological deficits in this cohort. Some may also concern about spinal stability, as a piece of facet joint was removed. On postoperative CT images, most of what we removed is lamina and only a small portion is articular process. The axial load is mainly supported by the lumbar disc, and the facet joints play a minor role in load bearing [38]. In a cadaveric model, Cyron et al. [39] investigated the resistance of facet joints against shear forces and revealed that facet joints can resist about one third of the applied shear forces. While unilateral facetectomy of greater than 75% alter the translational displacement and flexibilities of the spinal motion segment [40], only less than a third facet joint was resected in laminotomy. Furthermore, previous studies reported that endoscopic decompression achieved significant relief of pain symptoms without affecting segmental stability [30,41,42]. In addition, cadaver study demonstrated that unilateral and bilateral facetectomies had little effect on mechanics of the spinal segment under physiologic loading [43]. We thus postulated that laminotomy performed under endoscopy did not alter segmental stability.
This is a technical report and clinical analysis of a practical technique of laminotomy. It is a single center study, and the sample size is relatively small. While the lamina and lower articular process were partially resected, we did not study the long-term stability of the surgical segment. Similar to other spine surgeries which open the canal, care should be taken not to injure the neurological structures. With increased surgical experience and visible laminotomy under the endoscope, the rate of neurological complications is rare, as was in this study.
CONCLUSION
In summary, a convenient technique of laminotomy using a trephine was proposed for L4/5 IELD. This modified laminotomy can efficiently enlarge the L4/5 interlaminar entry by removing a portion of bony structure of the lamina. Under the guidance of endoscopy, this technique is efficient and safe, and is particularly suitable for LDH patients with concomitant spinal stenosis.
Supplementary Material
Supplementary Material: Supplementary videoclip 1 can be found via https://doi.org/10.14245/ns.2346572.286.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This project was supported by National Natural Science Foundation of China (Grant No. 82102599), Medicine and health science and technology plan in Zhejiang province (Grant No. 2020RC058). No relevant financial activities outside the submitted work.
Author Contribution
Conceptualization: ZF, ZC, YW; Data curation: ZF, YW, HW, YY, ZC, YW; Formal analysis: YW, TGJ; Funding acquisition: ZF, YW; Methodology: ZF, YW, YY, ZC, YW; Project administration: YY; Visualization: YW, HW, TGJ; Writing - original draft: ZF, YW; Writing - review & editing: ZF, HW, TGJ, YW.