Extra-intradural Spinal Meningioma: A Case Report
Article information
Abstract
Extradural spinal meningiomas are uncommon, and their pathophysiology is not entirely understood. Here, we present the case of a 49-year-old woman with low back and left leg pain of 5 years duration. Magnetic resonance imaging revealed a mass, 1.8-cm in size, with rim enhancement in the spinal canal at the T12 level and extending into the left T12-L1 foramen. In the surgical field, the mass presented with the characteristics of an extra-intradural spinal meningioma. The patient underwent a T12 total laminectomy. A linear durotomy was performed at the midline, and the intradural portion was removed. The extradural portion was not separable from the adjacent dura and the left T12 root, and it was removed by dural excision. Pathological examination confirmed the diagnosis of psammomatous meningioma. We also conducted a literature review of similar cases. Based on our experience with this case, we believe that it is important to clearly distinguish extradural meningiomas from other types of tumors as misdiagnosis can change the operative plan. The long term prognosis of extradural meningiomas is not clear but total excision is thought to be essential.
INTRODUCTION
A meningioma is classified as a type of central nervous system tumor. The supratentorial region is the most frequent site for meningiomas; however, they are sometimes observed in other locations, such as the spine12). Meningiomas arise from meningothelial cells in the arachnoid villi and therefore are intradural. However, a few of these tumors are thought to arise from the extradural component12). Here, we have reported our experience involving a case of extra-intradural calcified meningioma. We also conducted a literature review of similar cases.
CASE REPORT
A 49-year-old woman presented with low back and left leg pain of 5 years duration and a history of medication use for treatment of hypertension and diabetes mellitus for over 20 years. Neurological examination revealed hypesthesia of the left leg and no motor deficit, with normal deep tendon reflexes. Magnetic resonance imaging (MRI) showed an extradural mass, 1.8 cm in size, with rim enhancement in the spinal canal at the T12 level (Fig. 1). The mass extended to the midline and the left T12-L1 foramen. Computed tomography (CT) revealed dense calcification within the mass (Fig. 2). A T12 total laminectomy was performed with the patient in the prone position. The epidural mass was identified around the left T12 nerve root (Fig. 3A). A linear durotomy was performed at the midline, and the intradural portion was removed using an ultrasonic surgical aspirator and a Kerrison punch (Fig. 3B). The adjacent dura was severely ossified, and the left T12 nerve root sleeve was thickened. The extradural portion was not separable from the adjacent dura and the left T12 root, and it was removed by dural excision using a #15 blade and Edson forceps (Fig. 3C). The left T12 nerve root was sacrificed (Fig. 3D). The dural margin was coagulated using a bipolar coagulator, and the dural defect was repaired with artificial dura (Fig. 3E). Pathological examination of the tumor confirmed the diagnosis of psammomatous meningioma (Fig. 4). The Ki-67 labeling index of the tumor was less than 1%.
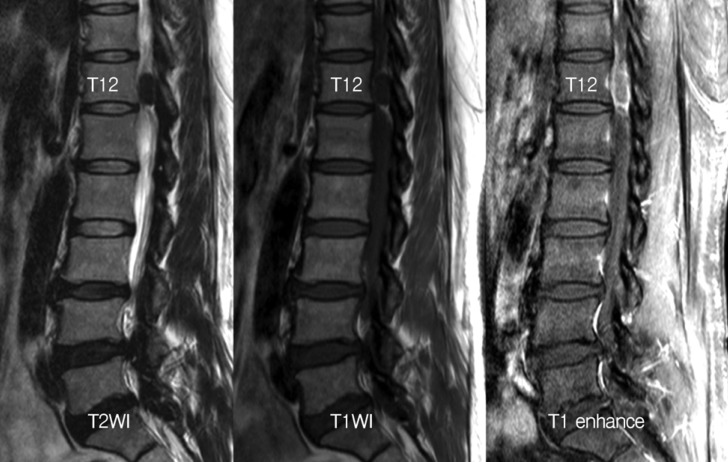
A sagittal T1-enhanced magnetic resonance (MR) image shows a low signal mass, 1.8 cm in size, with rim-enhancement in the spinal canal at the T12 level. Both T1- and T2-weighted MR images show a low signal mass.

The low signal mass with rim-enhancement passes into the left T12-L1 neural foramen on T1-enhanced axial magnetic resonance (MR) image. Calcification within the mass is shown on the computed tomography scan at the same level as that on the axial MR image.

Intraoperative images after T12 total laminectomy. (A) the epidural mass (white arrow) covering the left T12 nerve root, (B) the intradural mass is shown through the durotomy, (C) the residual extradural mass was strongly adherent to the left T12 nerve root (white arrow), (D) the sacrificed T12 nerve root (white arrow), (E) the dural defect was repaired with artificial dura after gross total resection.
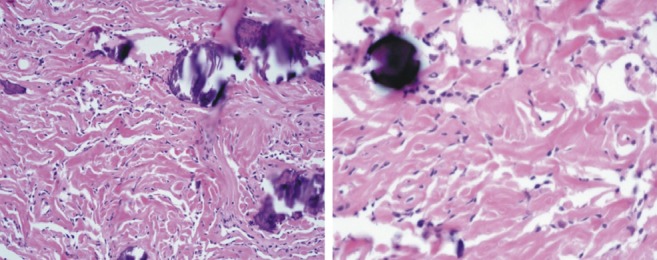
Tumor cells are largely uniform with spindle shape forming parallel, interlacing bundles in a collagen-rich matrix. Whorl formation is inconspicuous. Numerous calcified psammoma bodies are identified. On higher magnification, tumor cells have moderate eosinophilic cytoplasm. They show oval nuclei with delicate chromatin that on occasion have central clearing, or cytoplasmic-nuclear inclusions.
DISCUSSION
Spinal meningiomas account for 25-45% of all spinal tumors4) and 1.2-12.5% of all meningiomas10,12). Most spinal meningiomas are intradural, with only 3.3-21.4% of them being extradural12). A previous study reported that 85% of spinal meningiomas are intradural, 7% have an extradural extension, and 8% are entirely extradural10,11).
Spinal meningiomas generally arise from arachnoid cap cells or dural fibroblasts, and from the arachnoid in the case of cranial nerves and the choroid plexus1). Extradural meningiomas are difficult to explain. Some investigators have suggested the possibility of islands of arachnoid tissue migrating into the extradural spaces or aberrant arachnoid islets in the extradural spaces, as seen with other extracranial meningiomas such as those of the nose or skin3,9).
The differential diagnosis of meningioma includes schwannoma, metastatic tumors, lymphoma, and tuberculoma2). Misdiagnosis can lead to an incorrect operative plan. In our patient, the mass was more likely to be a schwannoma at the point of passing the path of the left T12 nerve, but the CT finding of ossification supported the diagnosis of meningioma. Intraoperative histologic examination is crucial for an optimal surgical plan and so are the preoperative imaging studies in tumors mimicking other pathology5).
The four common microscopic patterns of meningiomas are psammomatous, syncytial, transitional, and fibrous12). Of these, the most common type is the psammomatous meningioma in which calcification is comparatively more likely to be present12). In our patient, the pathological diagnosis was confirmed as psammomatous meningioma.
The long term prognosis of extradural spinal meningiomas is not clear, but recurrence rates might depend on whether total tumor excision was performed1). If a tumor is excised totally, recurrence-free survival rates are 93%, 80%, and 68% at 5, 10, and 15 years, respectively11,12). In cases of subtotal resection, recurrence-free-survival rates are 63%, 45%, and 9% at 5, 10, and 15 years, respectively1). Other factors associated with poor outcomes are the presence of an extradural component, young age (<50 years), multiple lesions, calcification, ossification, and anterior location of the lesion2,7,8).
The presence of an extradural component, calcification, and ossification make total resection of spinal meningiomas more difficult. In our patient, the adjacent dura was resected, and the left T12 nerve root was sacrificed for gross total resection. Although there is no consensus on the degree of total excision, Nakamura et al. reported that recurrence rates are significantly different according to the Simpson grade8). In our patient, Simpson grade I resection was performed. Although the left T12 nerve root was sacrificed, the patient experienced improvement in her leg pain. There was no aggravation of pain or development of any other complication observed at the 6-month follow up.
When surgical treatment is not feasible in case of recurrent, residual, or multiple lesions, radiosurgery can be considered as an option. Kufeld et al. reported 11 cases of spinal meningiomas (WHO grade I) treated with Cyberknife radiosurgery (CKRS). The median prescription dose was 14Gy (95% confidence interval 13-15Gy) and isodose was 70%6). After a median follow-up of 18 months (range, 6-50 months), no local tumor progression was observed. In 1 of the 3 patients with pain before CKRS, the initial symptom was improved. One patient developed a transient worsening of gait disturbance. No other patients developed any new clinical deficits.
CONCLUSION
In this report, we have presented our experience with a case of meningioma and a literature review of similar cases. Extradural meningiomas are uncommon, and it is important to clearly distinguish them from other types of tumors as misdiagnosis can lead to an incorrect operative plan. The long term prognosis of extradural meningiomas is not clear but total excision is thought to be essential.