Intraoperative Cone-Beam Computed Tomography Navigation Versus 2-Dimensional Fluoroscopy in Single-Level Lumbar Spinal Fusion: A Comparative Analysis
Article information
Abstract
Objective
Several studies have advocated for the higher accuracy of transpedicular screw placement under cone-beam computed tomography (CBCT) compared to conventional 2-dimensional (2D) fluoroscopy. The superiority of navigation systems in perioperative and postoperative outcomes remains a topic of debate. This study aimed to compare operative time, screw placement time and accuracy, total radiation dose, perioperative and postoperative outcomes in patients who underwent transpedicular screw fixation for degenerative lumbar spondylolisthesis (DLS) using intraoperative CBCT navigation versus 2D fluoroscopy.
Methods
A retrospective analysis was conducted on patients affected by single-level DLS who underwent posterior lumbar instrumentation with transpedicular screw fixation using surgical CBCT navigation (NV group) or 2D fluoroscopy-assisted freehand technique (FH group). Demographics, screw placement time and accuracy, operative time, total radiation dose, intraoperative blood loss, screw revision rate, complications, and length of stay (LOS) were assessed.
Results
The study included a total of 30 patients (NV group: n = 15; FH group: n = 15). The mean screw placement time, operative time, and LOS were significantly reduced in the NV group compared to the FH group (p < 0.05). The total radiation dose was significantly higher in the NV group (p < 0.0001). No significant difference was found in terms of blood loss and postoperative complications.
Conclusion
This study suggests that intraoperative CBCT-navigated single-level lumbar transpedicular screw fixation is superior in terms of mean screw placement time, operative time, and LOS compared to 2D fluoroscopy, despite a higher intraoperative radiation exposure.
INTRODUCTION
Recent studies have demonstrated a remarkable surge in the prevalence of lumbar fusion surgery (LFS) worldwide in the last 2 decades, as a result of the implementation of innovative surgical implants and advanced technologies in the field of spine surgery. According to recent estimates, the volume of elective LSFs in the United States increased by 62.3% from 2004 to 2015, exceeding aggregate annual hospital costs of $10 billion [1]. A parallel trend was observed in the United Kingdom, where the number of LFS procedures grew by 62% across the same decade [2].
Lumbar spinal fusion is primarily performed to address various conditions such as spondylolisthesis, scoliosis, lumbar stenosis, trauma, as well as oncological and infectious disorders [3-5]. The most prevalent fixation strategy for achieving stability in spinal segments and facilitating solid fusion is the placement of transpedicular screws. This approach is particularly indicated in cases of degenerative lumbar spondylolisthesis (DLS) when conservative treatments fails or when patients exhibit worsening neurological deficits [6]. Traditionally, pedicle screws are manually inserted along a trajectory based on anatomic landmarks with the aid of fluoroscopy. However, despite its established use, this procedure is not without complications, including pedicle breach, facet violation, and neurovascular damage [7]. Given the substantial increase in LFS procedures, there is a growing demand for innovative technologies that can enhance surgical outcomes while minimizing perioperative complications [8].
Recently, cone-beam computed tomography (CBCT) navigation and robot-assisted pedicle screw fixation have been reported to significantly improve screw placement accuracy, especially in cases with complex deformities, while being minimally invasive and reducing radiation exposure for the surgeon [9]. Nevertheless, other studies have also outlined the high costs associated with these technologies, as well as the steep learning curve and increased operative time due to software setup and issues, raising questions about the overall cost-benefit ratio of these systems [10].
The primary aim of this study was to compare operative and screw placement time, total radiation dose, and hospitalization time in patients who underwent posterior lumbar instrumentation with transpedicular screw fixation for single-level DLS using intraoperative CBCT navigation compared to 2-dimensional (2D) fluoroscopy. Moreover, screw accuracy, screw revision rate, and postoperative outcomes were assessed for the 2 techniques.
MATERIALS AND METHODS
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Fondazione Policlinico Universitario Campus Bio-Medico. Informed consent was collected from all study subjects upon enrolment.
1. Study Design and Population
A single-center retrospective study was carried out at the Department of Orthopaedic and Trauma Surgery of Fondazione Policlinico Universitario Campus Bio-Medico (Rome, Italy). Nonconsecutive, adult patients with single-level DLS (grades 1 or 2) and neurogenic claudication refractory to conservative treatment for ≥ 6 months who underwent transpedicular screw fixation between L3 and S1 from December 2020 to January 2022 with a follow-up of at least 2 years were included. Patients with a history of previous surgery, grade ≥ 3 DLS or spinal instability due to trauma, tumors, and infections were excluded. Patients were divided into 2 groups depending on the pedicle screw placement surgical technique. In patients operated before mid-2021 (freehand [FH] group), screws were placed with an open technique assisted by 2D fluoroscopy. In patients operated after mid-2021 (navigation [NV] group), screws were positioned percutaneously using 3-dimensional (3D) navigation through intraoperative CBCT imaging. Data were extrapolated from a prospectively maintained database, extracting continuous and discrete variables related to patients’ demographics, preoperative diagnosis, surgical approach, and outcomes.
2. Surgical Techniques
Under general anesthesia, the patient was positioned prone on the operating table supported by a Wilson frame with hips and knees slightly flexed to further increase interlaminar space and reduce nerve root tension, respectively.
In the FH group, a midline posterior approach to the lumbar spine was performed and the spinous and transverse processes, vertebral laminae and facet joints were carefully exposed. Pedicle screws (Reline MAS, Nuvasive, San Diego, CA, USA) were implanted using fluoroscopic anterior-posterior and lateral views to identify the pedicle under neuromonitoring (NVM5 Nerve Monitoring System, Nuvasive). Following placement, fluoroscopy was performed to confirm the correct positioning of the screws. When necessary, a subsequent decompression of the spinal canal was performed, and posterior lumbar interbody fusion was carried out with polyetheretherketone or titanium cages (Nuvasive). Eventually, screws were connected to appropriately sized titanium rods and tightened, and a final fluoroscopic assessment of the implant was performed.
In the NV group, the navigation reference frame was percutaneously secured to the posterior iliac crest and an intraoperative CBCT scan was performed using Loop-X (Brainlab, Munich, Germany). After image acquisition, pedicle screw length, diameter, and 3D orientation were planned using the Brainlab Elements Spine Planning software. Under real-time 3D navigation (Curve, Brainlab) and neuromonitoring (NVM5 Nerve Monitoring System, Nuvasive), K-wires were percutaneously inserted into along the planned transpedicular trajectory using a drill guide, then cannulated screws were implanted. Subsequently, a second CBCT scan was performed to ensure accurate screw placement. When necessary, decompression was performed via a small midline incision as detailed above. Percutaneous screws were connected to 2 titanium rods and a final 2D fluoroscopic assessment of the implant was performed.
Before closing the fascia, a surgical drain was placed in all cases. On postoperative day one, the drain was removed, and patients were allowed to ambulate while wearing a lumbar brace with the assistance of the healthcare personnel. Patients were discharged in stable conditions, afebrile, and without postoperative complications. Clinical and radiological reassessment was scheduled 1 at month after surgery.
3. Outcomes
The primary outcome of this study was to evaluate the mean time of screw placement and mean operative time between the 2 groups. Total radiation dose during surgery, intraoperative blood loss, screw placement accuracy, length of stay (LOS), and complications were secondary outcomes. Operative time was calculated as the time elapsed between skin incision and skin closure. The screw placement time and radiation exposure were calculated differently in the 2 groups.
In the FH group, the mean screw placement time was considered as the time between the first fluoroscopic image captured to assess pedicle location, and the final fluoroscopic image acquired to confirm the accurate position of screws, divided by the total number of implanted screws. The radiation dose was calculated by summing the dose of each fluoroscopic image taken throughout the surgical procedure.
In the NV group, the mean screw placement time was calculated as the time between the first CBCT scan acquired for navigation and the last CBCT scan acquired to assess the correct position of the implants, divided by the total number of implanted screws. The total radiation dose was calculated by summing all CBCT and fluoroscopic scans performed during the surgery. Screw placement accuracy was evaluated according to the Gertzbein-Robbins scale (GRS) [11] in the NV group and following the method reported my Kim et al. [12] on posteroanterior and lateral radiographs in the FH group.
4. Statistical Analysis
Continuous data were expressed as mean± standard deviation. The normality of data distribution was assessed with ShapiroWilk test. The independent-sample Student t-test and MannWhitney U-test were performed to compare investigated continuous variables between groups. Categorical variables were compared using the chi-square test. Statistical significance was set as p < 0.05. Formal analysis was performed using Prism 9 (GraphPad Software Inc., La Jolla, CA, USA).
RESULTS
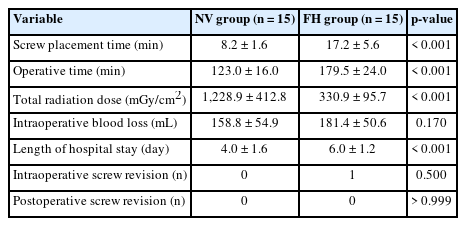
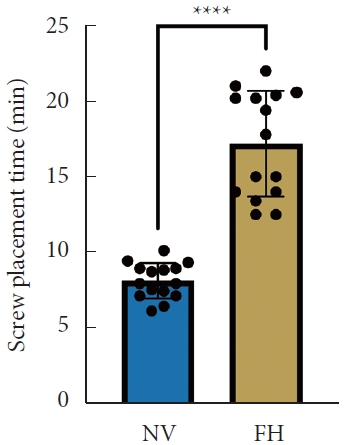
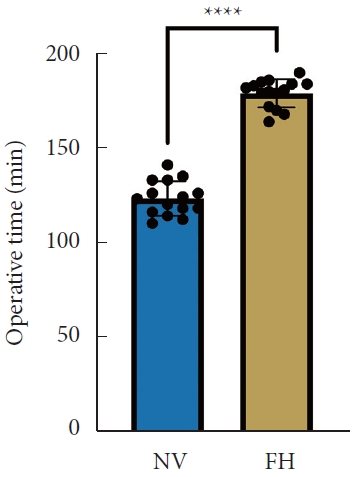
A total of 30 patients (17 males and 13 females) affected by single-level DLS were included in the study. Of these, 15 were enrolled in the NV group and 15 in the FH group, for a total of 120 screws. The mean age of the patients was 59.4± 14.8 years in the NV group and 57.3± 16.3 years in the FH group. In the FH group, 3 patients underwent screw fixation at L3–4, 10 patients at L4–5 and 2 patients at L5–S1. In the NV group, 2 patients underwent screw fixation at L3–4, 11 patients at L4–5 and 2 patients at L5–S1. All demographic data are depicted in Table 1. The mean screw placement time was 8.2± 1.6 minutes in the NV group compared to 17.2± 5.6 minutes in the FV group (p< 0.0001) (Fig. 1). Likewise, the operative time was significantly lower in the NV group (123.0± 16.0 minutes) compared to the FH group (179.5± 24.0 minutes, p< 0.0001) (Fig. 2). However, total radiation exposure was significantly higher in the NV group (1,228.9 ± 412.8 mGy/cm2) when compared to the FH cohort (330.9± 95.7 mGy/cm2, p< 0.0001) (Fig. 3). No significant intergroup differences were found in terms of intraoperative blood loss (158.8 ± 54.9 mL in the NV group vs. 181.4 ± 50.6 mL in the FH group). On the other hand, LOS was significantly reduced in the NV group (4.0 ± 1.6 days) compared to the FH group (6.0± 1.2 days) (p< 0.05) (Fig. 4). These results are summarized in Table 2. All screws in the NV group were successfully placed percutaneously, without the need to convert to open surgery in any case. One screw was intraoperatively revised in the FH group; no screws were revised in the NV group (p=0.500). According to the GRS, 50 screws (83.3%) in the NV group were grade A and the remaining were grade B (10, 16.7%), hence all considered clinically acceptable (grades A+B). In the FH group, 3 screws (5%) violated the lateral pedicle, while the majority were appropriately positioned. No postoperative revision was performed for any screw in either group. At 2 years postoperatively, no surgical site infection nor reoperation were reported. One patient in the FH group developed adjacent segment disease and was treated conservatively.
Mean screw placement time between NV and FH groups. NV, navigation; FH, fluoroscopy. ****p<0.0001, Mann-Whitney U-test.
Mean operative time between NV and FH groups. NV, navigation; FH, fluoroscopy. ****p<0.0001, Student t-test.
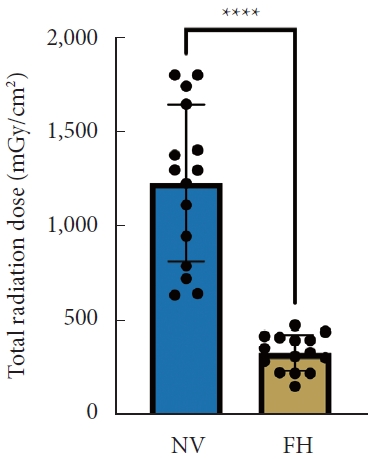
Total radiation dose between NV and FH groups. NV, navigation; FH, fluoroscopy. ****p<0.0001, Student t-test.
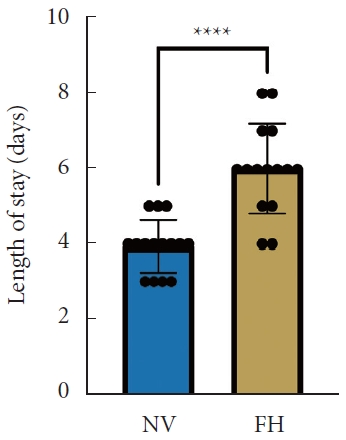
Length of stay between NV and FH groups. NV, navigation; FH, fluoroscopy. ****p<0.0001, Mann-Whitney U-test.
DISCUSSION
Modern technologies have significantly contributed to improving spine care in the last 20 years, making LFS a common and feasible procedure. Transpedicular screw fixation has been widely implemented to achieve a solid spinal fusion. During the last decade, the number of patients requiring LFS has been increasing, especially among the elderly [1]. Indeed, aging of the general population has contributed to the rise of degenerative spine disorders needing surgical treatment, such as DLS and lumbar stenosis [3]. In this context, technological advancements have fostered the development of novel strategies in spine fusion surgery, aiming to enhance precision while minimizing invasiveness. Minimally invasive spine surgery has been the focus of extensive clinical and scientific investigations over the last decade, with the aim to reduce tissue trauma and preserve neurovascular structures. Access to deep structures of the spine has been made easier, with the possibility to visualize the spine through CBCT images via 3D navigation, rendering surgical procedures more feasible and, more importantly, safer for the patients [8]. However, the advantages of CBCT navigation over conventional 2D-guided screw placement still need to be definitively confirmed.
The aim of this study was to compare the results of percutaneous transpedicular screw fixation using intraoperative 3D CBCT navigation vs. freehand open screw placement with 2D fluoroscopy in patients affected by single-level DLS. Our results showed that CBCT navigation provided significant improvements in screw placement time, operative time, and LOS compared to the conventional 2D fluoroscopic technique. These results can be easily explained by the fact that intraoperative navigation facilitates the surgical workflow by allowing for pre-implantation planning and defining the correct entry point and optimal screw trajectory. Moreover, screw malposition is less common, thus reducing the time needed for reimplantation.
Nevertheless, no substantial differences between navigated and conventional techniques have been reported in the recent literature [13,14]. A meta-analysis performed by Sun et al. [15] demonstrated significantly shorter operative times in the FH group, suggesting that intraoperative navigation might lengthen surgical procedures due to increased scanning time and screw planning. These results are in contrast with our data. While the setup time is usually longer for navigated procedures, optimization of the surgical workflow and involvement of a specialized multidisciplinary spine team, including surgeons, nurses, and radiologic technologists, may significantly minimize downtimes. Moreover, when using intraoperative CBCT navigation, the surgeon is ready to navigate right after image acquisition. The longer time required by software setup and CBCT acquisition is frequently counterbalanced by more efficient pedicle screw placement, making total operative time shorter than in conventional FH techniques as shown in the current study.
However, patient radiation exposure was significantly increased in the NV group in our study, although the surgical team was not exposed. Indeed, the surgical personnel exited from the operating room during CBCT acquisition, thus significantly abating the radiation dose absorbed by the scrubbed team [16]. This is crucial since it has been reported that spine surgeons may have up to a 13% greater risk of cancer due to intraoperative radiation exposure [17]. On the other hand, the greater patient radiation exposure during CBCT navigation compared to FH techniques cannot be adjusted but can be justified by a higher screw placement accuracy, which may reduce the risk of postoperative complications and overall morbidity [18].
Length of hospitalization was significantly reduced in the NV group compared to the FH group in our study. This is in line with Bovonratwet et al. [19], who showed a significantly shorter mean LOS in the navigated group compared to conventional single-level instrumented posterior lumbar fusion. This finding may be associated with the minimally invasive nature of navigated techniques, which are performed percutaneously, thus making surgical incisions smaller, reducing surgical exposure, and consequently minimizing intraoperative blood loss, as also shown by Wang et al. [20] Diminished blood loss and postoperative pain may result in lower rates of transfusion and complications, thus reducing the need for critical care and allowing for an early patient discharge [21].
Nonetheless, intraoperative navigation is also a valuable educational tool. Indeed, real-time exploration of the spine anatomy, planning pedicle screw trajectory and entry point, and assisting screw placement can provide the surgeon-in-training with immediate feedback on surgical gestures [9].
Despite all the abovementioned advantages, spine navigation is limited by the significant costs of the acquisition and maintenance of intraoperative imaging systems, navigation suites, and associated software. However, the overall increased accuracy results in shorter hospitalization times and lower revision rates compared to conventional FH approaches, thus possibly resulting in higher cost-effectiveness justifying the implementation of this technology [22].
This study has some limitations. First, the retrospective and single-center nature of the study, as well as the small sample size, inherently limit the reliability of our outcomes. Second, considering that the FH group did not routinely undergo postoperative CBCT at our institution, the 2 different systems utilized to assess screw placement accuracy in the NV and FH groups did not allow for a proper comparison and might thus introduce bias. Future multicentric and prospective studies are required to ultimately define the advantages of pedicle screw placement with the aid of intraoperative 3D navigation over conventional 2D FH techniques.
CONCLUSION
This study suggests that pedicle screw fixation under intraoperative CBCT navigation is superior in terms screw placement time, total operative time, and LOS compared to 2D fluoroscopy in single-level DLS, although exposing patients to a higher radiation dose.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This research was funded by the Research Grants BRIC-2018 ID3 (ACTIVE) and BRIC-2021 ID4 (Spine 4.0) of the National Institute against Accidents at Work (INAIL).
Author Contribution
Conceptualization: GV; Formal Analysis: GFP; Investigation: GV, FR, GFP, LA, PB; Methodology: GFP; Writing - Original Draft: GFP; Writing - Review & Editing: LA, GV, FR, RP, VD.