Migration of an Intracranial Subdural Hematoma to the Spinal Subdural Space: A Case Report
Article information
Abstract
A 57-year-old man complained of severe lower back pain and radicular pain in both legs for 1 week after falling from a ladder. Magnetic resonance imaging (MRI) of the spine showed a subdural hematoma (SDH), which was surgically removed. The patient had no back pain or the radicular leg pain at 2 weeks post-surgery. However, he complained of diffuse headaches upon follow-up. Brain computed tomography (CT) and MRI revealed an intracranial SDH, which was immediately removed by surgery. During his 1-year follow-up, he reported that the pain had resolved without recurrence. Simultaneous spinal and intracranial SDH are rare and no standard treatment exists for this condition. This case suggests that it is possible that an intracranial SDH can migrate into the cerebrospinal fluid (CSF) space through an arachnoid tear. CSF circulation allows the intracranial SDH to enter subarachnoid spaces encasing the spinal cord. In order to prevent irreversible damage, surgical intervention should be considered for case of spinal SDH with progressive neurological deficits.
INTRODUCTION
With technical advancements in magnetic resonance imaging (MRI), the reported incidence of spinal subdural hematoma (SDH) has been steadily increasing39). However, spinal SDH is still considered a rare hemorrhagic disorder7). Furthermore, simultaneous spinal and intracranial SDHs are extremely rare, having been reported in only about 35 cases to date6). This case report describes successful surgical treatment of simultaneous spinal and intracranial SDH.
CASE REPORT
The patient is a 57-year-old man, had been experiencing severe low back pain (Numeric Rating Scale: 5) and radicular pain in both legs for 1 week prior to visiting our institute. During this week, the patient did not take any medication. His symptoms were likely due to a fall, which had occurred 2 weeks prior. Upon neurological examination, he had weakness in both lower extremities (Manual Muscle Test: 3/5). The patient was alert, could orient well, and did not present any symptoms of cranial nerve palsy. Upon admission, MRI of the spine showed an elongated, lobulated mass encasing the cauda equina from L2 to S1 level. Sagittal and axial T1-weighted images showed high signal intensity in the spinal subdural space. Sagittal and axial T2-weighted images showed iso-intense in this space (Fig. 1). Based on these results, partial hemilaminectomy was performed (L3 subtotal, L5 left). At the time of surgery, the epidural space appeared normal without any observable hematoma. The dura mater was bluish in color and some bulging was observed. After incisions into dura and arachnoid membrane, the hematoma was identified and removed by aspiration and irrigation from the lower and upper levels of the laminectomy area. After successful removal of hematoma, the spinal cord and cauda equina appeared normal (Fig. 2). Post-operative spinal MRI performed 1 week later showed no sign of continued spinal SDH(Fig. 3).
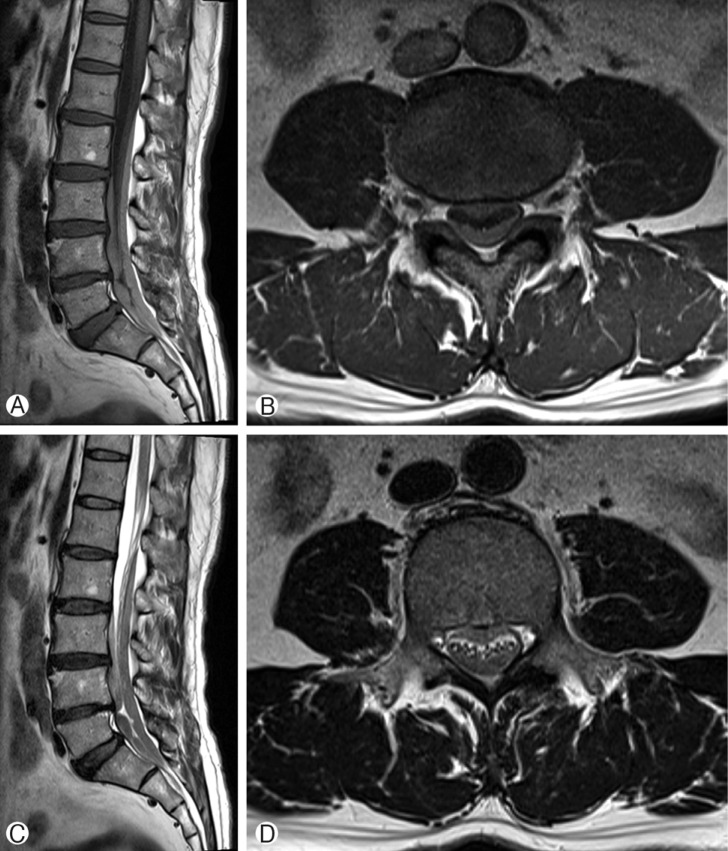
Pre-operative T1-weighted magnetic resonance images: sagittal (A) and axial (B) views. T2-weighted magnetic resonance images: sagittal (C) and axial (D) views. The images revealed a spinal subdural hematoma between L2 and S1.
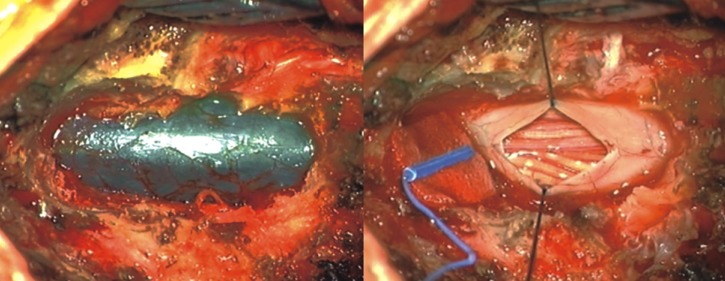
Intra-operative photograph shows the dura mater, which is bluish in color with some bulging. After excision of the dura and arachnoid membrane, the hematoma was revealed, and removed by aspiration and irrigation from the lower and upper levels of the laminectomy area.
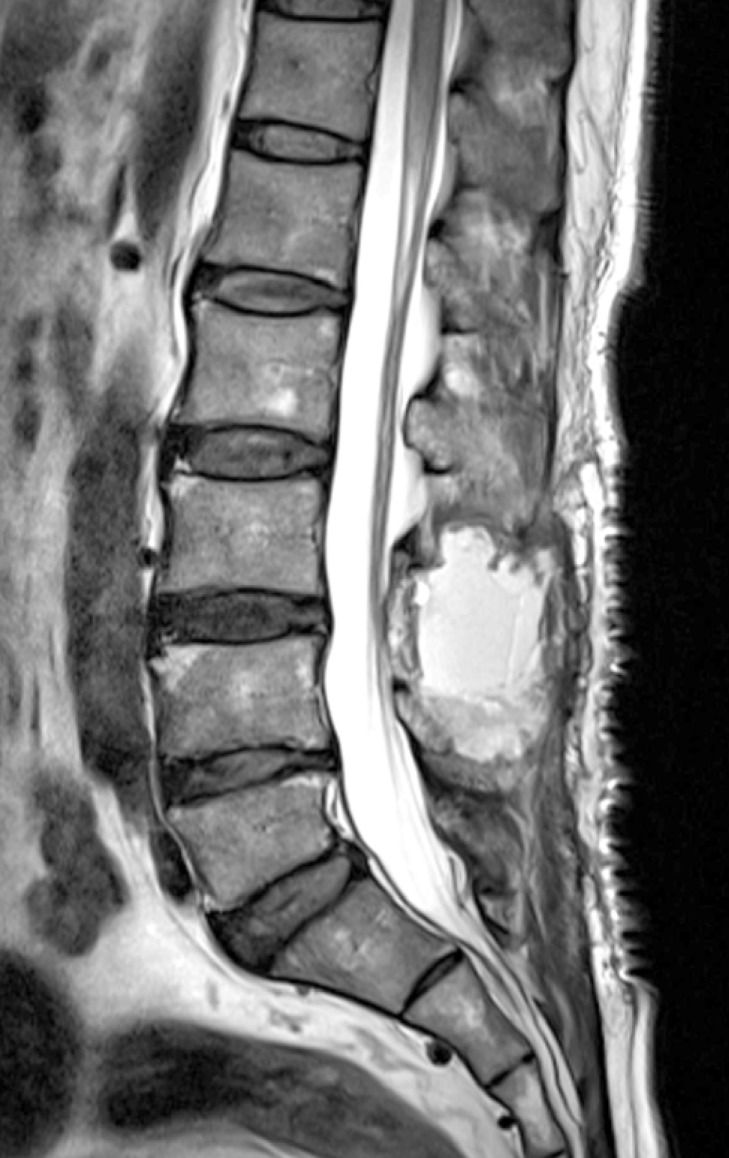
Sagittal T2-weighed magnetic resonance image reveals remarkable resolution of the hematoma after decompressive laminectomy.
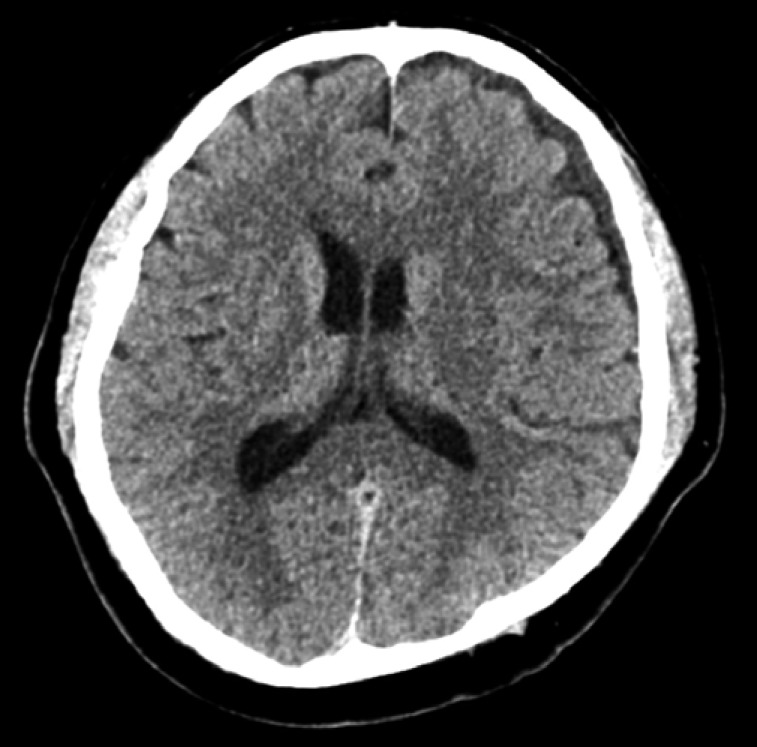
Two weeks after the operation, the patient began experiencing diffuse headaches. To determine the cause of these headaches, brain computed tomography (CT) and MRI were performed immediately. T1-weighted MRI of the brain showed iso-intense, while T2-weighted MRI showed low signal intensity in the left cerebral convexity and a midline shift to right with compression of the left lateral ventricle due to mass effect (Fig. 4). To evacuate the intracranial SDH, a burr-hole trephination with irrigation and drainage was conducted. Postoperative CT showed no residual hematoma (Fig. 5). Follow-up at 1 year after this surgery revealed that the patient's symptoms had resolved completely without any recurrence.

Pre-operative T1-weighted (A) and T2-weighted (B) magnetic resonance images of the brain. The T1- and T2-weighted images show iso- and low signal intensity in the left cerebral convexity, respectively, along with a midline shift to the right and compression of the left lateral ventricle due to a mass effect.
DISCUSSION
Spinal SDH is a rare hemorrhagic disorder6). Factors that predispose an individual to spinal SDH include trauma, surgery, lumbar puncture, spinal tumor, alcohol abuse, and coagulopathy2712). The pathophysiology underlying the simultaneous development of spinal and cranial SDH is poorly understood. It is possible that blood from the cranium leaks into the spine, or there may be two unique sources of bleeding.
At least two theories can explain the development of simultaneous spinal and cranial SDHs in our patient. The first theory is that the spinal SDH was caused by rupturing of vessels in the spinal space48). In this scenario, trauma due to falling from the ladder would be an important factor in spinal SDH development. However, since the spinal space does not contain any major blood vessels or bridging veins that can act as a source for a spinal SDH8), the incidence of spinal SDH is significantly lower than that of cranial SDH. The second theory is related to "the dilutional and water hammer like effect" previously proposed by Moscovici et al.10) and Bortolotti et al.1). Moscovici et al.10) reported a case of an 88-year-old man who received conservative management of an intracranial SDH due to head trauma. He subsequently developed cauda equina syndrome and laminectomy was performed. Bortolotti et al.1) reported a case of a 23-year-old woman who initially had blunt head trauma with an intracranial SDH. The patient began experiencing back pain 4 days after head injury. MRI revealed a spinal SDH. It is possible that there may be two unique sources of bleeding, but these cases suggest that cerebrospinal fluid (CSF) influx into the subdural space following a trauma-induced arachnoid tear can facilitate SDH migration into other parts of the spinal cord. We also hypothesized that an intracranial SDH can migrate into the CSF space through arachnoid tear. CSF circulation pathway can allow an intracranial SDH to enter the subarachnoid spaces encasing the spinal cord. Therefore, spinal SDH may occur as a sequel of cranial SDH via migration. Although we cannot completely exclude the possibility of intracranial hematoma result from cerebral hypotension induced by spinal decompression surgery, the patient showed no symptom of CSF leakage after surgical intervention. Furthermore, the age of intracranial SDH was estimated as more than 2 weeks on CT and MRI. Therefore, we think that intracranial SDH can migrate into the CSF space and enter the subarachnoid spaces encasing the spinal cord.
Regardless to the mechanisms of action, it was difficult to decide on spinal SDH treatment. Treatment options include conservative therapy and surgical intervention5). Spontaneous resolution of spinal SDH has been reported previously411), but, a meta-analysis demonstrates that most patients (85.5%) with symptomatic spinal SDHs undergo decompression surgery for symptom alleviation7). In the present case, surgery was performed to remove the spinal SDH, based on the aggravate symptoms, including gait disturbance and back pain (Numeric Rating Scale: 8). Thus, although patients with mild neurologic symptoms are likely to benefit from conservative therapy, prompt surgical intervention should be performed when the spinal SDH is massive or clinical symptoms are progressive.
CONCLUSION
We report a rare case of spinal SDH concurrent with intracranial SDH after trauma. Our case suggests the possibility that an intracranial SDH can migrate into the CSF space through an arachnoid tear and via CSF circulation, can enter the subarachnoid spaces encasing the spinal cord.