Propriospinal Myoclonus Induced by a Herniated Lumbar Intervertebral Disc at a Young Age: A Case Report
Article information
Abstract
The cause of propriospinal myoclonus (PSM) is idiopathic. Cervical trauma, ischemic myelopathy secondary to a spinal dural arteriovenous fistula, syringomyelia, Lyme neuroborreliosis, human immunodeficiency virus central nervous system infection, and cervical disc herniation can be the cause of PSM, but lumbar herniated intervertebral disc (HIVD) induced PSM has not been reported. We describe a patient who presented with PSM induced by HIVD and was treated with an epidural steroid injection using a transforaminal approach.
INTRODUCTION
Myoclonus is a brief, involuntary twitching of a muscle or a group of muscles. It has been classified into cortical, subcortical, spinal, and peripheral lesions12). Although many diseases have been reported as a cause of myoclonus8), only one recently reported a case of spinal myoclonus has been attributed to cervical herniated intervertebral disc (HIVD). The lumbar HIVD as a cause of myoclonus has not been reported yet.
Although spinal myoclonus can be difficult to manage, some drugs such as clonazepam and tetrabenazine hydrochloride may be effective5,8). Many authors have tried to treat myoclonus with spinal or epidural blocks in difficult cases, which would not infrequently cause worse results.
We report a case of propriospinal myoclonus (PSM) that might be induced by lumbar HIVD in a 15-year-old female who has been successfully treated with an epidural steroid injection using a transforaminal approach.
CASE REPORT
A 15-year-old female patient had the sudden onset of severe left sciatica. She could not stand up over 3 minutes due to pain. Her medical history was not contributable. At another hospital, she was suspected to have left lumbar HIVD at L5-S1 based on a physical examination, however, her pain was aggravated in spite of conservative management for 3 days. She also experienced a brief involuntary muscle twitching involving the left lower leg and trunk.
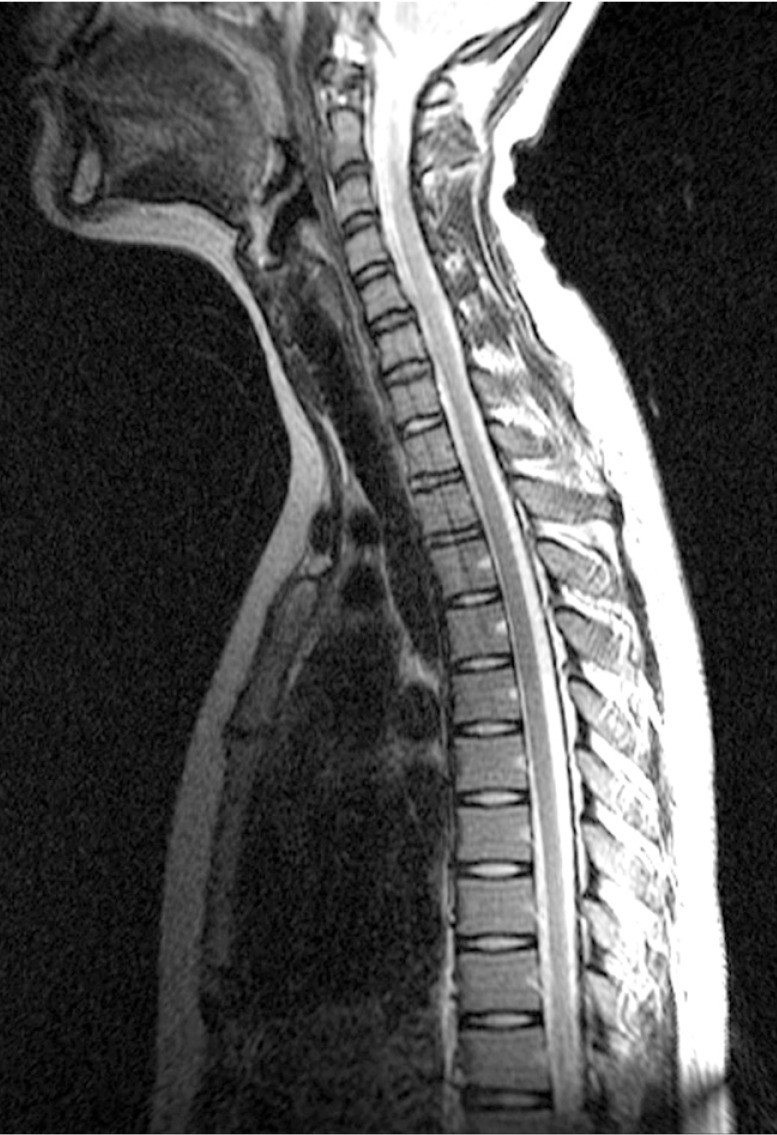
A whole spine magnetic resonance imaging (MRI) study showed HIVD between L5-S1 (Figs. 1,2,3). Electro-encephalography was normal. Routine laboratory, hematologic studies, electrolyte study included calcium, magnesium, copper and ceruloplasmin concentration and cerebrospinal fluid study were unremarkable. The nerve conduction test showed that the F-wave latency was prolonged and the H-reflex was normal in the left peroneal nerve, which is not a peripheral neuropathic finding. However, intermittent brief synchronous activity was noted in the left sternocleidomastoid, rectus abdominalis, gluteus medius, and gastrocnemius muscles based on surface electro-myography, which was consistent with PSM. The myoclonus was characterized by the hip and knee joints extending 1-2 times per second with the lower back extended. There was no facial muscle spasm. The body muscle spasms during sleep were not identified either. Myoclonus was decreased to 1-2 times per 3-4 seconds in frequency after administration of clonazepam (0.5mg), but there was no further improvement in symptoms.
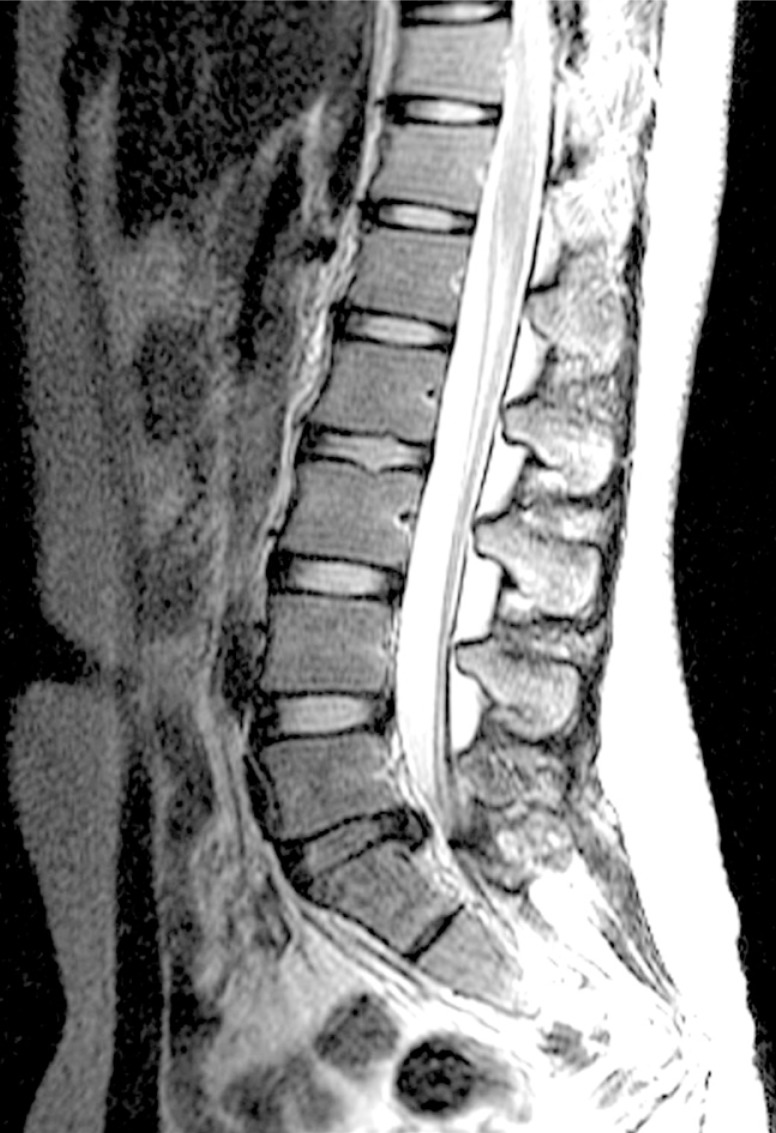
Lumbar spine MRI T2WI sagittal view shows L5-S1 disc degeneration and protruding disc compressing the thecal sac.
On physical examination, myoclonus and pain were aggravated in the straight leg raising test at approximately 50 degrees hip flexion or ankle dorsiflexion. Myoclonus was intensified during the one-leg standing and spread to the thoracic muscles.
A transforaminal epidural steroid injection was delivered with triamcinolone (20mg) and 0.3% lidocaine to the left L5, S1 root on one occasion (Fig. 4).
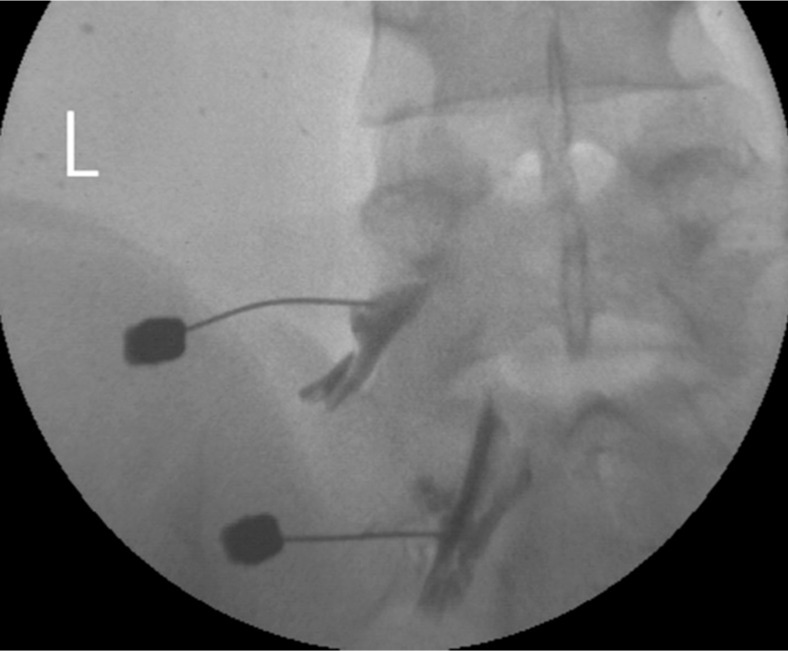
Epidural steroid injection via the transforaminal approach at the left L5, S1 root. Contrast media is spreading to the epidural space through the left L5, S1 root sleeves.
After the procedure, the sciatic pain was disappeared immediately and the myoclonus was decreased to 1-2 times per 10 seconds in frequency. After 2 weeks, the myoclonus improved gradually and disappeared completely. We observed her without medication in the outpatient clinic for 6 months after the procedure.
DISCUSSION
Brown and colleagues2) recommended that spinal myoclonus would be classified as segmental spinal myoclonus and PSM in 1991. Segmental spinal myoclonus is typically associated with a localized area of damaged spinal nerves. The affected area may include direct damage of the spinal cord or may cause abnormal changes in the function of the spinal cord. Segmental spinal myoclonus is usually rhythmic and not stimulus-sensitive. But PSM involves the following: all of the spinal cord, rather than a specific segment, complex muscles jerks; more extensive simultaneous involvement of muscles, such as the trunk and limbs; the pattern is usually arrhythmic; and stimulus-sensitive with a delay due to the slow conducting propriospinal nerve fibers.
According to a PSM study, diagnostic clues for PSM include widespread rhythmic or arrhythmic myoclonus prominently involving the trunk muscles and sparing the cranially-innervated muscles5).
In our case, the myoclonic jerks were arrhythmic, starting from the left leg and extending to the trunk, ascending, and aggravated with nerve stimulation (e.g., straight leg raising and one-leg standing). Furthermore, there are no myoclonic jerks in the cranial-innervated muscles. Thus, the clinical characteristics in this case would be a PSM.
Spinal myoclonus has been associated with laminectomies, the remote effects of cancer, spinal cord injuries, post-operative pseudomeningoceles, laparotomies, thoracic sympathectomies, poliomyelitis, herpes myelitis, lumbosacral radiculopathies, spinal epidural blocks, myelopathy due to demyelination, electrical injuries, acquired immunodeficiency syndrome, multiple sclerosis and cervical spondylosis8,9). In rare occasions, spinal myoclonus can be observed after peripheral nerve lesions17).
The cause of most PSM is thought to be idiopathic. Although various causes have been reported1,6,11,14,15), there has been only one report of a cervical disc as the cause of PSM resulting from disc disease. Capelle, et al.,4) described a patient who presented with right C6 radiculopathy due to HIVD C5-6 and myoclonic twitches predominantly affecting the abdominal muscles but spreading to adjacent muscles which was treated with discectomy and cage augmented fusion.
To the date, this report might be the first report of PSM with lumbar disc disease.
A variety of mechanisms have been proposed for spinal myoclonus, including dysfunction of segmental spinal cord circuitry, deficient inhibitory glycinergic transmission in the spinal cord, subsequent release of synchronous motor neuron oscillations within segments of the cord, and vitamin B12 deficiency causing vacuolar swelling of the myelin layers in the mid-thoracic cord and resulting in degeneration of the descending pyramidal and ascending posterior column traces18).
Also, according to Campos, et al.,3) there is evidence that various possible mechanisms can be involved: loss of inhibitory function of local dorsal horn interneurons, abnormal hyperactivity of local anterior horn neurons, aberrant local axons re-excitations, and loss of inhibition from supra-segmental descending pathways. Ogata, et al.,16) experienced that PSM due to injury to the L4 nerve root during epidural anesthesia was treated by L4 root block using 1.5ml of 1% lidocaine. He described that an injury to the left L4 nerve root possibly cause an abnormal transmission of the impulses or ectopic hyperexcitability in the nerve root, which might lead to the disturbance of the spinal inhibitory interneurons and hyperexcitability of the anterior horn cells causing myoclonus.
The authors assumed that local anesthetic agents which was administered to the spinal epidural space via a caudal or interlaminar injection may distribute more in the posterior lateral part than in the anterior part of spinal canal, in which the effect of the local anesthetic drug on inhibitory neurons might have led to a loss of inhibitory function in the spinal cord7,13). Thus, a transforaminal injection has an advantage over a caudal or trans-interlamina block. In the transforaminal injection, the injected drugs spread more onto the ventral surface of the spinal cord and can block the lesional root at the same time (Fig. 4). As a result, symptoms of myoclonus have been subsided by avoiding the disturbance of the spinal inhibitory interneurons activity.
CONCLUSION
This case shows that HIVD may be the one of the causes of myoclonus, and transforaminal epidural injection can be option of treatments, in which can approach the ventral surface of the spinal cord and affected root in order to avoid the disturbance of the spinal inhibitory interneurons activity. We recommend epidural steroid injections via the transforaminal approach as a treatment option for PSM.