A Cost-Effectiveness Analysis of the Integration of Robotic Spine Technology in Spine Surgery
Article information
Abstract
Objective
We investigate the cost-effectiveness of adding robotic technology in spine surgery to an active neurosurgical practice.
Methods
The time of operative procedures, infection rates, revision rates, length of stay, and possible conversion of open to minimally invasive spine surgery (MIS) secondary to robotic image guidance technology were calculated using a combination of institution-specific and national data points. This cost matrix was subsequently applied to 1 year of elective clinical case volume at an academic practice with regard to payor mix, procedural mix, and procedural revenue.
Results
A total of 1,985 elective cases were analyzed over a 1-year period; of these, 557 thoracolumbar cases (28%) were analyzed. Fifty-eight (10.4%) were MIS fusions. Independent review determined an additional ~10% cases (50) to be candidates for MIS fusion. Furthermore, 41.4% patients had governmental insurance, while 58.6% had commercial insurance. The weighted average diagnosis-related group reimbursement for thoracolumbar procedures for the hospital system was calculated to be $25,057 for Medicare and $42,096 for commercial insurance. Time savings averaged 3.4 minutes per 1-level MIS procedure with robotic technology, resulting in annual savings of $5,713. Improved pedicle screw accuracy secondary to robotic technology would have resulted in 9.47 revisions being avoided, with cost savings of $314,661. Under appropriate payor mix components, robotic technology would have converted 31 Medicare and 18 commercial patients from open to MIS. This would have resulted in 140 fewer total hospital admission days ($251,860) and avoided 2.3 infections ($36,312). Robotic surgery resulted in immediate conservative savings estimate of $608,546 during a 1-year period at an academic center performing 557 elective thoracolumbar instrumentation cases.
Conclusion
Application of robotic spine surgery is cost-effective, resulting in lesser revision surgery, lower infection rates, reduced length of stay, and shorter operative time. Further research is warranted, evaluating the financial impact of robotic spine surgery.
INTRODUCTION
Applications of robotics have demonstrated utility across a wide spectrum of surgical specialties [1]. Still, robotic surgery is in its nascent stages in the field of spinal surgery. Recent literature pertaining to the application of robotics in spinal instrumentation has revealed that robotics has the potential to revolutionize this aspect of spinal surgery in terms of better accuracy rates for screw placements, decreased operative time, less fluoroscopic exposure to the surgical team, and possible more conversion to minimally invasive techniques [1]. In addition to these aspects, the cost-effectiveness of robotics in spinal surgery is likely to play an important role in its eventual assimilation into everyday practice.
Between 2001 and 2010, approximately 3.6 million spinal fusions were performed, with an estimated total cost of $287 billion in the United States alone [2]. Considering these high costs, spine surgeons are coping with pricing pressures and increased accountability for their performance [3]. Amidst this, there is an initiative to improve the value that we, as spine surgeons, deliver to our patients: better outcomes and lower costs. The application of robotic systems to spinal surgery may play a vital role in implementation of such a value-based spinal care. Although the initial capital investment would be large (current pricing on urologic robotics is approximately $1.5 to 2 million), the benefit in patient volume, better accuracy and improved long-term outcomes can justify the utilization of these systems [4].
Robotic surgery has significantly changed practice patterns in urologic surgery [5]. The available literature on the cost-effectiveness of robotic urologic surgery however reveals that although post-operative outcomes are similar, robotic-assisted laparoscopic surgery is more costly than laparoscopic and open surgery [4,5]. Whether long-term outcomes may be more cost-effective with robotic-assisted systems remains to be proven [5]. Such cost-effectiveness analysis are imperative to understand the problems and accordingly implement solutions [5].
Here we apply a cost-effectiveness analysis on the application of robotic technology to spine surgery across a busy academic neurosurgical practice. The purpose is to gain an early insight towards utilization of the robotic platform in the value-based healthcare model for spinal surgery.
MATERIALS AND METHODS
The impact of robotic technology on spinal surgery was modeling using a combination of institution specific and nationally related data points.
1. Patient Cohort
This study was approved by the Institutional Review Board of LSUHSC, Shrevport (approval number: MODCR0000472_H13-020). Data was integrated retrospectively to 1985 consecutive patients whose data was previously prospectively gathered across six different neurosurgical providers at Louisiana State University Health Sciences Center in Shreveport, LA, USA. This was done during the period of July 1, 2014 to June 30, 2015. Five hundred and fifty-seven patients specifically underwent thoracolumbar instrumentation procedures. This temporal cohort specifically was analyzed due to the detailed procedural log, clinical information, and socioeconomic information available. Data was intentionally applied to only elective cases to ensure that planned utilization of possible robotic technology could be reasonably applied to the surgeon’s decision-making. Emergent cases and hospital consultation requiring immediate in-house surgical procedures were not considered in this study by the basis of economic evaluation. All patients scheduled for neurosurgical procedures who presented through the outpatient clinic were considered for this study regardless of attending, surgical procedure, or hospital location [6].
2. Payer Mix and Procedural Mix
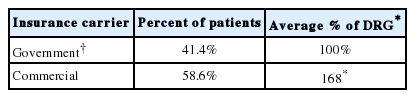
The appropriate payer mix (Medicare, Medicaid, Commercial, or other insurance) for the cases performed was recorded from the patient specific dataset. This has been previously documented [6]. The construct of governmental insurance included Medicare, Medicaid, and military insurance (Tricare). Despite being a managed care platform, Tricare insurance reimburses closer to Medicare rates than commercial insurance and as such was included in that cohort. Medicaid (1%) and Tricare (3.4%) represented only 4.4% of patients in the series and as such, the gross assumption was made to equate all governmental reimbursement to Medicare rates. For purposes of this study, the diagnosis related group (DRG) was considered to be a reimbursement of 100% [6]. Medicare reimbursement served as a benchmark with reimbursement for those with commercial insurance, which reimburses 168% for Medicare severity-DRG (MS-DRG) [7].
Procedural revenue was calculated using the appropriate national averages for MS-DRG payment for specific spinal procedures [8]. In order to calculate and appropriately weighted average DRG estimate, national data involving the incidence of the utilization of spine specific DRG codes was performed. The average DRG for thoracolumbar procedures was calculated at $25,057 for Medicare and $42,096 for commercial insurance for the hospital system. This incorporated both disease pathology as well as medical comorbidities. The average weighted DRG was calculated from nationally available data illustrating the reimbursement for MS-DRG and the national distribution of MS-DRG incidence between different neurosurgical disease bundles [8].
Operating room (OR) cost was calculated at $18 per minute which is a hospital-generated specific institutional number combining both fixed and variable cost. Per hospital administration calculation, this was 50% fixed and 50% variable cost. This falls within the national estimates for OR cost of $15–20 [9]. Time savings with utilization of robotic technology was estimated from previously published data [10].
3. Clinical Data Points
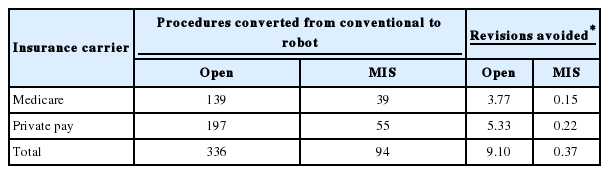
Reoperation rates secondary to pedicle screw misplacement were estimated from nationally available data. This included a 2.7% revision rate for open cases and a 0.4% revision rate for minimally invasive spine surgery (MIS) cases. The incidence of reoperation due to pedicle screw misplacement for surgeries with robotic assistance was constructed as 0% [11]. Average cost of the revision was adapted from earlier published data from Watkins et al. 2010 in the Cost Effectiveness of Image-Guided Spine Surgery in the The Open Orthopedics Journal as $23,762 for Medicare and $39,920 for private payors [12]. Previously presented institution specific infections rates for the comparison of open instrumentation (4.6%) and MIS instrumentation procedures (0%) were used [13]. Average cost of a spinal infection was adapted from earlier published data from McGirt et al. as $15,817 [14,15]. Difference in the average of length of stay (LOS) between MIS and open spinal procedures was estimated as 2.8 days from previously established systematic review of the literature [16]. Cost per hospital floor bed per day was noted as $1,799. This was adapted from Kaiser Family Foundation for data specific to Louisiana nonprofit hospitals [17].
The procedural impact of robotic technology was calculated. Cases previously performed underwent an independent review by a nonprimary surgeon and final review by the primary surgeon for the applicability of transition to minimally invasive technology by use of robotic technology. This was estimated to be at approximately 10% of cases in which the minimally invasive approach was abandoned due to the specific procedural challenges of percutaneous instrumentation.
Finally, based on previous historical integration of new image guided navigation technology in spine surgery at our institution, the utilization of the robot on otherwise open cases was estimated at 75%.
4. DRG and Cost
DRG was used as a surrogate for cost. Medicare adapted a prospective payment system in 1982. DRG represented a bundled prospective payment per each encounter with modifiers for patient severity. The vital distinction is that DRG represents how much a hospital is paid for the treatment of a certain pathology for a certain patient. It attempts to link the costs the hospital incurs for treatment of a certain patient classification. It does not necessarily represent the true cost the hospital requires for treatment of the patient [18].
As such, the concept of estimating cost on the basis of weighted DRG proposal has been established [19]. There exists for forward thinking value based cost algorithms [20]. However, on the current basis of volume based payment the DRG is used here.
RESULTS
During the academic year 557 patients underwent elective thoracolumbar fusion out of a total of 1985 elective cases (28%). Fifty-eight of 557 (10.4%) were minimally invasive fusions in the thoracic or lumbar spine. Independent review noted that approximately another 10% of cases would be candidates for minimally invasive fusion. Data regarding specific payor mix components is noted in Table 1. This included 63.0% of patients being governmental insurance based. The weighted average DRG is calculated at $25,057 for Medicare and $42,096 for commercial insurance. Time savings was averaged at 3.4 minutes per average 1-level MIS procedure. A total of 50 patients were determined to be candidates for conversion from open to MIS procedure due to robotic technology. Under the appropriate payor mix algorithm, this would have resulted in 31 new Medicare MIS cases and 18 new private payor MIS cases. Table 2 illustrates the procedural impact of robotic technology. Table 3 illustrates the impact on revision surgery.
Overall, the estimated cost of infections was substantial with an additional $363,116 of cost. Adaption of robotic surgery could lower that cost to $326,804 with a savings of $36,312. The economic impact of infection reduction can found in Table 4. Robotic surgery resulted in an immediate conservative estimate cost savings of $608,546. This is summarized in Table 5.
DISCUSSION
To our knowledge, our study is the first to critically analyze the economic impact of robotic technology on a practicing healthcare system as it relates to spinal surgery. Specifically, improved screw accuracy due to computer-assisted navigation inherent to robotic surgery results in decreased revision surgeries, and it allows for the conversion of more cases to MIS. This results in fewer postoperative infections and reduced LOS in the hospital. Furthermore, robotic surgery can also reduce operative time.
Based on these assumptions, which have been documented in literature, we created a model in which robotic-assisted spine surgery was utilized to treat those patients who were amenable for this technology. Robotic surgery led to savings of $608,546 over a 1-year period, considering a case-load of 557 cases. Even though the initial cost of acquisition of the system and maintenance charges need to be considered, it can be hypothesized that robotic-assisted spine surgery is likely to result in cost benefits over the long term. This is in addition to mitigating much of the harmful radiation exposure in MIS to which the patient, surgeon, and ancillary OR staff are subjected.
1. Robotics in Surgery
Advances in robotic surgery have been taking place since the 1980’s. Research by private and governmental entities ultimately resulted the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA). Approved by the U.S. Food and Drug Administration (FDA) in 2000, the da Vinci robot has been shown to offer safety, efficiency, cost reduction, and even immunological benefits for surgical procedures [21]. By 2015, over 700,000 surgeries had been performed with the da Vinci System, demonstrating widespread acceptance and utilization of surgical robotics.
Of particular interest at this moment in healthcare history is the cost-savings associated with the use of surgical robots. While the initial capital investment is large (current pricing on a single da Vinci Robot is approximately $1.5 to 2 million), the benefit in patient volume and improved outcomes can justify the utilization of these systems. Cost assessment of robotics in different surgical specialties is a recent and on-going endeavor and present evidence suggests that robotic surgery, under specific conditions, has the potential to become cost-effective [22]. Large number of cases, presence of industry competition, and multidisciplinary team utilization are some of the factors that could make robotic-assisted surgery make more reasonable and cost-effective.
2. Robotics in Spine Surgery
Spine surgery has been revolutionized due to rapid technological advancements taking place in the last two decades. The goals of these improvements have been to achieve of a high degree of precision, minimize risks of damage to neurovascular structures, facilitate surgeon access and OR dynamics, and diminish harmful exposure to ionizing radiation in patients and the operative team [1]. Because the risks associated with spine surgery are plentiful, and limiting complications is imperative, implementing a robot-assisted technique has the potential to address many concerns associated with conventional surgery. At the same time, cost-effectiveness of this technology needs to be carefully reviewed in the context of current value-based care models with emphasis on judicious allocation of healthcare dollars [23]. This is of particular importance in the multibillion dollar spine surgery industry.
One of the pioneers, and by far the most studied of these robotic-assisted surgical devices for spine surgery, is the Spine-Assist/Renaissance robot (MAZOR Robotics Inc., Orlando, FL, USA) [23]. Several clinical studies have analyzed the translational accuracy and efficacy of the Spine-Assist robot (MAZOR Robotics Inc.) in vivo. Roser et al. [24], found a 99% accuracy rate of lumbosacral pedicle instrumentation using the Spine-Assist robot compared to 98% utilizing fluoroscopy guided, and 92% using traditional navigation techniques. Schizas et al., reported a 95% accuracy rate vs. 92% for robot-assisted vs. fluoroscopicguided lumbosacral pedicle screw instrumentation, and Kantelhardt et al. similarly showed 95% accuracy vs. 92% using Spine-Assist and conventional fluoroscopy, respectively [25,26]. The only study to date demonstrating a reduced accuracy of screw placement came from Ringel et al. [27] in a randomized controlled trials (RCT) that demonstrated a significantly reduced accuracy rate of lumbosacral pedicle screw instrumentation with the SpineAssist robot (85%) compared to fluoroscopic-guided screws (93%).
In a new application of an existing surgical robot, the ROSA robot by Medtech (Medtech S.A., Montpellier, France), may help mitigate concerns of fixation strength to bony anatomy like those encountered by Ringel et al. [23,27] In their preliminary study on the novel application of the ROSA robot for spinal surgery, Lonjon et al. [28], reported an accuracy rate of 97.3% for pedicle screw instrumentation compared to 92% in the free-hand (FH) group.
The Da Vinci Surgical System (Intuitive Surgical Inc.) has been utilized for laparoscopic anterior lumbar interbody fusion with promising results; however, its use is not FDA-approved for actual spinal instrumentation and more exploration is necessary to validate its utility in spine surgery [23].
3. Conversion to Minimally Invasive Technique
Our series estimated a 10% conversion rate to minimally invasive technology. MIS of the spine is increasing in popularity and widespread practice. The tubular systems, which were first used for laminectomy and discectomy, have now been adapted to perform multilevel fusions. In 2010, approximately one sixth of all instrumented spine procedures were performed by MIS techniques. By 2016, that number had increased to almost one third. It is projected that half of all spinal instrumentation surgeries will be performed MIS by 2020 [29]. A recently published meta-analysis of 602 patients across 10 studies demonstrates decreased LOS, reduced intraoperative blood loss, fewer postoperative complications, and similar long-term outcomes based on validated outcome measures [30]. A follow-up paper that studied 345 patients enrolled in a prospective, national spine registry, treated by both orthopedic and neurological surgeons at 11 institutions across the United States, demonstrated no difference with regard to 12-month patient reported outcomes, LOS, and 90-day return to work [31]. Parker et al. [32] also suggested that, at 2 years, overall cost-per-patient was reduced.
4. Quantifying the Intraoperative Benefits of Robotic Assistance in Spine Surgery
Across all studies, the same important metrics are analyzed: accuracy of hardware placement, radiation exposure, learning curves for surgeons, and patient outcomes. Of these, the major limiting factors for the application of MIS techniques include surgeon learning curves and physician radiation exposure [23]. The following sections discuss the current research into each of these metrics.
5. Radiation Exposure
Robotic surgery can help reduce radiation exposure. The standard metric for this is the fluoroscopy time (FT), and some papers analyze the actual doses in millisieverts (mSv). The recent paper by Joseph et al. [1] described 10 recent studies that analyzed this metric. Five of these studies compared FH to robotic placement, and all 5 found the FT to be similar or decreased in the robotic group as compared to FH group. Roser et al. [24] also included the standard navigation group and found this group to have the overall lowest FT. Sensakovic et al. [33] developed a low-dose computed tomography (CT) protocol for their patients for pre-operative imaging with significant reductions in mSv per patient. From this data, it seems likely that FT and mSv will both be reduced. However, this seems directly related to surgeon technique and the learning curve of robotic surgery.
Vaccaro et al. [10], in their comparative analysis of 40 pedicle screw insertions in each arm (conventional MIS screw placement vs. robotic-assisted screw placement), noted that the conventional MIS placement required 108 intraoperative fluoroscopic images, while the robotic group resulted in no radiographic images. Thus, surgeons and the OR staff were subjected to zero radiation in the robotic-assisted screw placement. With both improved screw accuracy and decreased radiation exposure, it remains plausible to see an increase in MIS techniques secondary to robotic technology.
6. Pedicle Screw Insertion Accuracy and Revision Rates
Verma et al. [34], conducted the first of several meta-analyses on the topic of pedicle screw accuracy and safety of implantation with computer-assisted navigation (CAN), and reviewed 23 studies evaluating 5,992 pedicle screws. They found a significantly higher rate of accuracy utilizing CAN; however, though the rate of neurological injury favored navigation, the group failed to demonstrate statistical significance. Gelalis et al. [35], reviewed 26 studies and 6,617 pedicle screws inserted FH, with fluoroscopic guidance or with CAN. While they found no significance between the fluoroscopic and navigation assisted methods, both exhibited statistically superior accuracy as compared to FH technique. However, their analysis failed to demonstrate a significantly lower rate of screw revision or total reoperation rates and no difference in neurological injury. In the most recent meta-analysis, Shin et al. [36] used stringent exclusion criteria, including exclusion of noncomparative studies using differing platforms for navigation guidance, studies without explicit complication data, and studies examining cervical pedicle screws resulting in only 12 studies evaluating 4,953 screws. They demonstrated significantly increased screw accuracy with CAN. Perhaps due to their strict inclusion criteria, they also reported a significantly lower rate of screw-related complications in the navigation cohort as compared to FH.
A recent systematic literature review identified 25 studies that characterized the role of robots in spinal surgery; 18 retrospective studies, 7 prospective studies, and 4 RCTs were identified [1]. Twenty-two of these studies evaluated the accuracy of instrumentation placement. Many of these studies relied on the postoperative, CT-based Gertzbein and Robbins system (GRS) to classify pedicle screw accuracy [37]. According to the GRS, screws completely within the pedicle are considered grade A; a breach of <2 mm is grade B; a breach of 2 to <4 mm is grade C; a breach of 4 to <6 mm is grade D; and a breach of >6 mm is grade E. In this system, both grades A and B are deemed acceptable for screw placement [38]. Across all studies, robot-assisted pedicle screw placement was highly accurate. Interestingly, the very first RCT by Ringel et al. [25] found a significantly lower rate of accuracy using the robot (GRS grade A or B : 85% in the robotic group vs. 93% in the FH group). Two follow-up RCTs by Kim et al. [39] and Hyun et al. [40] found similar accuracy between robotic placement and freehand placement, but both studies demonstrated no proximal facet disruption using the robot, whereas FH technique saw up to 15.9% violation. All other studies in this group saw an increase in accuracy using robotic assistance. Interestingly, the RCT conducted by Roser et al. [24] found both robotic and FH accuracy superior to standard navigated technique. Limitations of these studies were usually related to small sample sizes, with most studies having less than 100 patients enrolled. There have been newer studies published this year with larger sample sizes. Molliqaj et al. [37] described a cohort of 169 patients with 880 screws places (439 in the robot group vs. 441 in the freehand group). They found that perfectly placed screws (GRS grade A) were found in 83.4% of robotically placed screws versus 76% in the freehand technique. A recent publication from Kantelhardt’s group retrospectively analyzed implantation of 2,067 robotically assisted pedicle screws in 406 patients [41]. They classified 1,799 screws (96.9%) as having an acceptable or good position, whereas 38 screws (2%) showed deviations of 3–6 mm and 20 screws (1.1%) had deviations >6 mm. They quoted a FH accuracy rate ranging from 65%–94% based on literature review. With the exception of a single study, most studies demonstrate improved accuracy and lower revision rates among robotically placed pedicle screws. Clearly, improved pedicle screw accuracy improves clinical outcomes and would reduce overall cost.
7. OR Time
OR time is a valuable resource of any surgeon or hospital system. Expert spinal surgeons in both neurosurgery and orthopedic surgery performed a cadaveric study illustrating the accuracy and timing of robot-assisted pedicle screw placement [10]. Eight pedicle screws were inserted by each surgeon from L2–5 with one side being traditional MIS fluoroscopic guided and the other being robotic guided using a leading spinal robot. Record time for both techniques included the technical exercise of screw placement (incision, drill, tap, and insertion) as well as equipment set-up. Conventional MIS screw placement was 36 minutes while robotic assistance was 32.6 minutes for four screws. This was inclusive of the 18.3 minutes required for the set-up of the robot. The MIS pedicle screw took 7.6 minutes for physician insertion. The robotic screw took 3.6 minutes for physical insertion independent of set-up time. Zero breaches were seen in the robotic arm. A 17.5% breach rate (7 of 40) was seen in the traditional MIS C-arm group. On average, 108 radiographs were used for the placement of traditional MIS screws [10].
It is worth noting, as with implementation of any new technology, there is a learning curve associated with robotic-assisted spine surgery. Joseph et al. [1], analyzed the learning process in 8 studies. Across all 8 studies, a notable reduction in per-screw time and FT was noted. Devito et al. [42], noted an improvement in accuracy from 83.7% to 90.8% from early to later cases, as well as a minutes-per-level decrease from 13.5 to 10.6. Kim et al. [39], and Hyun et al. [40], also noted a surprisingly similar rate of improvement. Thus, over time OR time is likely improve with any new technology including robotic-assisted surgery.
8. Limitations
As within any modeling algorithm, discrepancies in factual minutia are apparent. Most glaringly, the operative time-savings was grossly applied from MIS pedicle screw placement comparison across only 10 different surgeons for specific MIS cases. This however is the only available data-set involving time measurements of the robotic time for placement of pedicle screws. It provides some insight into the utilization of time in the operative theatre. Moreover, the importance of the data focuses on the application of minimally invasive technology to a new cohort of patients. This, by previous study, has been shown to reduce infection, reduce LOS, and reduces revision surgery.
Beyond the scope of this paper is the real and significant cost of robotic surgery as a capital item purchase. The cost of robotic technology can be taken directly from industry disclosed information. This includes both fixed costs of capital and service charge as well as variable costs per case. Cost of spinal instrumentation to the hospital system can also vary between healthcare enterprises. This however is not in the scope of societal cost savings as differing health systems can use robotic technology as a marketing tool to capture patient and payor mix in a regional fashion. The assumed model is used to look specifically at the possibility of hospital-based societal cost savings in the delivery of health care. The purpose is to illustrate how robotic technology, not a specific robotic device, can generate a cost savings through real patient and simulated cost data at a specific institution. Different robotic devices, and different generations of robotic devices, will have variable start-up costs. This is a variable can be manipulated at the hospital level based on things like negotiation and scale.
Further limitations of this analysis include lack of inferential statistics, and biases relating to descriptive analysis. Additionally, the cost estimates are derived from previously published literature, and therefore may not be reflective of the current era given the lack of inflation adjustments.
Further investigation is sincerely warranted regarding the application and cost effectiveness of robotic imaging guidance in specific clinical procedures. This includes lateral spine surgery and deformity surgery. Beyond modeling, direct clinical observation of the application of robotic technology is warranted.
CONCLUSION
The application of robotic surgery can be a cost-effective emerging technology resulting in decreasing revision surgery, decreasing infection rates, reducing LOS, and shortening operative time. Modeling at a major academic center resulted in an estimated $608,546 of savings in one academic year. Future investigations are essential in understanding the impact of robotic technology on specific procedures.
Notes
The authors have nothing to disclose.
Acknowledgements
The authors wish to thank Lance Butler Imaging, Navigation & Robotics at Globus Medical Incorporated (Audubon, PA) for his assistance in the acquisition of industry specific data.