Does Preservation of Ligamentum Flavum in Percutaneous Endoscopic Lumbar Interlaminar Discectomy Improve Clinical Outcomes?
Article information
Abstract
Objective
Ligamentum flavum (LF) is an important anatomical structure for prevention of postoperative adhesions, but the opening of LF is necessary for percutaneous endoscopic lumbar interlaminar discectomy (PEID). Although the defect in LF is small with conventional PEID, the defect could be minimized with LF splitting technique. The objective of this study was to compare clinical outcomes of PEID with opening of LF versus splitting of LF.
Methods
A retrospective study was performed for patients underwent PEID for L5–S1. PEID with the opening of LF (open-group) was performed for 55 patients and with splitting of LF (split-group) was performed for 34 patients. The defect of LF in Open-group was 3–5 mm, but the defect was negligible in split-group because the split LF was reapproximated by its elasticity. Clinical outcomes were evaluated with Korean version of the Oswestry Disability Index (K-ODI) and visual analogue pain scores for back (VASB) and leg (VASL). The changes of clinical outcomes during postoperative 24 months between groups were evaluated with linear mixed-effects model.
Results
The clinical outcomes were similar between groups for K-ODI (p=0.98), VASB (p=0.52), and VASL (p=0.59). Each outcome demonstrated significant improvement from preoperative baseline throughout the postoperative 24 months (p<0.05). Complications included recurrence in 4 patients and dural tear in 1 in open-group (9.1%), and residual disc herniation in 2 patients and transient weakness in 1 in split-group (8.8%).
Conclusion
Splitting versus opening LF in PEID may be left to the surgeon’s discretion. The potential risks and benefits of LF handling should be considered when performing this surgical technique in PEID.
INTRODUCTION
Surgical treatment is recommended for medically intractable lumbar disc herniation (LDH) and open discectomy is a standard surgical technique. More recently, minimally invasive surgery (MIS) has been popularized and percutaneous endoscopic lumbar discectomy (PELD) is one such MIS surgical technique [1,2]. A prospective study showed that the clinical outcomes of PELD is not inferior to conventional open discectomy for LDH [3]. The distinct advantages of PELD are decreased bony and soft tissue trauma, as well as reduced postoperative scar formation [3]. Less trauma may improve clinical outcomes and reduce postoperative adhesions [4,5]. Two approaches are utilized for PELD: transforaminal (PETD) and interlaminar approaches (PEID). Ligamentum flavum (LF) is an important anatomical structure for prevention of postoperative adhesions, but the opening of LF is necessary for PEID [3,6,7]. Although the defect in LF is small with conventional PEID, the defect could be minimized with LF splitting technique [8-11]. Preservation of LF may lead to better clinical outcomes [4,5,12,13]. However, the advantage of LF preservation may not be clinically significant amid the surgical complexity of PEID. The objective of this study was to compare clinical outcomes of PEID with opening of LF versus splitting of LF in a retrospective study design.
MATERIALS AND METHODS
1. Patient Selection
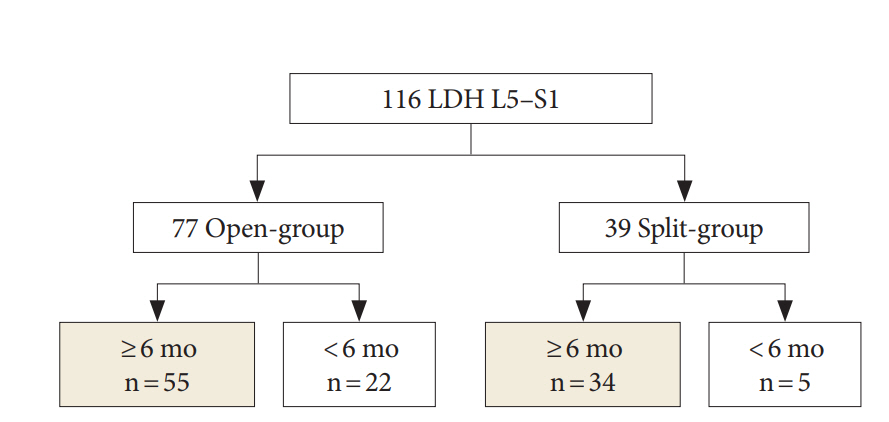
After approval of Institutional Review Board (IRB) at the Seoul National University Hospital for this study (H-1611-015-803), medical records were retrospectively reviewed. This study was a retrospective study and all patients' personal information were deleted. Therefore, informed consent from patients were wavered by the IRB. This study included patients who underwent PEID for L5–S1 from April 2009 to April 2016. PEID with splitting of LF was performed from April 2009 to October 2012. PEID with the opening of LF was performed from November 2012 to April 2016. Although the surgical indication was same between 2 surgical techniques, steps for introduction of the working cannula and endoscope were less complex with opening of LF than with splitting of LF at our teaching hospital. Therefore, splitting of LF was switched to the opening of LF in November 2012. The present study included patients (1) aged between 18–60 years, (2) LDH at L5–S1, (3) pain recalcitrant to medical treatment for more than 6 weeks, (4) without instability at any lumbar spinal segment, and follow-up more than 6 months. This study excluded patients with (1) neurological disease such as Parkinson disease or myelopathy, (2) trauma related LDH, (3) previous lumbar spinal surgery, (4) concomitant cancer, and (5) combined lumbar spinal stenosis. In total, 89 patients selected, and LF was opened in 55 patients (open-group) and was split in 34 patients (split-group) (Fig. 1).
2. Surgical Procedure and Postoperative Management
All operations were performed under general anesthesia. PEID was performed for all patients with LDH at L5–S1 [14]. Patient positioning and surgical instruments were same in both groups. A skin incision of 8 mm was made at the center of L5–S1 interlaminar space and a dilator was inserted until it contacted with LF under fluoroscopic guidance. After LF identification and clearing out soft tissue around LF under endoscopic visualization (Vertebris system; Richard Wolf, Knittlingen, Germany), LF was opened in about 3–5 mm with endoscopic scissors and forceps and the opening was enlarged with working tube in opengroup. In the split-group, LF was split with blunt dissector and scissors, and the small opening at the LF was enlarged with working tube (Fig. 2) [11,15,16]. After identification of epidural fat and neural tissue, the spinal endoscope was advanced into the spinal canal. Discectomy was performed at either the shoulder or axilla of the traversing nerve root in both groups according to the location of herniated disc material. After confirmation of sufficient nerve root decompression and hemostasis, the endoscope and working tube were removed. The defect of LF in opengroup was 3–5 mm, but the defect was negligible in split-group because the split LF was reapproximated by its elasticity. A skin was closed with 3-0 nylon. Patients were encouraged to ambulate from the day of surgery and discharged the next day. Patients were scheduled to visit the clinic at 1, 3, 6, and 12 months after the operation and yearly thereafter.
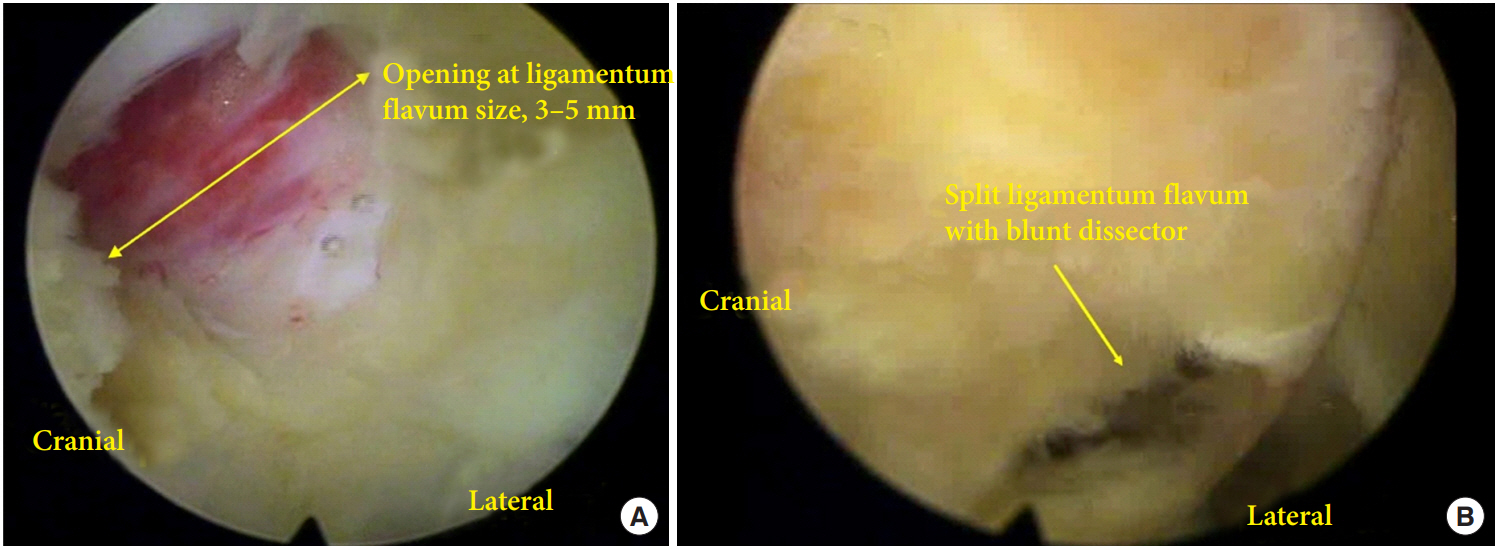
The size of opening in ligamentum flavum (LF) between opening of LF and splitting of LF. (A) The size of opening of LF is usually less than 5 mm and this opening is enlarged during insertion of working tube. (B) The opening at LF is made with blunt dissector and scissor at the junction of ligamentum and facet joint. The small opening is enlarged during insertion of working tube.
3. Clinical Outcomes
Clinical outcomes were evaluated with patient-reported outcomes questionnaires, which included the Korean version of the Oswestry Disability Index (K-ODI, ×/45) [17] and visual analogue pain scores for the back (VASB, ×/10) and leg (VASL, ×/10) [15,16,18]. All patients completed the questionnaires at each preoperative and postoperative clinic visit [15,16,18]. In addition, patients were encouraged to follow up in the outpatient clinic any time they experienced intractable pain [15,16]. Magnetic resonance imaging was not routinely performed in patients without recurrent symptoms. Median follow-up period was 47.5 months (range, 11–76 months) for split-group and 24 months (range, 6–81 months) for open-group.
4. Statistical Analysis
Clinical outcomes were assessed with K-ODI, VASB, and VASL. The changes of clinical outcomes during postoperative 24 months between groups were evaluated with linear mixed-effects model. A model that contains both fixed and random effects is called a mixed model. The fixed effects included group, time, the interaction between group and time, and factors with p-values less than 0.1 for comparison of baseline characteristics present in Table 1. The random effect was the subjects. A post hoc analysis was planned using a stepdown Bonferroni method with the following significant interaction effects: the time trend in each group, testing the changes between preoperative and post-operative (1, 3, 6, 12, and 24 months) time points for the groups that showed significant temporal trends, and group differences at each time point [15,16]. The considered factors were age, sex, overall duration of symptoms, body mass index (BMI; overweight, BMI >25 kg/m2), smoking status (yes vs. no), side of symptom, disc type (bulging/protrusion vs. extrusion/sequestration) [19], high grade migration [20,21], high canal compromise of disc material (>50% of spinal canal) [21,22], Pfirrmann grade at the index level (grades 1–3 vs. 4–5) [23,24], and the presence of Modic change (yes vs. no) [25]. Comparisons between continuous and noncontinuous values were performed using Mann-Whitney U-tests (or t-tests) and chi-square tests, respectively. All statistical analyses were performed using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA). Statistical significance was defined as p<0.05 (2-sided).
RESULTS
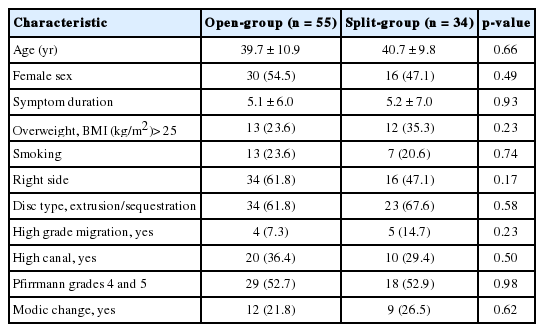
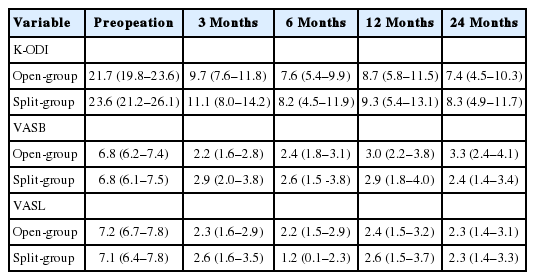
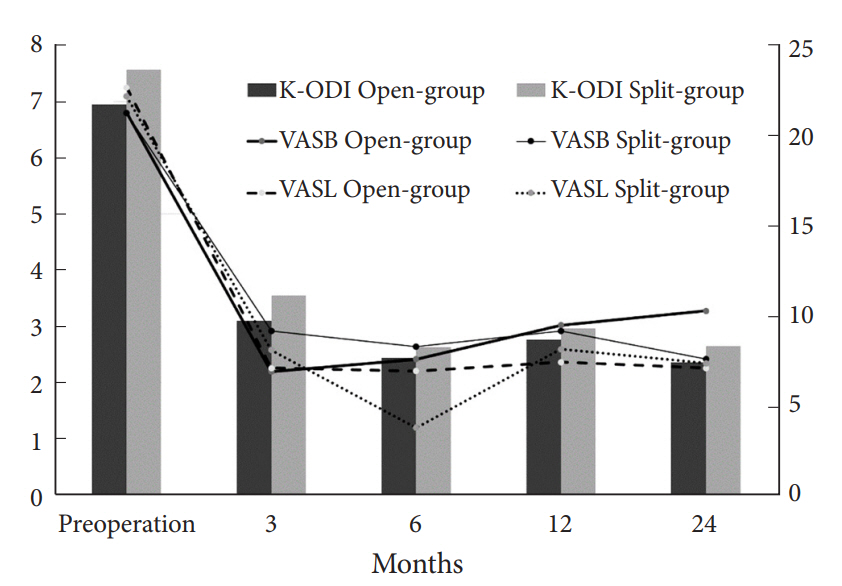
The baseline characteristics of patients are described in Table 1. There were no significant differences between groups regarding age, sex, duration of symptoms, the proportion of BMI>25 kg/m2, smoker, side of symptom, disc type, high canal compromise, severe disc degeneration and Modic change (p>0.1). Therefore, those factors were not included in the fixed effects of LMM. The clinical outcomes were similar during entire follow-up period between groups for K-ODI (p=0.98), VASB (p=0.52), and VASL (p=0.59). The difference of mean value between groups did not exceed mean clinically significant difference of K-ODI, VASB, and VASL, which were 6.4, 1.2, and 1.6, respectively [26]. Each outcome demonstrated significant improvement from preoperative baseline at 1 month after surgery and the improvement was maintained during the first 24 months (p<0.05) (Table 2, Fig. 3). Complications included recurrence with reoperation at 1, 24, and 39 months in 3 patients, recurrence without reoperation in 1 patient, and dural tear in 1 patient in open-group (9.1%), and asymptomatic residual disc material in 2 patients and transient weakness of ankle plantar flexion in 1 patient in split-group (8.8%). The incidence of complications was not different between groups (p=0.97).
DISCUSSION
The primary objective of this study was to compare clinical outcomes between LF open and splitting techniques. The results showed that the clinical outcomes were not different between groups during the follow-up period.
1. The LF in Discectomy
The number of spine surgeries has been increasing with the development of diagnostic modalities, improved surgical techniques, increased options for minimally invasive surgeries and increased number of patients with spinal disease [27]. LF is an important anatomical barrier for prevention of postoperative scar tissue [5,12,13,28,29] and mechanical stabilization of the lumbar segment [30,31]. Because most patients with LDH do not have spinal instability, preservation of LF may be beneficial considering long-term stability of the lumbar spinal segment and prevention of postoperative adhesions. In this regard, LF preservation has been emphasized in open discectomy [5,13,32]. Surgical techniques have been improving and various technical modifications have been proposed regarding preservation of LF in PEID [3,8,10,11]. However, there is controversy on the clinical significance of LF splitting technique given its technical complexity. The opening size of the opening at LF is merely less than 5 mm and the clinical significance has been questionable [6]. Moreover, the opening of LF may be a less complex surgical procedure than splitting of LF in education and in practice. The authors switched to LF opening because of difficulty in education and questionable clinical significance with LF splitting. The present results showed that clinical outcomes of LF splitting were not better than those of conventional opening of LF. The influence of adhesion through a small defect in LF may not be significant enough to make clinical differences. Nonetheless, the outcomes may be different with a long-term follow-up and the adhesion may be problematic when revision surgery is necessary. Therefore, preservation of LF may still be a desirable surgical technique, if possible. However, the occurrence of insufficient removal of ruptured disc materials in split-group showed that blind spots and decreased visualization may be a shortcoming of the LF splitting technique. The blind spot created due to split LF was might have caused missed disc fragment. Although transient weakness occurred in 1 patient of split-group, the nerve root may be injured during splitting of LF. On the other hand, reoperations and dural tear only occurred in open-group in this study. The potential advantages and disadvantages of each method should be considered in selecting the surgical technique for LF handling. However, this study was underpowered due to small number of patients, and a study with larger number of patients would be beneficial in further study of LF handling.
2. Limitations and Meaning of This Study
There were several limitations in this study. First, the present result may not be generalizable. This study was a retrospective design and had an inherent selection bias between the 2 groups. The number of subjects was too small to draw a conclusive answer for the issue of reoperation, dural tear, and residual disc. The present results were obtained from a single institution. A further study with a large number of subjects is required to address the lack of statistical power, and a multicenter study would help make the results more generalizable [33]. Second, the opening of LF and splitting LF was applied before and after October 2012, respectively, and adjusting the period effect was impossible. However, the period effect may be negligible since the differences in clinical outcomes between the 2 groups were less than established clinically significant difference values. Third, the purpose of this study was to compare the clinical outcomes regarding preservation of LF in PEID alone and only patients with L5–S1 LDH were included. The questionable significance of LF preservation in PEID had not been studied, and this study may incur further study to find scenarios with clinical significance of preserving LF.
CONCLUSION
The selection of splitting LF in PEID may be up to the surgeon’s discretion. The potential risks and benefits of LF handling should be considered in selecting the surgical technique.
Notes
The corresponding author (CHK) is a consultant of RIWOspine GmbH. The other authors have nothing to disclose.
Acknowledgements
A grant from the Korea Health Technology R&D Project supported this work through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HC15C1320).