INTRODUCTION TO DEGENERATIVE CERVICAL MYELOPATHY: A NEW OVERARCHING CONCEPT
Degenerative cervical myelopathy (DCM) is the most common etiology of spinal cord dysfunction among adults globally [1]. As an overarching clinicopathological entity, DCM encompasses a host of degenerative conditions of the cervical spinal column, including osteoarthritic degeneration (i.e., spondylosis) and ligamentous aberrations (i.e., ossification of the posterior longitudinal ligament [OPLL], ossification of the ligamentum flavum), that culminate in chronic compression of the cervical spinal cord, neural tissue destruction, and ultimately clinical loss of functional ability [2,3]. Even though cervical spondylotic myelopathy (CSM) and myelopathy secondary to OPLL have historically been segregated, these entities are unified under the umbrella of DCM. Given the limited potential of the spinal cord for repair, expeditious diagnosis and treatment of DCM are critical, so as to reduce the risk of permanent disability. With the continued aging of the global population, DCM has become an important public health priority. In fact, 3 of the top 100 national priorities for comparative effectiveness research identified by the Institute of Medicine are related to DCM [4]. The current article aims to provide a concise and widely accessible review of the latest advances and future directions in the treatment of DCM.
CURRENT CONCEPTS ON THE PATHOBIOLOGY OF DCM
Study of the pathobiology of DCM has been limited in the past owing to lack of a robust animal model [2,5]. However, the recent development of animal models that recreate the progressive spinal cord compression seen in humans have led to significant advances in our understanding of the pathobiological processes underpinning DCM, including ischemia, neuroinflammation, and apoptosis [5-7].
Both regional and local spinal cord perfusion are compromised in DCM [8]. At the macrovascular level, degenerative changes to the cervical spinal column compress upon, and narrow the lumen of, the major feeding arteries of the spinal cord (i.e., vertebral arteries, anterior spinal artery) [9,10]. At the microvascular level, compression and deformation of the spinal cord leads to stretching, flattening, and eventual loss of penetrating branches of the lateral pial arterial plexus [9,11]. The blood-spinal cord barrier (BSCB) is disrupted owing to loss and dysfunction of endothelial cells, which is further potentiated by ischemia [5,11]. Disruption of the BSCB in DCM may be mediated by matrix metalloproteinase-9 [12]. With this disruption of the BSCB, there is an influx of inflammatory cells into the spinal cord parenchyma from the peripheral circulation; this initiates an inflammatory cascade characterized by activation of microglia and recruitment of macrophages [11,13,14]. Ischemia, BSCB disruption, and neuroinflammation produce activation of apoptotic pathways resulting in progressive neuronal and oligodendroglial cell death [11,15,16]. This apoptosis may be mediated by Fas [13,15], tumor necrosis factor-α [17], and mitogen-activated protein kinase [18] pathways.
The role of glutamate excitotoxicity in DCM is akin to traumatic spinal cord injury. Specifically, there is an influx of Na+ owing to activation of neuronal voltage-gated Na+ channels [19]. This leads to cytotoxic edema and an influx of Ca2+ through the Na+-Ca2+ exchange pump [20,21]. This, in turn, triggers the release of glutamate into the extracellular space, causing an increase in local cell death through excitotoxic mechanisms [22,23].
ADVANCES IN CLINICAL AND IMAGING ASSESSMENT OF DCM
1. Ancillary Clinical Tests
The modified Japanese Orthopaedic Association (mJOA) remains the ‘gold standard’ for assessing patients with DCM [24]. Nonetheless, the mJOA is an insensitive scale with only modest inter-rater and intrarater reliability; the reported minimum detectable change is 2 points [25,26]. The Nurick grade, likewise, exhibits low sensitivity and poor responsiveness [27]. Additional assessment methods include the 30-m walk test, QuickDASH, Berg Balance Scale, Graded Redefined Assessment of Strength Sensibility and Prehension Myelopathy (GRASSP-M), Grip Dynamometer, and GAITRite Analysis [28]. Indeed, there is a need for quantitative, objective assessment measures in the setting of DCM, both as research tools, but perhaps more importantly, as clinical instruments that may practically be applied at the bedside or clinic. This has spurred interest in the development of smartphone-based assessments that patients may self-administer at home, analogous to what has been done for Parkinson’s disease [29].
2. Biomarkers
The possibility of using laboratory tests, electrophysiology (EP) examinations, or imaging data as biomarkers that improve diagnostic accuracy, quantify the severity of disease, and/or offer prognostic information has sparked tremendous research interest. A number of studies have attempted to identify candidate serum or cerebrospinal fluid biomarkers, but these remain in the early stages of investigation [30]. One promising approach is to measure serum microRNAs, which reflect specific genes that are expressed during spinal cord compression, such as miR-21 for neuroinflammation, miR-34a for neuronal apoptosis, and miR-10a for ossified posterior longitudinal ligament [31,32]. Others have investigated novel EP techniques such as contact heat evoked potentials, demonstrating excellent diagnostic accuracy [33]. Signal changes on T2-weighted and T1-weighted magnetic resonance imaging (MRI) images have been shown to correlate with increasing disability in DCM, while T1-weighted signal change is a negative prognostic factor for postsurgical recovery [34].
3. Quantitative Microstructural MRI
More recently, advanced MRI techniques that interrogate specific aspects of microstructure such as axonal integrity, demyelination, and tract-specific atrophy have been used [35]. These modalities include diffusion tensor imaging, magnetization transfer, functional MRI, myelin water fraction, MR spectroscopy, and T2*-weighted imaging (T2*WI) [35-37]. Metrics derived from these modalities, including spinal cord morphometric measures (e.g., cross-sectional area), fractional anisotropy, magnetization transfer ratio, and T2*WI white matter-to-gray matter signal intensity ratio (WM/GM), have shown to be sensitive in detecting myelopathy progression and appear to provide more specific and accurate information about spinal cord tissue injury than conventional MRI [36,38,39]. To date, fractional anisotropy and T2*WI WM/GM have shown the strongest results as biomarkers of white matter injury [35,40]. However, the complex data that are produced by these methods requires robust fully automated image analysis and multivariate modeling, which has seen tremendous advances but remains a work in progress [41].
4. Machine Learning
With the movement toward personalized medicine approaches, and the simultaneous spurt in artificial intelligence, there has been an interest in applying machine learning algorithms to generate high-performance prediction models that may more accurately predict the prognosis of a patient with DCM [42]. Machine learning techniques, for example, have been applied in the setting of DCM to identify patients, particularly those with mild DCM, who may be good surgical candidates and respond favourably to surgical decompression [43,44].
LATEST ADVANCES AND FUTURE DIRECTIONS OF NONOPERATIVE TREATMENTS
In the context of DCM, the role of nonoperative management has been studied as a comparison to surgical management. The majority of these studies are retrospective case series with the exception of the Kadanka randomized control trial, that was a trial comparing the natural history of DCM versus surgical intervention, rather than directly comparing nonoperative management [45,46]. Studies comparing nonoperative treatments compared to the natural history of the disease do not exist [47]. The role of pharmacological interventions as an adjunct to surgery to maximize postoperative recovery has, however, become a topic of great interest.
1. Riluzole
Riluzole was originally conceived in the 1980s as an anticonvulsant, and is currently licensed by the U.S. Food and Drug Administration for the treatment of amyotrophic lateral sclerosis [48,49]. It is a sodium channel blocking agent, which in animal models of DCM has been shown to reduce glutamatergic excitotoxicity and improve functional outcomes [50-52]. Given this success in the animal model, a phase 3, multicenter, double-blinded, randomized control trial has been completed looking at the benefits of riluzole in outcomes of surgery in DCM (the CSM-PROTECT study - NCT01257828). Preliminary results of this study have been reported in conference proceedings, and have been reported to show no benefit above the net improvement in mJOA, Nurick and American Spinal Injuries Association scores seen with decompressive surgery [53]. Despite these results, there did appear to be a significant reduction in the postoperative neck and neuropathic pain that was sustained 6 and 12 months after surgery. Therefore, delineating the impact of riluzole on the outcomes of surgery for DCM is a future research priority.
2. Corticosteroids
In DCM animal models there is an established increase in the production of inflammatory cytokines within the spinal cord following decompressive surgery, which is sustained in delayed decompression [54]. In experimental studies this inflammatory response has been shown to result in impaired functional outcomes and diminished neural repair [51,54-57]. In animal trials, the addition of methylprednisolone to decompression for DCM demonstrated a reduced inflammatory response, enhanced neuronal preservation and accelerated locomotor recovery without changes to the peripheral immune cell populations [58]. While the use of corticosteroids has been studied clinically in traumatic spinal cord injury with some controversy [59-61], there are a lack of studies on the role of corticosteroids in DCM, the most common form of nontraumatic spinal cord injury. In addition to their role in neuroprotection from inflammatory cytokines, corticosteroids can also have other beneficial impacts. In patients undergoing anterior cervical discectomy and fusion (ACDF) perioperative dexamethasone administration results in reduced airway edema, improved swallowing function and reduced hospital stay but without affecting overall fusion rates [62]. Furthermore, corticosteroids can potentially reduce postoperative pain and reduce hospital stay [63,64].
3. Disc Regeneration
Cervical stenosis secondary to progressive degenerative disc disease (DDD) is often the initial insult behind the development of DCM [65]. Several advances have been made in animal models which make it possible to study DDD and the impact of interventions [66,67]. One avenue to halt disc degeneration is with therapeutic protein injections aimed at stimulating cell growth. These injections have been carried out in rabbit [68], rat [69,70], and sheep models [71] with some initial promising results, however, they have a short duration of the therapeutic effect and further investigation will require slower release carriers.
Gene therapy can also be used with the aim of changing the intradiscal gene expression to upregulate anabolic cascades and downregulate harmful physiological changes. Genes of interest include MMPs, TIMPs, LMP-1, and AD-Sox9 [72-76]. Several in vivo models have demonstrated successful therapeutic expression of these genes leading to delayed degeneration, however future success hinges on the development of nonviral vectors [77,78].
Cell therapy with the injection of stem cells can also be used to decelerate the degenerative process even in advanced DDD. Mesenchymal stem cells are currently the most common lineage used, and preliminary animal studies have shown promising results [79,80]. Cell therapy has also been the focus of several clinical trials in lumbar DDD that have shown improvement in postoperative pain and MRI findings [81-84]. These early experimental and clinical results on the use of cell therapy are promising and require further research and application to DCM.
4. Multimodality Pain Management
Adequate perioperative and postoperative pain control in spine surgery allows for faster recovery, improved patient satisfaction, and reduced complications [85]. Opioids are commonly used in the management of severe acute pain, but can be associated with severe complications, particularly in the aging population. As a result, multimodality regiments can be used to utilize the synergistic mechanisms of nonopioid agents and reduce opioid requirements. There is grade I evidence that the use of multimodality agents, given pre-emptively, including gabapentinoids, local anesthetics, acetaminophen and nonsteroidal antiinflammatory drugs (NSAIDs) result in reduced narcotic use and improved postoperative pain in spine surgery [86-88]. Consideration should be given to NSAIDs, once thought to significantly impair bony fusion, as they have now repeatedly been shown to be a safe and effective analgesic adjunct in spine surgery [86,89-92].
5. Anticonvulsants
Both Gabapentin and Pregabalin are routinely used in the treatment of neuropathic pain associated with DCM, based on their success in treating other forms of neuropathy [93,94]. Observational studies in cervical spondylosis have demonstrated a significant reduction in pain from baseline with pregabalin, however, vigilance is advised to monitor patients for adverse effects [95]. Further research is needed to justify the routine use of anticonvulsants for the management of neuropathic pain in the context of surgery for DCM.
LATEST ADVANCES AND FUTURE DIRECTIONS OF SURGICAL TREATMENTS
The ultimate aims of any surgical intervention for DCM are to provide adequate decompression of the neural elements and ensure mechanical stability. The decision making in order to achieve these goals safely, with the least morbidity and best long-term outcome, is difficult and is best tailored to individual cases and the surgeon’s abilities. Due to the heterogeneous nature of DCM, there exists a number of approaches and interventions that can be utilized including ACDF, cervical artificial disc (or ‘arthroplasty’ - CAD), anterior cervical corpectomy and fusion (ACCF), hybrid ACDF/ACCF procedures as well as posterior laminectomy, with or without posterior instrumented fusion, and laminoplasty techniques.
1. Anterior Versus Posterior
A number of considerations exist when attempting to decide which approach is optimal for DCM patients. Firstly, presentations such as focal single or 2-level disease from spondylitic disc degeneration in a younger patient will always lend themselves towards anterior management compared to older patients with multilevel stenosis that would be best served through a posterior approach [96,97]. However, amongst the patients who display equipoise between both approaches, attempts have been made to determine whether superiority exists (in complications or outcomes). A prospective observational multicenter AOSpine study in 264 patients demonstrated no significant differences in the rates of complications or the improvement seen in functional and quality of life outcomes between anterior or posterior groups [96]. A more recent study, based specifically on those patients undergoing 3–5 level surgery, from 245 patients in the Quality Outcome Database, provided a similar conclusion, and added that readmission and reoperation (within 1 year) were also equivalent [97]. A more robust, logistic regression model analysis of both CSM North America and CSM International combined datasets also proved equivalent outcomes and complication rates up to 2 years after surgery [98]. The CSM-S trial, a randomized control trial to assess anterior versus posterior decompression in equivalent patients in DCM, completed patient enrolment in 2018 and the results are expected soon after follow up is collected [99]. This should provide a more definitive insight, but the evidence to date suggests surgeon’s experience in choosing the correct approach provides equivalent outcomes and complications whether anterior or posterior [96-98].
2. Decisions in Surgical Management
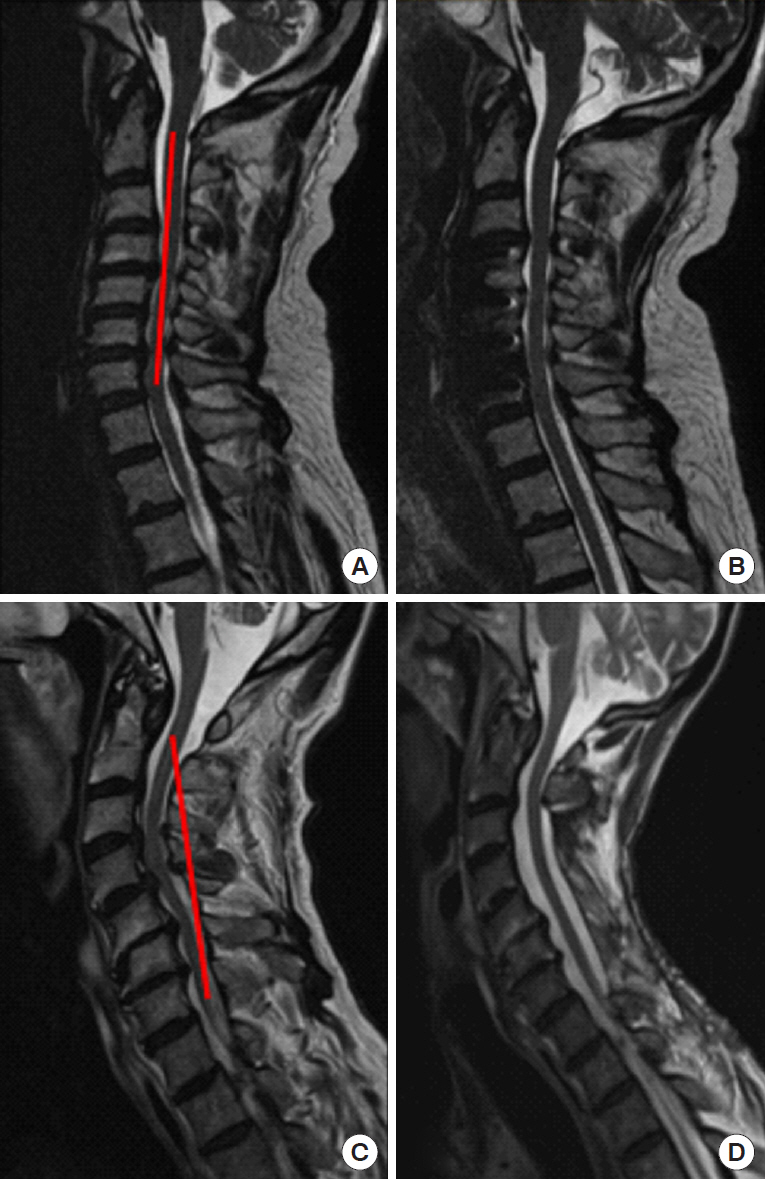
Preoperative consideration should be given to a number of factors, including age, comorbidities, cervical deformity, bone density, etc. that are proven to affect patient outcomes after DCM surgery [100]. Of these, the restoration of postoperative sagittal alignment is an important factor associated with postoperative outcomes [101]. The concept of the modified K-line and the minimum interval distance (at least 4 mm from the K-line to the anterior compressive elements) needs to be applied to every case, as failure to properly address cervical kyphosis is associated with higher risk of postoperative residual compression [100,102,103]. If the minimum interval distance is <4 mm, then an anterior approach or combined anterior/posterior approach should be strongly considered (Fig. 1), and is associated with producing better postoperative outcomes compared to posterior alone [104].
ACDF is the well-established mainstay to treat focal spondylitic cervical disease. With a predictable side effect profile and ability to treat multilevel disc disease, it has become a popular choice for day surgery intervention [105,106]. ACCF offers the ability to provide ventral decompression of retrovertebral disease or correction of kyphotic deformity with a cage or allograft construct to achieve fusion, through the same anterior approach and with a similar side effect profile [107,108]. Although no direct comparison or prospective trial exists, systematic reviews have shown multilevel ACDF is favorable above multilevel ACCF, in terms of outcome measures and sagittal alignment [109]. Hybrid constructs, using a combination of ACDF and ACCF, have also emerged as a useful tool and may be superior over a long-segment ACCF based on retrospective evidence [109-111]. Another emerging concept is the oblique corpectomy without fusion, aiming to decompress the ventral cord whilst maintaining more than 50% of the vertebral body [112]. This can be achieved using conventional ACCF techniques from a lateral approach, and advocates suggest the absence of instrumentation reduces adjacent segment degeneration but produces similar neurological outcomes compared to ACCF [113,114]. At present, however, direct comparisons between conventional ACCF and oblique corpectomy have not been performed.
For the posterior approach, laminectomy remains an excellent option for long-segment decompression, now most often combined with laminectomy and fusion (LF) to avoid the unacceptably high postlaminectomy kyphosis rates that emerged in the 1970s/1980s [115]. It is associated with a high fusion rate (>98%), with a revision rate of only 1%, and complication rate of ~9% [116]. For more focal disease amenable to the posterior approach, techniques such as ‘split’ or ‘skip’ laminectomies have become popular as a method to achieve decompression with posterior ligament and muscle attachment preservation [117,118]. These can be applied in increasingly frail patients, with similar outcomes reported compared to standard laminectomy in the limited literature that is available [118]. Laminoplasty (the technique by which the lamina is removed, ligamental decompression achieved and then the lamina ‘island’ is replaced or fused in position) was originally seen as a solution to prevent postlaminectomy kyphosis whilst avoiding the need for instrumented fusion, and remains a popular option worldwide, particularly for specific indications such as OPLL [115,119]. LF and laminoplasty have both been proven to produce neurological improvement in functional and quality of life outcome measures (up to 2 years after surgery), but comparison between the 2 techniques is difficult. A multicenter, prospective observational study comparing 166 LF patients to 100 laminoplasty showed similar patient outcomes and rates of complications, but with shorter hospital stay in the LF group [120]. Other comparisons, from systematic reviews, have favored LF to preserve cervical lordosis and to reduce neck pain, but have not found a difference in functional or quality of life metrics [87,121]. The improved lordosis with LF, however, needs to be balanced against the increased cost compared to laminoplasty, but ultimately the surgeon’s experience with either technique should be the leading discriminator [122,123].
3. Cervical Disc Arthroplasty
Cervical artificial discs (or arthroplasty) aim to preserve motion across operated segments in an effort to reduce the incidence of adjacent level disease, but have limited indications in the treatment of DCM. For soft discs and radiculopathy symptoms, it is gathering an increasing body of evidence, with increasingly long follow-up periods [124]. However, this has to be balanced against a revision rate as high as 7.7% (compared to the 2% in ACDF), revision surgeries that have increased morbidity and cost, with complications such as heterotopic ossification occurring in as high as 47% of patients [118,125-128]. These reasons, and the limitations of using CAD for more than 2 levels, have meant that there is currently only a very limited role for CAD in the treatment of DCM [100]. In addition, many surgeons believe that removing the motion across a diseased or spondilytic segment is a key component of the effectiveness of surgery and CAD is therefore contrary to this paradigm. A relatively new addition is the promotion of ‘hybrid’ constructs where CAD is used in combination with ACDF, or as an adjunct to a previous ACDF [128]. Proponents suggest that different cervical levels are subject to different mechanical stressors, and that Hybrid constructs can be used to reflect this heterogeneity between levels, however, there is a paucity of evidence to suggest Hybrid surgery is equivalent or produces different outcomes compared to CAD or ACDF.
4. Stereotactic Navigation, Robotics, and Minimally Invasive Techniques
Despite the abundance of new technology in the application of robotics, minimally invasive surgery (MIS) and stereotactic navigation in spine surgery, very little has been published with regards to improving outcomes in DCM surgery. Stereotactic navigation has been used to improve the accuracy (and therefore safety) of both cervical pedicle and lateral mass screws, in addition to aiding anterior decompression in complex craniocervical junction cases [129-131]. Robotic-assisted devices utilize stereotactic navigation to aid with pedicle screw placement, but despite their increasing popularity in North America, they currently have no role in DCM surgery [132]. The use of minimally invasive or endoscopic techniques for posterior foraminotomy have been well described, but recent reports and case series have illustrated the use of these techniques to achieve single or 2-level posterior decompression [133,134]. Tubular retractors have also been described in the application of MIS ACDF surgery, which allows for a smaller incision, less traction and greater protection from iatrogenic injury on the prevertebral soft tissues [135]. This does however come at a cost of a restricted working space and inability to use an anterior plate. Similar techniques have also been described to produce ‘tunnel’ corpectomies, however, the exact benefits of these techniques over conventional methods remain to be proven [136].
CONCLUSION AND FUTURE RESEARCH
The pathophysiology of DCM is diverse, and the range of available nonoperative and operative interventions are a testament to that fact. A large body of evidence has accumulated in recent years to demonstrate the safety and efficacy of surgical decompression in DCM, with significant gains in the functional and quality of life outcomes measured. Despite these gains, significant knowledge gaps still exist that should become the focus of future research. The current clinical assessment tools, such as the mJOA, contain a number of subjective elements and are therefore subject to interobserver discrepancies. There is a real and urgent need to develop a more objective tool to assess the severity of DCM, and the use of specialized ancillary testing (such as the GRASSP-M tool) together with quantitative imaging assessments may suit this purpose. In a similar vein, patients with ‘mild’ DCM (mJOA 15–17) often pose difficult clinical conundrums. Whereas the use of surgery is clear in moderate-severe disease, the natural history of mild DCM (or those with asymptomatic cord compression on imaging) is much more difficult to predict and therefore this cohort has become the target of recent prospective observational studies. Further still, evidence for the safe use of physiotherapy treatments and continued exercise (or elite athletic activity) in the context of mild DCM needs to be clarified. A collaborative, global effort to decide the future research priorities in DCM is currently underway [137].