Cervical Spondylotic Myelopathy: Natural Course and the Value of Diagnostic Techniques –WFNS Spine Committee Recommendations
Article information
Abstract
Objective
This study presents the results of a systematic literature review conducted to determine most up-to-date information on the natural outcome of cervical spondylotic myelopathy (CSM) and the most reliable diagnostic techniques.
Methods
A literature search was performed for articles published during the last 10 years.
Results
The natural course of patients with cervical stenosis and signs of myelopathy is quite variable. In patients with no symptoms, but significant stenosis, the risk of developing myelopathy with cervical stenosis is approximately 3% per year. Myelopathic signs are useful for the clinical diagnosis of CSM. However, they are not highly sensitive and may be absent in approximately one-fifth of patients with myelopathy. The electrophysiological tests to be used in CSM patients are motor evoked potential (MEP), spinal cord evoked potential, somatosensory evoked potential, and electromyography (EMG). The differential diagnosis of CSM from other neurological conditions can be accomplished by those tests. MEP and EMG monitoring are useful to reduce C5 root palsy during CSM surgery. Notable spinal cord T2 hyperintensity on cervical magnetic resonance imaging (MRI) is correlated with a worse outcome, whereas lighter signal changes may predict better outcomes. T1 hypointensity should be considered a sign of more advanced disease.
Conclusion
The natural course of CSM is quite variable. Signal changes on MRI and some electrophysiological tests are valuable adjuncts to diagnosis.
INTRODUCTION
Cervical spondylotic myelopathy (CSM) is the most common cause of spinal cord dysfunction. It is the result of static or dynamic repeated compression of the spinal cord. This review paper will present current knowledge on its natural course and the value of diagnostic techniques. This study is a summary of the consensus meeting of the World Federation of Neurosurgical Societies Spine Committee.
CLINICAL PRESENTATION OF CSM
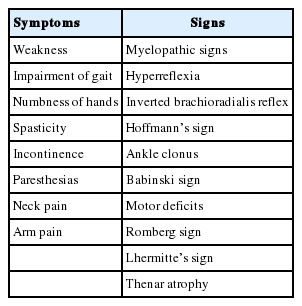
The diagnosis of CSM is based on specific symptoms, physical signs, and imaging findings. Clinical symptoms of CSM are summarized in Table 1 [1]. Neck pain, arm pain, limited motion of neck, diminished function of hands (clumsiness or difficulty with buttoning buttons, using keys, or changes in hand writing), wasting of the intrinsic muscles, spasticity, walking difficulty which can be tested by “heel-to-toe tandem walking,” “heelwalking,” or “toe-walking.”
Myelopathic signs can be defined as hyperreflexia (grade 3 or 4) or provocative signs (clonus [>3 beats], Babinski’s sign, Hoffmann sign, inverted brachioradialis reflex) [2].
Gibson et al. [3] have examined the prevalence of myelopathic signs in cervical myelopathy. They have compared CSM patients with control group for sensitivity and specificity of those clinical finding. Hoffman’s sign had 59% sensitivity and 84% specificity. Inverted brachioradialis reflex had 51% sensitivity and 81% specificity. Babinski sign and clonus had 13% sensitivity and 100% specificity. Hyperreflexia had 72% sensitivity and 43% specificity.
Although the physical signs on neurologic examination are well known, the symptoms are vague. The myelopathy signs (Hoffman’s sign, hyperreflexia, inverted brachioradialis reflex, Babinski, and clonus) are important for the diagnosis [2]. However, it has been reported that these signs are absence in 21% of cervical myelopathy patients [4].
An adequate decompression is expected to revert these myelopathy signs to normal. Acharya et al. [2] have examined those signs in severe CSM cases (mean Nurick score ≥3). They have found that 38% of patients have still one sign at the end of 1 year. The most resolved sign was inverted brachioradialis reflex, Babinski, and clonus with only 5% had positive signs. Hoffman did not resolve much, 38% of the patients still had a positive test at the end of 1 year. Although triceps and biceps changed to normal in all patients, patella reflex (10%) and Achilles (14%) were still hyperactive. The maximum recovery was seen in the first 6 months after surgery. After 6 months, a plateau was seen [2].
1. Scores Defining the Severity of the Disease
There are multiple classifications and measurements of disease severity. The Nurick classification system for myelopathy [5] is the oldest classification first proposed in 1972 (Table 2).
Japanese Orthopaedic Association (JOA) scale for CSM was introduced by Hukuda et al. [6] in 1985. It consists of a total of 17 points. In 1991, Benzel et al. [7] have modified Japanese Orthopaedic Association Scoring System (mJOA) and this scoring system has been the most widely used system (Table 3). It has total of 20 points.
Since the Nurick grade and mJOA scores assess different modes of functionality, in 11.8% of patients there was disagreement. The correlation of Nurick grade mJOA was best in patients with moderate myelopathy than in those with mild or severe ones.
In conclusions, we should continue to incorporate both Nurick scale and mJOA score in evaluation of patients with CSM till there is a more comprehensive scoring system that shows all aspects of function in a patient [8].
A study by Tetreault et al. [9] has reviewed 91 articles commenting on the predictive value of clinical factors (16 excellent, 38 good, 37 poor papers). Most important outcome predictors were preoperative severity and duration of symptoms. This review also reported many other valuable predictors including signs, symptoms, comorbidities and smoking status.
NATURAL COURSE OF CSM
Natural course of the CSM is still not well known. There are mainly 3 symptom complexes: Neck pain, radiculopathy, myelopathy. A combination of those are very common, and it is not clear if we should call the disease CSM in case there is no myelopathy. Patients usually experience long and constant periods of disease with a series of new episodes during which they experience new symptoms and signs [1]. A steady progression of symptoms is not common. Instead, myelopathic disease has silent periods of time with intermittent periods of rapid decline in neurological function [10].
Natural history of CSM can be reported on different stages of the disease:
1. Moderate to Severe CSM Patients (mJOA Scores Less Than 13)
There is a consensus that patients with severe progressive myelopathy need a surgical treatment [11]. However, what is the best option for patients with very subtle signs of myelopathy, or no myelopathy is not well known.
Clarke and Robinson [12] have reported 120 patients with CSM. They have treated 26 of them conservatively. They showed that almost 75% of patients had symptom progression episodes with subsequent stability. A slow deterioration happened in 20% of them. In 5%, after initial symptoms and signs, they had a long stable period. Approximately 50% of the conservatively managed patients improved [12].
Although many authors have reported that CSM has a progressive course over time [11,13], there are contrary reports. One of them has been reported by Lees and Turner [10]. They have described patients with CSM (n=44) and patients with spondylosis without myelopathy (n=51). Some of the patients with CSM (n=28) were managed conservatively and 17 of them have improvement over time.
2. Mild CSM Patients
The CSM patients with mJOA scores between 13–17 can be considered as mild CSM. Those patients with mild CSM have no limitations on daily activities. Hence, it is controversial as to whether surgical intervention is optimal treatment. Sumi et al. [14] have followed 60 patients with mild CSM with conservative treatment, and found that the outcomes of mild CSM without surgical treatment is fairly good with a tolerance rate of 70%.
In a systematic review, 20% to 62% of patients with symptomatic myelopathy were found to worsen if not managed surgically [13,15].
Those patients with pure radiculopathy with no sensory or motor deficits do have nerve root compression mostly recover with conservative treatment (85% resolve in 4 weeks) [16]. Pure radiculopathy with sensory and motor changes have also great tendency to recover (75%–90%). Best results are reported with soft disc herniations and shorter history. However, in case of hard spondylotic changes, longer history, those patients do have moderate results. Return of muscle power is better with soft disc (60%) in comparison to hard disc herniation (40%).
Matsumoto et al. [17] has reported a series with mild CSM in which 35% of patients had progression of symptoms during conservative management.
Kadanka et al. [18] have reported that most of the patients with mild myelopathy (80%) will improve with or without surgery. Shimomura et al. [19] have reported 80% of patients were stable during a 3-year follow-up. Murphy et al. [20] have reported that in the case of conservative management, subjective self-assessment and general health can worsen by the time.
In most analyses, approximately 20% of patients who had conservative treatment initially, had to go a surgical treatment during follow-up.
3. Patients With Myelopathy Signs but No Symptoms
Significant cord compression may present on magnetic resonance imaging (MRI) without any symptoms in approximately 5% of patients [21]. Some authors use the term “nonmyelopathic spondylotic cervical cord compression” (NMSCCC) in this condition [22].
Wilson et al. [23] have searched the predictors of deterioration in patients who had no myelopathy but radiological findings of cervical stenosis. They found that the incidence of myelopathy development at 1-year follow-up is 8%, and at 4 years, it is 23%.
4. Patients With No Symptoms Having Significant Stenosis (Premyelopathic)
If the patient has no symptoms and on examination no signs of myelopathy, it is widely accepted to follow-up them. However, minor trauma may cause worsening of such patients.
Matsunaga et al. [24] have reported 323 ossification of the posterior longitudinal ligament (OPLL) patients with significant cervical stenosis but having no symptoms. They found that during 17.6-year average follow-up time only 17% developed myelopathy. In general, there are 2 studies examining the rate of progression to myelopathy in patients with significant canal stenosis having no symptoms [24,25]. So, the risk of developing myelopathy with cervical stenosis can be estimated as approximately 3% per year (range, 1% to 5%) [26].
In conclusion, the clinical course of cervical myelopathy is quite variable. In the majority of patients with mild symptoms, conservative management may result in stability or improvement of symptoms [12,13,15]. Predicting the clinical course of a single patient remains difficult, though some evidence suggests that patients in younger ages and patients with mild symptoms are more likely to improve [27].
DIAGNOSTIC TESTS FOR CSM
In this session, we will discuss the value of electrophysiological tests and imaging techniques for CSM.
1. Value of Electrophysiology
Electrophysiological tests help for localizing diagnosis of a lesion, they do not give etiologic diagnosis. Such as they can tell the lesion is in anterior horn cells, they cannot tell it is amyotrophic lateral sclerosis (ALS). Most common electrophysiological tests used for cervical spinal cord and radicular syndromes are somatosensory evoked potential (SEP), motor evoked potential (MEP), electromyography (EMG), and nerve conduction study (NCS). They usefully provide additional information to clinical and neuroimaging findings in assessing the spinal cord injury severity. They were also found useful in follow-up evaluation after surgical treatment and rehabilitation. In CSM, they are expected to help in differential diagnosis and provide early signs in patients with mild symptoms.
1) Somatosensory evoked potential
SEP reflects the dorsal column function. Segmental cervical cord dysfunction can be shown by an abnormal spinal N13 response, and the P14 potential indicates a dorsal column dysfunction. These 2 peaks are reflections of the spinal cord function.
2) Motor evoked potential
Magnetic or electrical motor cortex stimulation and recording from distal muscles are procedure to elicit MEP. Other than central motor conduction time (CMCT), recruitment curve for MEP and silent period provide more information on the functional status of the cord [28].
3) EMG and NCS are sensitive tests for cervical radiculopathy, useful to differentiate peripheral nerve disorders that can cause clinical symptoms similar to CSM.
Cutaneous silent period (CSP) and contact heat evoked potentials (CHEPs) may be added to the list although not considered as routine tests. CSP has been reported to have a high sensitivity for detecting CSM [29]. CHEPs have shown the highest sensitivity (approximately 95%) to disclose at-level impairments in CSM patients [30].
4) Electrophysiology in differential diagnosis of CSM from other neurologic diseases
Differentiation of ALS, polyneuropathy, and radiculopathy is not easy with routin needle EMG and nerve conduction velocity studies. Root stimulation, triple stimulation MEP have been proposed for differentiation [31]. In early phases of ALS, it is harder to diagnose the disease. Cervical root stimulation may allow a clear distinction between motor neuronopathy and demyelinating polyneuropathy [32].
5) Electrophysiological tests in the prediction of outcomes of CSM
In earlier studies, electrophysiological tests before surgery by evoked potentials have been reported as useful for outcome prediction after surgery [33-35]. In cervical myelopathy after a decompressive surgery, De Mattei et al. [33] have first demonstrated a significant CMCT improvement by MEPs in 11 of 12 patients. Similar findings have been reported. In another study, preoperative MEPs were not found to give predictive information for clinical outcome, but the potentials have also improved after surgery [36].
Besides, in patients with mild CSM, a decompressive surgery was resulted with neurological improvement and increased thoracolumbar spinal cord CMCT [34].
The timing of surgery had also positive effects on potentials. Early surgery for CSM was found to produce a beneficial effect on MEPs. In a retrospective study, MEP changes were correlated with clinical findings [35].
SEPs also provide important preoperative information. In severe CSM patients, median SEP N9-N20 interval can predict good functional outcome [37]. Besides, N13 abnormalities have predicted good surgical outcomes. SEPs have been found useful in outcome prediction in many studies [37,38].
6) MEPs and CMCT measurement
Earlier reports indicated that CMCT is a useful measurement to assess preoperative CSM severity [33,39]. But, it is not well known whether an upper limb CMCT or lower limb CMCT prolongation is more valuable.
CMCT has been found to be useful [33], or not useful [36] to assess CSM severity, or useful only in mild CSM [34].
Clinical findings, multimodal spinal cord evoked potentials (SCEPs), and surgical outcomes have been correlated in a study [39]. The results have shown that patients with prolonged CMCT-thoracic level (TL) had affection of lateral corticospinal tract, posterior funiculus and gray matter. In patients with severe myelopathy and prolonged CMCT-TL, the prognosis may be poor even after surgery [39].
In 34 patients operated with anterior decompressions ascending SCEP and descending SCEP recordings in addition to MRI, have been found useful in localization of the lesion segments of cervical myelopathy [40]. That has helped identification of clinically silent compressions and avoid surgical interventions.
SCEP can give accurate functional localization [41]. In a retrospective study, SCEPs were found useful to learn the responsible level in multilevel OPLL [42].
7) Electrophysiological tests versus magnetic resonance changes in prediction of outcome
Patients with CSM with pathologic SEPs had also reduction in myelin water fraction (MWF) in 3 T magnetic resonance (MR) [43]. MWF was also correlated with SEP latencies. The authors have commented that decreased myelin content in the spinal cord is together with impaired spinal cord conduction.
It was interesting to see that patients with significant cervical cord compression on MRI, but having no clinical myelopathy signs, risk of early progression into symptomatic CMS (<1 year) was predicted by the presence of symptomatic radiculopathy and abnormal SEPs and MEPs [44]. However, hyperintense signal changes on MRI predicted the later (>1 year) development of CSM [44]. In another report, the lower limbs CMCTs, but not the diameters of the spinal canal on MRI, were correlated with long-term outcomes. They concluded that electrophysiology is predicting outcomes better than MRI changes [44]. Transcranial motor cortex stimulation was found more valuable in determining the localization of the lesion than MRI [45]. Anteroposterior (AP) diameter of the spinal cord, flattening at the lesion level and CMCT were well correlated [46].
MRI using diffusion tensor imaging (DTI) at the lesion level can provide a more sensitive information regarding spinal tract damage than the clinical and electrophysiological assessments [47].
8) Presymptomatic CSM evaluation
Bednarik et al. [48] have examined canal compression area, MR T2 hyperintensity, SEP, MEP, EMG findings in a group of 199 presymptomatic patients. Inclusion criteria were axial pain or radiculopathy, absence of any clinical signs attributed to cervical cord involvement. After 2-year follow-up, 45 patients (22.6%) have developed clinical signs of CSM. If there was a positive electrophysiology, time-to-CSM development was earlier (<12 months). If there was an MRI hyperintensity, time-to-CSM development was later (>12 months) [48]. In conclusion, asymptomatic patients with abnormal SEPs and radiculopathy tend to progress to clinical myelopathy [25,48,49]. Interestingly, the amount of compression at the AP diameter divided by transverse diameter has not affected the development of CSM [25].
9) Electrophysiological tests in monitoring of surgeries for CSM
Intraoperative electrophysiological monitoring has an important role in high-risk spinal cord injury surgery.
(1) SEP monitoring
SEPs provide effective information to evaluate the functional integrity of the spinal cord [50]. SEPs can be obtained very simply, they are noninvasive, they do not have much interference with inhaled anesthetics. The main disadvantage of SEP is that it reflects the posterior pathways not the anterior motor pathways. However, in a series with 462 patients with intraoperative SEP monitoring, only one patient had no intraoperative SEP changes, but developed a partial central cord syndrome [51].
(2) MEP monitoring
The shortcomings of SEPs can be answered by employing MEPs. MEP intraoperative recording techniques are as below:
(a) Cranial electrical stimulation and recording from the intradiscal electrodes [52] is not used anymore. With this method, it was possible to localize the conduction block at spinal cord level, but it was an invasive technique.
(b) Cranial or spinal (orthodromic, antidromic) electrical stimulation and recording from very thin epidural electrodes [53]. In this technique, direct (D) waves may be elicited. This technique allows a more reliable functional localization. It also correlates with functional outcomes [53].
(c) Cranial electrical stimulation and recording from muscles [54]. This can be done with constant-voltage stimulators using a short train of stimuli in anesthetized patients [54]. Some authors call those potentials myogenic MEP. Instead of halogenated volatile anesthetic agents, total intravenous anesthesia should be preferred for MEP monitoring (Fig. 1).
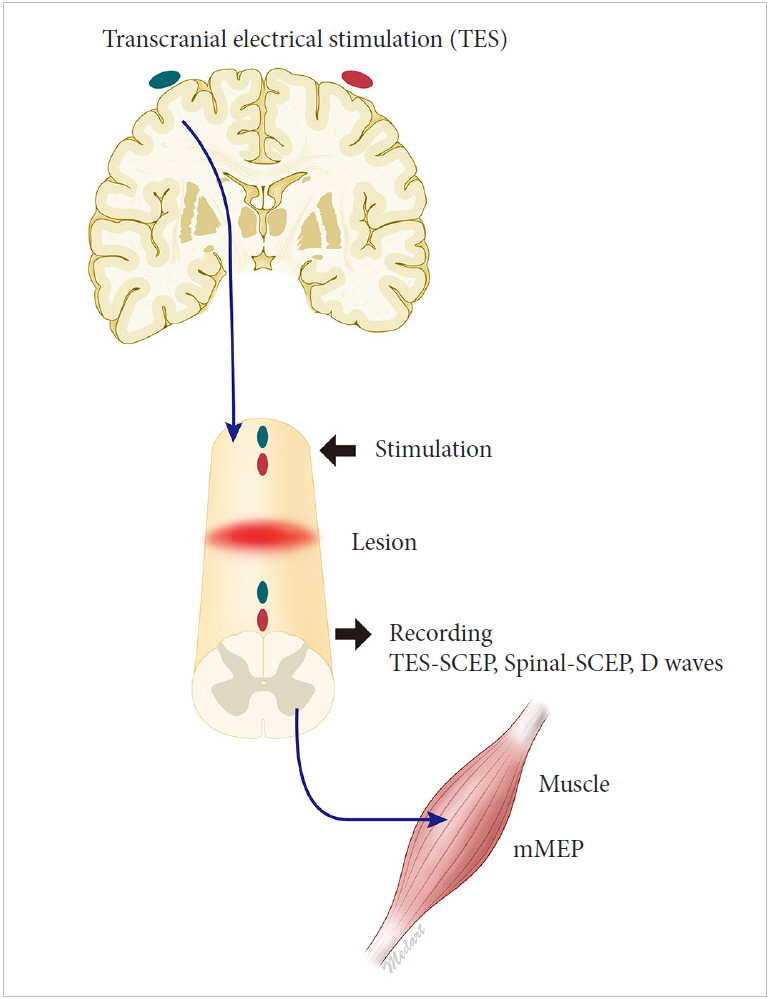
Techniques to record motor evoked potentials (MEPs) during surgery. Transcranial electrical stimulation (TES) results a volley on descending tracts mostly via corticospinal tract. Recording from the spinal cord is called TES spinal cord evoked potential (TES-SCEP). If the stimulation is applied to rostral spinal cord via epidural electrodes, it is called Spinal-SCEP. D waves can be recorded with both of these techniques. If the potentials are recorded from distal muscles, it is called myogenic MEP (mMEP).
10) Prediction of intraoperative neurological worsening by MEPs
In a study with 427 patients MEPs were found very sensitive for showing motor tract function during cervical surgery [55]. The authors strongly recommend to use MEPS during surgery of patients with CSM, especially on those with OPLL [55]. However, the literature is full with controversial results. For prediction of intraoperative neurological worsening MEPs were found sensitive [55], not sensitive especially for segmental paralysis. MEPs had unnecessary alerts in 18.4% of 1,445 patients who did not have any deficits after surgery [56].
Traynelis et al. [57] have reported 720 patients using different approaches of cervical spine without monitoring. A postoperative neurological deficit was reported in only 3 patients (0.4%). Two of them had myelopathy, but 1 had radiculopathy only. In all 3 patients, postoperative deficits completely resolved without any treatment. They commented that monitoring during decompression for symptomatic cervical disease is not necessary [57]. The surgeons operating on such patients without monitoring could well reduce the costs of surgery without risking patient safety.
Haghighi et al. [58] have reported the results of MEP and SEP monitoring in 100 patients and found that MEPs are more sensitive for detecting myelopathy and this is correlated well with abnormal MR images.
A systematic review searching the utility of intraoperative monitoring in CSM has concluded similar results for anterior cervical surgery [59]. MEP and SEP monitoring may be sensitive for diagnosis of neurological injury during such surgeries, however intraoperative MEP/SEP worsening is not specific [59]. Such changes may happen without clinical worsening and it does not always prevent neurological injury [29,59].
Ajiboye et al. [60] have monitored 2,627 cases during surgery for anterior cervical discectomy and fusion (ACDF). Neurological injuries occurred in 0.23% and 0.27% of patients with and without monitoring. The usage of intraoperative monitoring for ACDFs has dropped from 22.8% in 2007 to 4.3% in 2014. They have concluded that the utility of routine intraoperative monitoring for ACDFs is questionable.
Positive changes in MEP during monitoring may reflect a functional improvement 1 month after surgery in CSM patients [61]. However, the neuropathic pain and long-term functional outcomes are not affected. Improvement of MEPs during surgery may reflect better outcomes of motor power.
11) C5 root monitoring
During cervical posterior decompression, especially patients with CSM at the C4–5 levels are susceptible to C5 injury [62]. EMG and MEP monitoring during cervical laminoplasty has decreased C5 root palsy [62,63]. Myogenic MEP recordings from deltoid and biceps and spontaneous EMG are more sensitive than any other electrophysiologic tests [63].
Bose et al. [63] have reported in a retrospective study who underwent anterior cervical decompression MEPs were more sensitive to C5 deficits in comparison to spontaneous EMG activity alone, since the false-negative findings during EMG activity only are more often.
Oya et al. [64] have examined 131 cases during cervical laminoplasty with multimodality potentials. C5 palsy after surgery was in 3 patients. Incidence of C5 palsy (2.2%) and other neurologic deficits while using multimodality potentials were relatively low. MEPs elicited from deltoids or biceps had 100% sensitivity for predicting a postoperative deficit. SEPs were not helpful in predicting postoperative deficits. They have commented that SEP monitoring is not useful, MEP monitoring is not useful for lower extremity power, but it is useful for C5 palsy [64].
Ando et al. [65] have examined the efficacy of MEP to prevent deltoid weakness in 278 patients operated with laminoplasty technique. 7 patients (2.5%) developed deltoid weakness (2 acute, 5 delayed onsets). Persistent monitoring alerts occurred in 2 patients with acute-onset. In 1 patient deltoid weakness was prevented by foraminotomy. No alerts in delayed onset palsies. MEPs by cortical stimulation and recording from deltoid muscle is valuable in detecting early onset C5 nerve palsy [65].
In conclusion, electrophysiological tests are useful for differential diagnosis of other neurological disorders from CSM. MEP and SEP are valuable tests to predict outcomes of CSM surgery. But no evidence that they are more valuable than clinical parameters. The use of MEP/SEP monitoring may be considered as a sensitive technique to diagnose potential neurological injury during surgery for CSM. However, intraoperative MEP/SEP worsening is not specific, and it does not show clinical worsening in every incidence. Intraoperative MEP/SEP changes do not necessarily prevent neurological injury and improve the outcomes. MEP by recording from deltoid muscle is valuable in detecting C5 nerve palsy. The value of monitoring during ACDF surgery and lower extremity function are questionable.
2. Value of Canal Diameters in Computed Tomography and MRI
The main component of the CSM is the narrowing of the spinal canal. It results from a combination of intervertebral disc herniation, osteophytes and OPLL [66]. However, the mere presence of cord compression on MRI is inadequate for the diagnosis of CSM as it can be demonstrated in approximately 5% of asymptomatic patients [21], lending the diagnosis of NMSCCC [22]. Specificity of cord compression seen on MRI is limited for the diagnosis of CSM [67]. This session assesses the impact of canal diameters on clinical outcomes in patients with CSM. Twenty-four key articles published in the last 10 years were reviewed.
The early studies assessing the canal diameters were based on radiographs [68,69]. They assessed the size of the cervical spinal canal using the developmental segmental sagittal diameter (DSSD). This was measured from the vertebral body posterior surface to the closest point on the spinolaminar line at the level of the pedicle [68]. DSSD less than 10 mm was predicted to cause myelopathy [68]. However, variations in magnification and distance from the X-ray source to film can confound such measurements [69]. The Torg-Pavlov ratio – the ratio between the canal diameter and the vertebral body width was used to overcome this limitation. Torg-Pavlov ratio <0.8 was used as an indicator of spinal canal stenosis [69]. However, this ratio had a low positive predictive value due to false positives arising from errors of patient positioning [70].
With the advent of computed tomography (CT) and MRI, the use of plain radiography for assessing the spinal canal fell out of favor [71]. The MRI had benefit of being able to measure the soft tissue components (herniated disc and hypertrophied ligamentum flavum) contributing to canal stenosis [66]. Earlier MRI based techniques relied mainly on qualitative or semiquantitative data such as percentage of flattening, cross-sectional cord shape, shape of the cord on midsagittal T1- and T2-weighted imaging (T2WI) [14,19,72]. Over time however, they became more quantitative and thus less influenced by subjective bias. Currently AP diameter, transverse area (TA), and compression ratio (CR) are the measurements of choice in evaluating spondylotic cord compression [48,66].
1) Semiquantitative scales
As the degree of segmental pathologic changes and cord compression exist on a spectrum, the need for assessment of the severity of these processes resulted in the development of grading systems. Muhle et al. [73] graded cervical stenosis on a 4-point scale as follows.
Grade 0: no obliteration of anterior or posterior subarachnoid space
Grade 1: partial obliteration of the anterior or posterior subarachnoid space
Grade 2: complete obliteration of the anterior or posterior subarachnoid space
Grade 3: cervical cord compression or displacement
Kang et al. [74] modified Muhle’s grading to include signal intensity (SI) changes on T2WI. Their proposed classification was as follows:
Grade 0: absence of spinal canal stenosis
Grade 1: subarachnoid space obliteration exceeding 50%
Grade 2: presence of spinal cord deformity
Grade 3: presence of spinal cord SI change
The grade of stenosis in study of Kang et al. [74] was found to have a positive correlation with neurologic deterioration and with the percent of patients who had undergone surgery. Park et al. [75] validated Kang classification and found it to correlate with clinical manifestations of CSM.
Nagata et al. [76] used sagittal T1 weighted MRI for assessing the severity of the latter. They have divided cervical cord compression on MRI into 3 classes:
Class 1: slight compression, cord width decrease by <1/3
Class 2: moderate compression, cord width decrease by >1/3
Class 3: severe compression, cord width decrease by >2/3
2) Quantitative measurements
While grading systems allow for a calibrated estimation of disease severity, they are only semiquantitative, and hence not free from bias. To develop more quantitative methods, MRI based measurements of cord and canal dimensions were investigated. A good correlation of 2 variables measured on CT myelography – TA of the spinal cord, CR with the degree of pathologic changes have been reported in cadavers [48].
(1) Compression ratio
The CR is defined as the ratio between the AP diameter and the transverse diameter of the cord on axial imaging [22]. A CR <0.436 is associated with an unfavorable prognosis. The CR is a more objective measure of cord compression, but is not without limitations. It fails to be useful in cases with circumferential cord compression. Another shortcoming is that since the cord is not rectangular in shape the CR may overestimate sagittal compression when compression is measured laterally [48]. In a systematic review, no excellent quality studies were found correlating CR with postoperative outcomes [77].
(2) Cross-sectional area of the spinal cord
The other variable is cross-sectional area of the spinal cord (CSA) measured on axial images. The CSA has been validated by multiple studies as a marker of severity of myelopathy, postsurgical recovery, and pathological cord changes [22,48,78,79]. The cord loses its function when its CSA is <45 mm2 (also known as CSAcritical) and surgery on patients with a CSA lower than this value was shown to have worse functional outcomes in spite of adequate morphological decompression [78]. CSA on MRI correlates with recovery ratio, but not with postoperative functional score assessed by JOA/mJOA Scores (Level of evidence: 2) [77]. A more recent systematic review reported that patients with greater CSA had better neurological outcomes after surgery [80].
(3) Maximum canal compromise/Maximum spinal cord compression
Another commonly employed tool for assessing the severity of cervical stenosis is called “maximum canal compromise” (MCC). Its equivalent for assessing cord compression is known as “maximum spinal cord compression” (MSCC). They are first described for spinal cord injury [81], then now commonly used to evaluate CSM [66]. The MCC is the ratio of the AP diameter of the spinal canal at the region of interest to the AP diameter of the spinal canal at the average of the normal sites above and below it [81]. The MSCC is a tool for assessing cord compression that is derived in the same manner
While MSCC/MCC eliminates the need for standardization of measurements across patient populations, they are not free from limitations. The variation in cord size within the cervical spine is not well quantified. Therefore, any estimate of the diameter of a segment that is derived by interpolation from the segments above and below it has doubtful accuracy [66]. Their inability to pick up lateral stenosis as the assessment is carried out in the midsagittal plane, is another limitation [66].
Nouri et al. [66] have found a good correlation between worse outcomes (mJOA<16) at 6 months and MCC/MSCC. Arvin et al. [82] have also found a correlation between MSCC and 6 months and 1-year postoperative mJOA recovery rate, walking test times.
(4) Canal diameter
The AP diameter of the canal is another measure of the degree of canal compromise. It is the distance between the most posterior point on the posterior aspect of the vertebral body and the nearest point on the spinolaminar line. It has been shown to be able to differentiate between patients with normal anatomy, NMSCCC, and CSM [22], but no significant association with prognosis has been demonstrated (Table 4) [77,83,84].
(5) Occupation rate
The proportion of the spinal canal occupied by the cord measured as the ratio between the AP diameters of the cord and the canal in the midsagittal plane is occupation rate of the spinal cord [21]. The authors have found that the border value for the occupation rate in asymptomatic persons is at 72.3%. So, they proposed an occupation rate >75% as a cutoff for developmental cervical stenosis.
3. Value of Signal Intensity Changes in MRI
CSM is known to lead to T1 and T2 signal changes on cervical MRI [85]. T1, T2 changes and histology are associated with spinal cord compression and constriction. Those signal changes have been attributed to myelomalacia and cord gliosis secondary to a long-standing compressive effect [86]. Al-Mefty et al. [87] have speculated high signal T2 shall be related to edema, inflammation, gliosis, and myelomalacia. On the other hand, low signal T1 can be related to cystic necrosis and syrinx. Signal changes in gray matter may indicate temporal progression of CSM. Though the anatomical translation of T1/T2 signal changes is established, its effect on outcome is still under study [88].
Several grading systems for SI changes have been proposed according to the extent, SI types, axial appearance or combination of those. SI can be assessed both qualitatively (presence vs absence) and quantitatively (SI ratio) [79], sagittal extents, TA [66,89]. Most of them contain T2 changes, some also include T1. No grading system is yet accepted as the increase (Fig. 2).
High signal in T2: A simple and most widely used grading of T2 SI has been proposed by Chen et al. [90]: Grade 0 is no increase of signal. Grade 1 is faint, fuzzy bordered intensity increase. Grade 2 is intense, well-defined bordered intensity increase.
There is a significant correlation between the degree of compression (axial area of spinal cord) and high signal in T2WI [91,92]. Sagittal extent and area of T2 hyperintensity have also been found significant predictors of surgical outcome at 6 months [89].
Approximately one-third of the CSM patients have T2 hypersignal [67]. Pyramidal signs correlated with SI changes on T1 and T2WI [91]. Also, Hoffmann sign was related to a greater degree of signal change (T1 and T2) [92].
Dynamic factors have been investigated and found to have a role in high signals in T2WI. Increased segmental range of motion is a risk factor for T2 signal changes in CSM, and segmental hyperextension is correlated with higher hyperintensity on T2 [85].
Cervical cord available space was examined in a prospective study using flexion and extension dynamic MR images [93]. The space was found very narrow on extension, T2 intensity was found more pronounced on flexion. The authors have commented that despite reduced canal diameter in extension, higher rate of signal changes in flexion occurs because of expansion effect [93].
1) High signal in T2 and outcomes
This change in intensity is known to be associated with more severe disease and a worse prognosis [48,82,89,94]. There was a significant correlation of SI change and presence of clinical myelopathy and a correlation of SI ratio with recovery rate [67]. A correlation of worse outcomes at 6 months and 12 months with presence of hypointensity on T1-weighted imaging (T1WI), hyperintensity on T2WI, area on T2WI, sagittal extent on T2WI were reported [82,92]. However, there was no significant correlation between the SI change and severity of clinical symptoms [79] or deterioration of myelopathy [14], or exacerbation of CSM in patients managed conservatively [84]. Instead, in a retrospective study, Oshima et al. [83] have found that among patients with clinically mild CSM with SI change on MRI, only 44% had neurologic deterioration or undergone surgery over the course of 10 years. They suggest that the presence of SI change in otherwise mild CSM may not necessarily warrant an operative intervention.
In a meta-analysis investigating high signal changes in T2WI and outcomes [95], multisegmental and sharp increase in T2 signal changes have been found to end with poorer outcomes (Class II evidence). If the T2WI signal changes are regressing after surgery, better postoperative outcomes should be expected (Class II evidence) [95]. Higher preoperative signal change ratio correlates with worse clinical outcome [96].
In a retrospective study including 197 patients [97], T2 SI has been graded according to Chen et al. [90] Grade 2 signal changes on T2 were associated with lower rates of cure. T1 hypointensity was also related with lower rates of cure [97].
Mastronardi et al. [98] have reported disappearance rates of the T2WI after surgery. They have found 52% had regressed after decompressive surgery, of which 17.4% regressed in the immediate postoperative MRI. That means T2 signal changes tend to be reversible.
On the other hand, T2 high signal changes may also be found in asymptomatic canal stenosis. Kato et al. [21] have investigated 1,211 healthy volunteers. Sixty-four volunteers had significant radiological compression, 28 volunteers (2.3%) presented T2 signal changes. In conclusion, T2 signal changes do not necessarily translate into clinical disease.
T2WI signal hyperintensity is not specific and can reflect reversible or irreversible structural changes. We know that patients may have both weak and strong T2WI signal hyperintensity parts (Table 5) [66,99].
2) Low signal in T1
Low-intensity signal on T1WI is considered as a sign of advanced disease or significant neural tissue loss [66,77,87,90,99]. Hyposignal in T1 rarely appears without increase in T2 signal.
Low-intensity signal on preop T1WI correlates with poor postoperative neurological outcome [41]. In a prospective study by Salem et al. [100] 93 patients operated by either anterior or posterior approach and 12 months of follow-up, signal changes in the T1 cervical MRI have been found to predict worse outcome after surgery.
In conclusion, high SI on T2WI alone cannot be considered as a predictor of worse outcome [99]. However, if there is T2WI strong signal hyperintensity with sharp, clear border, if there are long high-intensity segments on T2WI, low SI segments on TIWI or combined T1 and T2 SI changes, if T2WI high intensity after surgery persists, those can be considered as negative predictors of surgical outcome. Those patients have more severe histological changes and worse recovery after surgery.
4. New Imaging Techniques for CSM
Conventional MRI can only give the structural information of spinal cord. However, advanced techniques such as DTI, MWF, magnetization transfer (MT), MR spectroscopy (MRS), and functional MRI (fMRI) can provide additional information about spinal cord metabolism and structure [101]. Ischemia due to direct compression of vessels, as well as impaired microcirculation, free radical-mediated cell injury, glutamatergic toxicity, and apoptosis are the changes which may be reflected by new imaging techniques [101-105].
1) Dynamic MRI
Dynamic MRI (dMRI) can find more spinal cord compression than static MRI [73]. In a report using extension MRI it was found that compression levels were quite more with extension MRI in comparison to the static MRI [106].
2) Fiber tracking
MR DTI and spinal cord fiber tracking are useful tools for CSM imaging. It was reported that when compression of the spinal cord white matter increases, the mean apparent diffusion coefficient (ADC) has decreased [107]. A slightly elevated fractional anisotropy (FA) at the compression level was found.
At the compression site, almost half of the CSM patients (46%) are having normal to decreased ADC, or normal to elevated FA [108]. Clinical studies have clearly documented that an increase in ADC and decrease in FA are signs of the late stages of chronic spinal cord compression [105]. The reason of these pathological changes are the elevation of ADC after an increase in extracellular water and suppression of FA due to absence of directional organization inside the cord [109].
A recent study has also shown that if the FA before surgery is high at the site of compression these patients are having better functional recovery [110]. That means FA at the compression site is a biomarker for determining good surgical candidates.
3) MR spectroscopy
Although MRS provides important information about cellular biochemistry and neural function, studies examining MRS changes in CSM patients are very few.
N-acetyl aspartate (NAA), choline, lactate, and creatine (Cr) are some metabolites examined. NAA is an indicator of axonal integrity and it was found mostly in axons and neurons. Lactate is an indicator of metabolic dysfunction after central nervous system injury. The NAA/Cr ratio is decreased in CSM patients which reflects increased neuronal injury to these patients [111]. In about 1/3 of CSM patients an abnormal lactate signal was found [101].
On the other hand, there are many limitations to MRS study. Since the spinal cord is smaller in volume, adjacent tissues can contaminate the results. Patient movement during scan acquisition can also affect MRS and DTI. Cardiac pulsations and the respiratory movements also cause physiological movements of the spinal cord, so the suppression of those movements by cardiac gating, special radiofrequency coils are necessary to enhance the quality of MRS and DTI in the spinal cord.
Future applications of DTI and MRS can be speculated [112]. Some of them are inhibition, such as the cell apoptosis inhibition with antibody, neurotropin release via genetically altered fibroblasts and repairing injured plasma membranes by dietary therapy. Some noninvasive modalities would be necessary to understand the efficacy of these therapies.
There it comes new MR technologies to have more information about spinal cord function. Some of those techniques are; chemical exchange saturation transfer imaging, dynamic susceptibility contrast perfusion MRI, MT imaging (MTI), dynamic contrast-enhanced perfusion MRI, arterial spin labeling, and spinal cord fMRI. Especially MTI, which has the ability to quantify myelin integrity was found useful for detecting changes of multiple sclerosis and ALS patients.
RECOMMENDATIONS
1. Recommendations for Clinical Presentation of CSM
• Myelopathic signs (hyperreflexia, inverted brachioradialis reflex, Hoffmann sign, Babinski, and clonus) are an integral component of clinical diagnosis of cervical myelopathy. However, they are not very sensitive and may be absent in about 20% of myelopathic patients.
• Individual myelopathic signs taken alone cannot diagnose cervical myelopathy in all patients but at least one is present in severe myelopathy.
• Clinical diagnosis of CSM relies heavily on characteristic symptoms and signs elicited during history and physical exam which prompt further investigation with cervical spine imaging.
• In severe myelopathic patients, after laminoplasty, major recovery in myelopathic signs occurs during the first 6 months and there after it plateaus.
• In patients with myelopathic signs, if there are no alternative explanations, a combination of clinical symptoms and imaging studies must form the basis of our treatment decisions. The absence of myelopathic signs does not preclude the diagnosis of CSM nor its successful surgical treatment.
2. Recommendations for Natural Course of CSM
• Natural course of patients with cervical stenosis and signs of myelopathy greatly vary.
• Progression of the disease is possible, but prediction of those patients is not well known. Some patients may remain static for lengthy periods, and some patients with severe disability can improve without treatment.
• For patients with no symptoms but having significant stenosis (premyelopathic), risk of developing myelopathy with cervical stenosis is approximately 3% per year.
3. Recommendations for Value of Electrophysiology
• Electrophysiological tests to be used in CSM patients are (in order of benefits): MEP, SCEP, SEP, and EMG.
• Routine electrophysiological tests are useful in differential diagnosis of CSM from other neurological conditions. However, especially during the early course of the disease differential diagnosis is very difficult, specific tests are necessary and mild forms of ALS and polyneuropathy may not be differentiated easily.
• Although MEP and SEP have been found as valuable tests to predict outcomes of CSM surgery, there is no evidence that they are more valuable than clinical parameters.
• Electrophysiological tests may have better outcome predictions than MR changes.
• Electrophysiological tests are not very useful in monitoring lower extremity power, and the value of monitoring during ACDF surgery is questionable.
• EMG and MEP monitoring have been found to be useful to decrease C5 root palsy during CSM surgery.
• Intraoperative MEP/SEP worsening is not specific, and it does not show clinical worsening in every incidence. Intraoperative MEP/SEP changes do not necessarily prevent neurological injury and improve the outcomes.
4. Recommendations for Value of Canal Diameters in CT and MRI
• In spite of conflicting evidence, MRI morphometric analysis of the spine has a significant role in evaluation and prognostication of CSM and it should be included in the preoperative workup.
• Among the many variables assessed using MRI – compression ratio, maximum canal compromise, and transverse area are most importantly correlated with functional outcomes following surgery in patients with CSM. Each parameter has its own strengths and limitations, therefore a combined assessment of MR parameters has a greater predictive yield.
5. Recommendations for Value of Signal Intensity Changes in MRI
• Spinal cord T2 hyperintensity on cervical MRI may be correlated with a worse outcome in CSM.
• Patients with lighter signal changes in T2 on cervical MRI should not be excluded from surgical treatment of CSM.
• More studies are needed to validate proposed grading systems, or to create new ones.
• T1 hyposignal should be considered as a sign of more advanced disease, with worse outcome.
• More studies are needed to assess the effect of sagittal and axial extension of T1 signal changes on outcome.
6. Recommendations for New Imaging Techniques for CSM
• Diffusion MRI, MR Spectroscopy, and dMRI may be a part of MR examinations for CSM protocol apart from conventional MRI. We suggest their usage for outcome studies.
• With data pooling of clinical and imaging findings, we will be able to prognosticate better and identify patients earlier before the changes and permanent damage sets in.
Notes
The authors have nothing to disclose.