 |
 |
- Search
|
|
||
Abstract
Objective
Cervical spondylotic amyotrophy (CSA) is a relatively rare entity caused by cervical degenerative spinal diseases and characterized by motor weakness accompanied by remarkable muscle atrophy in the upper extremities without significant sensory deficits or spastic paraparesis in the lower extremities. Postoperative outcomes and predictive prognostic factors vary among previous reports. In the present report, we describe the surgical results in patients who were surgically treated for CSA and present a literature review.
Methods
In total, 33 patients with CSA were retrospectively analyzed. Correlations between the surgical outcome and the following factors were statistically analyzed: age, sex, type of impaired muscle, preoperative severity of motor weakness, number of levels of cord or root compression, presence of a T2 high-intensity area in the spinal cord, cervical kyphosis, and methods of surgical procedure.
Results
On postoperative neurological evaluation, 25 patients (75.8%) had favorable outcomes and 8 had unfavorable outcomes (proximal type, 72.2%; distal type, 78.6%). Patients with favorable outcomes were significantly younger than those with unfavorable outcomes (p=0.013). PatientŌĆÖs characteristics except for age and radiological factors were not correlated to surgical outcome.
Conclusion
The present study focused on the surgical results in patients who were surgically treated for CSA along with updated information from a literature review. Improvement of motor weakness is expected with acceptable prevalence although higher age can be a negative factor. Surgical outcomes and predictive factors related to a poor prognosis were determined and compared with those of previous articles.
Cervical spondylotic amyotrophy (CSA) is as relatively rare clinical manifestation among symptoms associated with cervical spondylotic myelopathy or radiculopathy. CSA is characterized by motor weakness accompanied by remarkable muscle atrophy in the upper extremities without significant sensory deficits or spastic paraparesis in the lower extremities. Surgical management is required in some patients with progressive or severe neurological deterioration; usually, however, the symptoms stabilize or improve by conservative treatment alone. Several reports have described the surgical indications and outcomes in patients with CSA, but the most appropriate surgical timing and optimal methods remain unclear. Additionally, postoperative outcomes and predictive prognostic factors vary among previous literature reviews [1-16].
In the present report, we describe surgical results in patients who were treated for CSA in our institute. Operative outcomes and predictive factors related to a poor prognosis were investigated and compared with those of previous articles. We also updated overview of CSA including the histological background, pathophysiology, clinical manifestations, examination findings, differential diagnosis, and treatment of CSA with a literature review.
In total, 644 patients underwent operations for cervical degenerative diseases from 2009 to 2018 in our institute. Among them, 33 patients (27 men, 6 women) with a diagnosis of CSA were retrospectively analyzed. CSA is divided into a proximal type (scapular, deltoid, and biceps), distal type (triceps, forearm, and hand), and combined type according to the distribution of muscle atrophy in the upper extremities. The institutional human ethics review board of our facility approved this study protocol (FHR 2019-2) with patientŌĆÖs informed consent. The inclusion criteria for the patients were as follows: (1) the presence of cervical spondylosis (including ossification of the posterior longitudinal ligament and disc protrusion of <4 mm), (2) the presence of diffuse or localized muscle atrophy of the upper extremity with a manual muscle test (MMT) grade of <3, (3) mild or no sensory disturbance in the upper extremity, (4) either the presence or absence of spastic deep tendon reflex in the lower extremities, (5) absence of gait disturbance and sphincter dysfunction, and (6) either minimal or no radicular pain (numerical rating scale score of <3) of the upper extremities (however, severe radicular pain at onset was included if it improved at operation). The patients were classified into 3 subgroups according to the distribution of the atrophic muscle: proximal type, distal type, and combined type. Any cases of CSA related to a spinal tumor, infection, trauma, inflammatory disease, or cervical reoperation were excluded. Preoperative electromyography (EMG), nerve conduction studies, and consultation with a neurologist were conducted if CSA was difficult to discriminate from other diseases such as peripheral entrapment neuropathy and motor neuron disease. Patients who suspected to have noncervical neurological diseases and joint disorders in the upper extremities were also excluded. The radiological findings were evaluated by plain radiographs, magnetic resonance imaging (MRI), myelography, and computed tomography myelography (CTM) and were considered to correspond to the patientŌĆÖs neurological symptoms. Muscle strength was measured 12 months postoperatively. Improvement of the muscle strength of the most atrophic and impaired muscles was divided into 4 grades: ŌĆ£excellent,ŌĆØ full recovery or recovery to an MMT grade of 2; ŌĆ£good,ŌĆØ recovery by one grade; ŌĆ£fair,ŌĆØ no improvement; and ŌĆ£poor,ŌĆØ decreased muscle strength. According to the MMT assessment, the surgical result was defined as either a favorable outcome (ŌĆ£excellentŌĆØ and ŌĆ£goodŌĆØ MMT results) or an unfavorable outcome (ŌĆ£fairŌĆØ and ŌĆ£poorŌĆØ MMT results).
Correlations between the surgical outcome and the following factors were statistically analyzed: age, sex, duration of illness, diabetes mellitus, smoking, body mass index, presence of severe pain at onset, type of impaired muscle, preoperative MMT grade of the most atrophic and impaired muscles, number of levels of cord or root compression, presence of a high-intensity area (HIA) in the spinal cord on T2-weighted images, cervical kyphosis (C2ŌĆō7 angle of <-5┬░), and methods of surgical procedure.
The statistical analysis was performed using JMP statistical software ver. 13 (SAS Institute Inc., Cary, NC, USA). The chi-square test, Mann-Whitney U-test, and Fisher exact probability test were applied for all of the statistical assessments. Significance of the obtained results was judged at the 5% level.
The average age at the time of the operation was 59.7 years (range, 30ŌĆō80 years), and the mean duration of illness was 7.1 months (range, 1ŌĆō24 months). Ten of all cases presented motor weakness and atrophy within 3 months. Proximal, distal, and combined type CSA was present in 18, 14, and 1 patient, respectively (Table 1). Based on the radiological data, 28 cases of all patients demonstrated multilevel lesion with compressed cord or stenosis of cervical foramen which mainly affected to the anterior horn and/or the ventral roots. Intracanal lesion, foraminal lesion, and combined lesions was observed in 12, 12, and 9 cases, respectively. The average number of levels of neural compression was 2.8. An HIA in the spinal cord on T2-weighted images and cervical kyphosis were observed in 13 and 7 patients, respectively. Anterior cervical discectomy or corpectomy with fusion was performed in 18 patients. Anterior foraminotomy without fusion was performed in 3 patients. Cervical laminectomy or laminoplasty was carried out in 10 patients. Among these 10 patients, posterior foraminotomy or posterior fixation was added in 3 and 2 patients, respectively. Anterior and posterior combined decompression with fusion was performed in 2 patients. At the postoperative neurological evaluation, 25 patients (75.8%) had a favorable outcome (excellent in 12, good in 13), and 8 patients had an unfavorable outcome (fair in 8, poor in 0). Table 2 shows the detailed data between patients with proximal and distal type CSA. The rate of a favorable outcome was not significantly different between patients with proximal and distal type CSA (72.2% vs. 78.6%, respectively; p=0.68). The data between patients with favorable and unfavorable outcomes are compared in Table 3. Patients with a favorable outcome were significantly younger than those with an unfavorable outcome (p=0.013). There was no statistical significance between the 2 groups in sex, duration of illness, diabetes mellitus, smoking, body mass index, presence of severe pain at onset, type of impaired muscle, preoperative MMT grade of the most atrophic and impaired muscles, number of levels of cord or root compression, presence of a HIA in the spinal cord on T2-weighted images, cervical kyphosis (C2ŌĆō7 angle of <-5┬░), and methods of surgical procedure.
Age was the only factor with a significant difference between the 2 groups.
In 1952, Brain et al. [17] initially reported rare cases of cervical spondylosis characterized by motor weakness and atrophy of the upper extremities without obvious sensory disturbance and the presence of long tract signs. Crandall and Batzdorf [18] reported that the incidence of motor-dominant symptoms with minimal sensation loss in patients with cervical spondylotic myelopathy was slightly less than 7% and described this condition as a ŌĆ£motor system type of myelopathy.ŌĆØ Keegan [19] reported a case of ŌĆ£dissociated motor loss syndromeŌĆØ in a patient with atrophy of the deltoid and brachialis biceps muscles in the right upper extremity with mild sensory deficits at first examination. Four years later, this patient had developed acute phase atrophy of the same muscles on the left side. The etiology of this patientŌĆÖs condition was finally confirmed by autopsy. In 1975, Sobue et al. [20] and Yanagi et al. [21] proposed a definition of CSA and suggested that segmental myelopathy can be related to the clinical manifestations of CSA. According to their criteria, the main subjective symptoms were severe muscular atrophy in the upper extremities with an absent or insignificant sensory deficit in patients with cervical spondylosis. Proximal upper extremity muscle atrophy was slightly more frequent than distal upper extremity muscle atrophy. Muscle atrophy was commonly lateralized; however, diffuse atrophy in the upper extremity was rare. Transient and minor radicular pain could be contained and was often influenced by neck motion. The presence of objective signs of hyperreflexia in the lower extremities was also acceptable. Ebara et al. [3] summarized a case series of distal type CSA as ŌĆ£amyotrophic type of myelopathy handŌĆØ with clinical features of especially localized wasting and weakness of the extrinsic and intrinsic hand muscles. Sonoo et al. [22-24] clearly established identification of the impaired neurological segment and differentiation from other diseases resembling clinical findings, especially using appropriate electrophysiological examination.
The pathophysiology of CSA is presumed to involve several relevant factors. Damage to either the ventral nerve roots or the anterior horn by compression or impingement due to the degenerative spinal changes associated with cervical spondylosis may play an important role in CSA. Keegan [19] reported intradural compression of the ventral nerve roots by a posterolateral osteophyte in an autopsy case of CSA. Ota and Ono [25] also described this mechanism, demonstrating that the ventral root was compromised by LuschkaŌĆÖs joints in their autopsy series. Tanaka et al. [26] reported that the ventral root was located more caudally than the dorsal root from the origin of the dura mater into the intervertebral foramen in their microscopic cadaver study. From this anatomical viewpoint, selective compression against the ventral nerve roots might occur around the intervertebral foramen. This study also showed that anatomical differences between the nerve roots and their corresponding discs in the intervertebral foramina depend on the spinal level [26].
Sobue et al. [20] and Yanagi et al. [21] originally suggested that the etiology of CSA was a sort of segmental myelopathy caused by regional damage around the anterior horn. In an autopsy case of amyotrophic type of myelopathy hand, Ebara et al. [3] found that involvement in the gray matter seemed to be predominant. Based on this pathological finding, they concluded that the regional motor atrophy in the upper extremity resulted in greater vulnerability of the anterior horn cells in the gray matter than in the pyramidal tract. Kameyama et al. [27] also supported this pathology by showing MRI findings in which a symmetric intramedullary change in the signal intensity, a so-called snake-eye appearance, was observed in 3 cases. They stated that multisegmental cord damage and dynamic cord compression in the flexion and extension positions must be associated with the pathology of CSA. Multisegmental lesions of the anterior horn are highly likely to cause severe muscle atrophy because the motor cell columns that supply the muscles have reservoir pools extending to more than one segment. Similarly, Fujiwara [28] reported a case of distal type CSA in a patient in whom cavitation within the anterior horn on the affected side was evident by delayed CTM. The anterior horn cells may be also damaged by a vascular insufficiency mechanism, in which blood supply from the sulcal arteries becomes insufficient by paramedian compression of the spinal cord.
Although the 2 above-mentioned pathophysiological mechanisms have been advocated as causes of CSA, the definitive mechanism cannot always be determined individual cases [9]. Actually, Fujiwara et al. [4] reported that more than half of patients with CSA had impingement against both the anterior horn and the ventral nerve root in their radiological study.
The average age at onset of neurological symptoms in patients with CSA is around 50 to 60 years, and men are more frequently affected than women. As mentioned above, CSA is characterized by weakness and wasting in the upper extremities without apparent sensory deficits, lower extremity symptoms, or sphincter dysfunction (Fig. 1). CSA is usually unilateral and occasionally bilateral [19,27,29]. Sonoo [23] reported that symptoms progressed within 1 month in 79% and 84% of patients with proximal and distal type CSA, respectively. In patients with proximal type CSA, impairment of shoulder abduction and elbow flexion is commonly found because of C5 or C6 segmental disorders. Dominant muscle atrophy in the deltoid and infraspinous muscles indicates that C5 is the main responsible level. Hand joint extension and pronator teres muscle function are frequently impaired when C6 is involved. In patients with distal type CSA, wasting and weakness of the extrinsic and intrinsic hand muscles occur in the presence of C7ŌĆōTh1 lesions. Among these symptoms, a positive finger drop sign is the most frequent disability with an incidence of about 60%, in which the C8 segment is speculated to be predominantly involved. Atrophy of the triceps brachii muscle is often also present [23]. Distal type CSA is characterized by a lower incidence, longer preoperative period, higher number of stenotic canal levels, and higher incidence of an HIA on T2-weighted MRI [4,30]. Dropped head syndrome resulting from posterior neck and shoulder girdle muscle atrophy has rarely been reported among patients with CSA [31,32].
Some authors have reported the prevalence of various compression sites in patients with CSA (Fig. 2). Compression sites are divided into several groups, including the foraminal area, preforaminal area (ventral nerve root), paramedian area (anterior horn), the region medial to the spinal canal, and combinations of these. However, especially in patients who are older or have severe degenerative change of the cervical spine, the compression site may be difficult to determine. Furthermore, neural compression around the foraminal area is often difficult to evaluate on MRI alone. Fujiwara et al. [4] reported that 53% of patients had impingement against both the anterior horn and ventral nerve root in CTM and MRI studies. They also indicated that the surgical outcome in patients with impingement against the anterior horn or combined impingement against both the anterior horn and ventral nerve root was inferior to that in patients with impingement of only the ventral nerve root. When the responsible lesions are in the spinal cord, there is a discrepancy between the spinal cord segment and the intervertebral level. Therefore, in this situation, patients with proximal type CSA have a cord lesion at the C4/5 disc level (indicating the anterior horn at the C5/6 cord level), and patients with distal type CSA have a cord lesion at the C5/6 and C6/7 disc levels (indicating a C7ŌĆōTh1 cord lesion) [3,7].
Electrophysiological examination has several important roles in the diagnosis and treatment of CSA. Nerve conduction velocity, EMG, motor and sensory evoked potentials can be helpful to obtain a definite diagnosis of CSA and to differentiate CSA from similar diseases. Some of these examinations also be proved to predict prognosis in patients with CSA after conservative and surgical treatment.
Standard needle EMG in patients with CSA demonstrates denervation potentials and decreased motor unit potentials in the atrophic muscles without abnormal findings in the other muscles. These pathological findings may be observed in paraspinal muscle corresponding segmental cervical level without abnormal findings in thoracic level [23]. Fibrillation and positive sharp waves on EMG indicate anterior radix or nerve root injury of the atrophic muscle, whereas fasciculation and synchronization implicate lower motor neuron injury of the anterior horn.
Some authors have suggested that the preoperative value of the compound muscle action potential (CMAP) stimulated at ErbŌĆÖs point is a good indicator when deciding whether to perform surgery and predicting the treatment efficacy [4,6]. Fujiwara et al. [4] described the relationship between the percentage amplitude of CMAPs in the most severely atrophic muscle and the surgical result in patients with proximal and distal types of CSA. Patients who had a percentage amplitude of CMAPs of Ōēź10% on the affected side than the normal side were able to recover muscle function [4]. Imajo et al. [33] reported that patients with proximal type CSA who had an average CMAP amplitude of >50% on the affected side compared with the normal side had good recovery even after conservative treatment. They also found that a CMAP amplitude of >10 mV on the normal side was a favorable factor indicating no involvement of the anterior horn. In another article, Imajo et al. [6] demonstrated that a CMAP amplitude of 30% to 50%, evaluated in the same manner, was associated with favorable surgical outcomes in patients with proximal type CSA. The sensory nerve action potential in the upper extremity is basically intact in patients with CSA because the main lesion of CSA is more proximal than the dorsal root ganglion [24].
Motor neuron disease is the most critical condition to distinguish from CSA. Some differential diagnoses for motor function disorders include regional cerebral infarction at shoulder or hand motor cortex, muscle dystrophy, posterior interosseous nerve palsy, rupture of the distal extensor tendons, Subtype of Guillain-Barr├® syndrome such as acute motor axonal neuropathy, and Hirayama disease. Other diseases that can cause muscle wasting and atrophy, such as neuralgic amyotrophy, thoracic outlet syndrome, cubital tunnel syndrome, and rotator cuff lesions, may be considered if sensory deficits and pain are minimal.
Symptoms of CSA usually stabilize or improve by conservative treatment alone. Previous studies showed that conservative treatment including cervical traction, immobilization of the neck using a collar, physical therapy, and administration of vitamin B12 or E was effective with a 58% recovery rate in patients with proximal type CSA [34,35]. However, surgical management is generally indicated in cases of progressive or severe neurological deterioration in spite of conservative management.
In our study, a favorable outcome was seen 76% of all patients (proximal type, 72.2%; distal type, 78.6%). This result seems to be acceptable compared to previous reports. In previous articles, several authors reported the surgical outcomes in patients with CSA and analyzed predictive factors related to unfavorable surgical results (Tables 4, 5) [3-16]. The total incidence of favorable surgical outcomes, including all types of CSA, ranged from 70% to 96%. This incidence in patients with proximal and distal type CSA was 58% to 100% and 38% to 86%, respectively. There was no statistical significance between proximal type and distal type CSA in the present study. However, certain difference of mean age at operation between two groups had a possibility to influence. Some articles have indicated distal type CSA is a negative prognostic factor because of the presence of intramedullary gray matter lesions around the anterior horn and a much longer distance to the atrophied hand muscle than in proximal type CSA [4,8,13,14,24]. From analysis of predictive factor related to prognosis, older age was the only factor associated with an unfavorable outcome whereas no significant differences were found in the other factors. In an overview of previous reports, older age at operation was not an indicator of poor prognosis. The reason of this result is questionable, however, mean age in the unfavorable outcome group had a gap more than 10 years in comparison with that in the favorable outcome group. And in the unfavorable outcome group there was a tendency of a longer duration and severe preoperative MMT grade in spite of no statistical difference. We found that a longer duration of illness and preoperative MMT grade were poor prognostic factors among various patient characteristics. In their multivariate analysis, Tauchi et al. [13] found that the symptom duration was a factor that affects the prognosis. According to the receiver operating characteristic analysis of a different study, Tauchi et al. [15] recommended 4.3 months from symptom onset to surgery as the most appropriate timing with which to avoid an unfavorable postoperative course in patients with proximal type CSA. With respect to the preoperative physical status, the severity of the MMT grade and presence of long tract signs were related to poor recovery in some studies [5,13,15]. Electrophysiological examination has also shown that lower preoperative CMAP on the affected side than normal side is a predictably useful finding [4,6]. No clear evidence supports which approach or surgical method is the most effective for patients with CSA. According to one review article, the anterior approach is preferred for patients with a ventral cord lesion involving one or two intervertebral levels, whereas posterior decompression is preferred for lesions involving more than 2 intervertebral levels in the presence of a narrow spinal canal [30]. Thus, the surgical strategy is basically similar to that for degenerative cervical disease. And previous reports showed various approach and operation for patient with CSA considering their responsible lesions and condition of the cervical spine. Although additional anterior or posterior foraminotomy is performed in many cases, whether these procedures are necessary remains unclear.
The present study has several limitations. First, this study was retrospective and consisted of a small number of patients. This may explain the differences between our study and previous reports with regard to prognostic surgical factors for CSA despite the fact that the patientsŌĆÖ clinical features were similar to those in previous studies. Second, the patient selection process was biased. We did not use a prescribed protocol of conservative management or consistent surgical indications. Therefore, the surgical timing and methods varied, and these factors undoubtedly influenced the surgical outcome and risk factor analysis. Third, evaluation of postoperative muscle strength was performed 12 months after surgery. The follow-up periods in other reports are longer than ours. We believe that the timing of the examination was sufficient to judge maximum recovery of muscle strength; however, it was not always adequate for distinguishing CSA from similar diseases. The number of clinical series to date that have focused on CSA is insufficient to investigate the most appropriate timing and choice of surgical treatment. Therefore, further studies are needed to clarify the prognostic factors that lead to better surgical outcomes. However, the current article is a helpful updated overview of CSA and provides important information regarding treatment and prognosis.
The present study examined the clinical characteristics and surgical results in patients who were treated for CSA in our institute, including updated information from a literature review. Surgical outcomes and predictive factors related to a poor prognosis were summarized and compared with those of previous articles. This article also identified several factors that might be related to the surgical prognosis in patients with CSA.
Fig.┬Ā1.
(A, B) Muscle atrophy in a patient with proximal type cervical spondylotic amyotrophy. Muscle atrophy was more remarkable on the affected side (A, arrows) than the normal side (B). (C, D) Finger drop finger sign and atrophy in the intrinsic hand muscles in a patient with distal type cervical spondylotic amyotrophy.
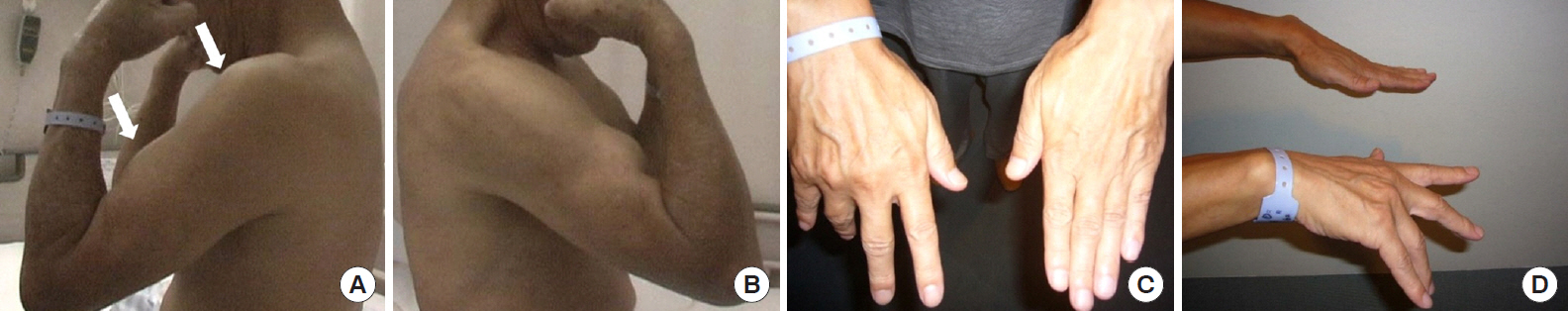
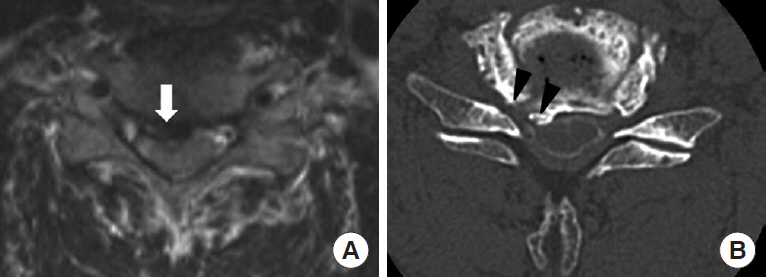
Fig.┬Ā2.
(A) Magnetic resonance imaging demonstrating compression of the unilateral ventral cord and root by degenerative change of the cervical spine (arrow). (B) Computed tomography myelography showing compression of the ventral nerve root due to an osteospur from the origin of the ventral rootlet to the entrance of the cervical foramen (arrowheads).
Table┬Ā1.
Demographic and clinical data of all patients with CSA (n=33)
Table┬Ā2.
Demographic and clinical data of patients with proximal type and distal type CSA
Table┬Ā3.
Demographic and clinical data of patients with favorable and unfavorable outcomes
Table┬Ā4.
Summary of clinical outcomes after surgery for CSA: literature review
| Study | No. of cases | Age (yr) | Sex | Duration (mo) | Type | Preop MMT | Postop MMT | Follow-up (mo) | Operation | Favorable outcomeŌĆĀ, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Matsunaga et al., [9] 1993 | 12* | 57.6 | M: 11, F: 1 | NA | P: 11, D: 1 | NA | NA | NA | LP+FR: 7 | 8 (89) |
| AD: 2 | ||||||||||
| Shinomiya et al., [10] 1994 | 10 | 51.7 | M: 8, F: 2 | NA | P | 2.6 | 4.7 | 52 | ADF | 10 (100) |
| Ebara et al., [3] 1988 | 15* | NA | NA | NA | D | NA | NA | 12ŌĆō24 | LP: 6 | 6 (86) |
| ADF: 1 | ||||||||||
| Kaneko et al., [8] 2004 | 6 | 69.5 | M: 2, F: 4 | NA | D | NA | NA | NA | LP: 6 | 4 (67)ŌĆĪ |
| Fujiwara et al., [4] 2006 | 32 | 59.8 | M: 31, F: 1 | 11 | P: 24, D: 8 | NA | NA | 78 | LP: 10 | P: 22 (92) |
| LP+FR: 22 | D: 3 (38) | |||||||||
| Srinivasa Rao and Rajshekhar, [11] 2009 | 7 | 46.4 | M: 7, F: 0 | 8.3 | D | NA | NA | 46.5 | ADF | 4 (67) |
| Uchida et al., [16] 2009 | 51 | 60.1 | M: 43, F: 8 | 12.6 | P: 37, D: 14 | NA | NA | 31 | LP+FR: 12 | P: 23 (62) |
| AD: 39 | D: 5 (36) | |||||||||
| Inui et al., [7] 2011 | 90* | 54.5 | M: 76, F: 14 | NA | P: 55, D: 24, C: 6 | NA | NA | 60 | LP: 5 | 28 (82) |
| LP+FR: 22 | ||||||||||
| ADF: 7 | ||||||||||
| Imajo et al., [6] 2012 | 24 | 61.2 | M: 22, F: 2 | 18.8 | P | NA | NA | 50 | LP: 2 | 14 (58) |
| LP+FR: 11 | ||||||||||
| ADF: 11 | ||||||||||
| Tauchi et al., [13] 2013 | 59 | 59.4 | M: 56, F: 3 | 11.4 | P: 41, D: 18 | 2.3 | NA | 32 | LP (┬▒ FR): 45 | 41 (70) |
| ADF: 8 | ||||||||||
| PF: 6 | ||||||||||
| Takebayashi et al., [12] 2013 | 28 | 50.6 | M: 25, F: 3 | NA | P: 19, D: 9 | NA | NA | 43.5 | LM+FR | 27 (96) |
| Iizuka et al., [5] 2014 | 47* | 61.5 | M: 38, F: 9 | 30.0 | P: 35, D: 12 | 1.7 | NA | 18.0 | LP: 14 | 15 (83) |
| ADF: 4 | ||||||||||
| Tauchi et al., [14] 2014 | 17 | 56.3 | M: 16, F: 1 | 11.8 | D | 2.4 | 3.4 | 35.2 | LP: 17 | 9 (53) |
| LP+FR: 1 | ||||||||||
| ADF: 3 | ||||||||||
| LP+FR+PF: 2 | ||||||||||
| Tauchi et al., [15] 2015 | 41 | 60.5 | M: 39, F: 2 | 12.0 | P | 2.2 | NA | 31.3 | LP (┬▒ FR): 22 | 31 (76) |
| ADF: 5 | ||||||||||
| PF (+LP or LM): 4 | ||||||||||
| Present study | 33 | 59.7 | M: 27, F: 6 | 7.00 | P: 18, D: 14, C: 1 | 2.7 | 3.8 | 12 | LP (┬▒ FR): 8 | 25 (76) |
| AD: 3, ADF: 18 | ||||||||||
| PF (+LP or LM): 2 | ||||||||||
| Combined AP: 2 |
CSA, cervical spondylotic amyotrophy; Duration, duration of illness; Preop, preoperative; Postop, postoperative; MMT, manual muscle test; NA, not available; P, proximal; D, distal; LP, laminoplasty; LM, laminectomy; FR, foraminotomy; AD, anterior decompression; ADF, anterior decompression and fusion; PF, posterior fusion; AP, anteroposterior.
Table┬Ā5.
Predictive factors of postoperative unfavorable results in patients with CSA
| Authors | Age (yr) | Sex | DM | Pain | Duration | Type | Preop MMT | Long tract signs | Preop CMAP | Levels of stenosis | Compression site | HIA on T2 MRI | LIA on T1 MRI | Preop kyphosis | Operation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fujiwara et al., [4] 2006 | Distal | Decreased* | |||||||||||||
| Uchida et al., [16] 2009 | NS | Longer | Distal | NS | NS | Medial | NS | ||||||||
| Inui et al., [7] 2011 | NS | NS | NS | NS | NS | NS | NS | ||||||||
| Imajo et al., [6] 2012 | Decreased* | ||||||||||||||
| Tauchi et al., [13] 2013 | NS | LongerŌĆĀ | DistalŌĆĀ | SevereŌĆĀ | NS | NS | NS | NS | NS | ||||||
| Iizuka et al., [5] 2014 | NS | NS | NS | NS | NS | NS | Positive | NS | NS | NS | |||||
| Tauchi et al., [13] 2015 | NS | LongerŌĆĀ | Severe | NS | NS | NS | NS | NS | NS | ||||||
| Present study | Older | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
CSA, cervical spondylotic amyotrophy; DM, diabetes mellitus; Duration, duration of illness; Preop, preoperative; Postop, postoperative; MMT, manual muscle test; CMAP, compound muscle action potential; HIA, high-intensity area; LIA, low-intensity area; NS, not significant; MRI, magnetic resonance imaging.
REFERENCES
1. Mori K, Yamamoto T, Nakao Y, et al. Cervical spondylotic amyotrophy treated by anterior decompression. Three case reports. Neurol Med Chir (Tokyo) 2006 46:366-70.


2. Zhang JT, Yang DL, Shen Y, et al. Anterior decompression in the management of unilateral cervical spondylotic amyotrophy. Orthopedics 2012 35:e1792-7.


3. Ebara S, Yonenobu K, Fujiwara K, et al. Myelopathy hand characterized by muscle wasting. A different type of myelopathy hand in patients with cervical spondylosis. Spine (Phila Pa 1976) 1988 13:785-91.


4. Fujiwara Y, Tanaka N, Fujimoto Y, et al. Surgical outcome of posterior decompression for cervical spondylosis with unilateral upper extremity amyotrophy. Spine (Phila Pa 1976) 2006 31:E728-32.


5. Iizuka Y, Iizuka H, Mieda T, et al. Prognostic factors for cervical spondylotic amyotrophy: are signs of spinal cord involvement associated with the neurological prognosis? Spinal Cord 2014 52:364-7.



6. Imajo Y, Kato Y, Kanchiku T, et al. Prediction of surgical outcome for proximal-type cervical spondylotic amyotrophy novel mode of assessment using compound action potentials of deltoid and biceps brachii and central motor conduction time. Spine (Phila Pa 1976) 2012 37:E1444-9.


7. Inui Y, Miyamoto H, Sumi M, et al. Clinical outcomes and predictive factors relating to prognosis of conservative and surgical treatments for cervical spondylotic amyotrophy. Spine (Phila Pa 1976) 2011 36:794-9.


8. Kaneko K, Taguchi T, Toyoda K, et al. Distal-type cervical spondylotic amyotrophy: assessment of pathophysiology from radiological findings on magnetic resonance imaging and epidurally recorded spinal cord responses. Spine (Phila Pa 1976) 2004 29:E185-8.


9. Matsunaga S, Sakou T, Imamura T, et al. Dissociated motor loss in the upper extremities. Clinical features and pathophysiology. Spine (Phila Pa 1976) 1993 18:1964-7.


10. Shinomiya K, Komori H, Matsuoka T, et al. Neuroradiologic and electrophysiologic assessment of cervical spondylotic amyotrophy. Spine (Phila Pa 1976) 1994 19:21-5.


11. Srinivasa Rao NV, Rajshekhar V. Distal-type cervical spondylotic amyotrophy: incidence and outcome after central corpectomy. J Neurosurg Spine 2009 10:374-9.


12. Takebayashi T, Yoshimoto M, Ida K, et al. Minimum invasive posterior decompression for cervical spondylotic amyotrophy. J Orthop Sci 2013 18:205-7.


13. Tauchi R, Imagama S, Inoh H, et al. Risk factors for a poor outcome following surgical treatment of cervical spondylotic amyotrophy: a multicenter study. Eur Spine J 2013 22:156-61.



14. Tauchi R, Imagama S, Inoh H, et al. Characteristics and surgical results of the distal type of cervical spondylotic amyotrophy. J Neurosurg Spine 2014 21:411-6.


15. Tauchi R, Imagama S, Inoh H, et al. Appropriate timing of surgical intervention for the proximal type of cervical spondylotic amyotrophy. Eur J Orthop Surg Traumatol 2015 25 Suppl 1:S107-13.



16. Uchida K, Nakajima H, Yayama T, et al. Anterior and posterior decompressive surgery for progressive amyotrophy associated with cervical spondylosis: a retrospective study of 51 patients. J Neurosurg Spine 2009 11:330-7.


17. Brain WR, Northfield D, Wilkinson M. The neurological manifestations of cervical spondylosis. Brain 1952 75:187-225.



19. Keegan JJ. The cause of dissociated motor loss in the upper extremity with cervical spondylosis. J Neurosurg 1965 23:528-36.


20. Sobue I, Kato H, Yanagi T. Clinical characteristics and classification of cervical spondylotic myelopathy. Rinsho Seikeigeka 1975 10:999. -1006. (Japanese).
21. Yanagi T, Kato H, Sobue I. Clinical characteristics of cervical spondylotic amyotrophy. Rinsho Shinkeigaku 1976 16:520-8.

22. Sonoo M, Kuwabara S, Shimizu T, et al. Utility of trapezius EMG for diagnosis of amyotrophic lateral sclerosis. Muscle Nerve 2009 39:63-70.


24. Sonoo M. Electrodiagnosis of cervical spondylotic amyotrophy. Spine Spinal cord 2017 30:547. -51. (Japanese).
25. Ota H, Ono K. Dissociated motor loss in the upper extremity with cervical spondylosis. Rinsho Seikei Geka 1975 10:114. -31. (Japanese).
26. Tanaka N, Fujimoto Y, An HS, et al. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine (Phila Pa 1976) 2000 25:286-91.


27. Kameyama T, Ando T, Yanagi T, et al. Cervical spondylotic amyotrophy. Magnetic resonance imaging demonstration of intrinsic cord pathology. Spine (Phila Pa 1976) 1998 23:448-52.


28. Fujiwara K. Cervical spondylotic amyotrophy with intramedullary cavity formation. Spine (Phila Pa 1976) 2001 26:E220-2.


29. Gebere-Michael SG, Johnston JC, Metaferia GZ, et al. Bilaterally symmetric cervical spondylotic amyotrophy: a novel presentation and review of the literature. J Neurol Sci 2010 290:142-5.


31. Ahdab R, Cr├®ange A, Benaderette S, et al. Cervical spondylotic amyotrophy presenting as dropped head syndrome. Clin Neurol Neurosurg 2009 111:874-6.


32. Taniguchi S, Takahashi H, Aoki Y, et al. Surgical treatment for dropped head syndrome with cervical spondylotic amyotrophy: a case report. BMC Res Notes 2018 11:500.




33. Imajo Y, Kato Y, Kanchiku T, et al. Pathology and prognosis of proximal-type cervical spondylotic amyotrophy: new assessment using compound muscle action potentials of deltoid and biceps brachii muscles. Spine (Phila Pa 1976) 2011 36:E476-81.


34. Nagata K, Ohashi T, Ishibashi K, et al. Effectiveness of conservative treatment for Keegan type upper extremity dysfunction. Seikei Saigai Geka 1996 39:131. -7. (Japanese).
35. Nagata K, Satou K, Tsuru M, et al. Clinical results of cervical spondylotic amyotrophy (Keegan type dissociated motor loss). Spine Spinal Cord 2009 22:1117. -23. (Japanese).




























