Opioids and Spinal Cord Stimulators: Pre- and Postoperative Opioid Use Patterns and Predictors of Prolonged Postoperative Opioid Use
Article information
Abstract
Objective
The aim of the study was to compare trends and differences in preoperative and prolonged postoperative opioid use following spinal cord stimulator (SCS) implantation and to determine factors associated with prolonged postoperative opioid use.
Methods
A database of private-payer insurance records was queried to identify patients who underwent a primary paddle lead SCS placement via a laminectomy (CPT-C3655) from 2008–2015. Our resulting cohort was stratified into those with prolonged postoperative opioid use, opioid use between 3- and 6-month postoperation, and those without. Multivariate logistic regression was used to determine the effect preoperative opioid use and other factors of interest had on prolonged postoperative opioid use. Subgroup analysis was performed on preoperative opioid users to further quantify the effect of differing magnitudes of preoperative opioid use.
Results
A total of 2,374 patients who underwent SCS placement were identified. Of all patients, 1,890 patients (79.6%) were identified as having prolonged narcotic use. Annual rates of preoperative (p = 0.023) and prolonged postoperative narcotic use (p < 0.001) decreased over the study period. Significant independent predictors of prolonged postoperative opioid use were age < 65 years (odds ratio [OR], 1.52; p = 0.004), male sex (OR, 1.33; p = 0.037), preoperative anxiolytic (OR, 1.55; p = 0.004) and muscle relaxant (OR, 1.42; p = 0.033), and narcotic use (OR, 15.04; p < 0.001). Increased number of preoperative narcotic prescriptions correlated with increased odds of prolonged postoperative use.
Conclusion
Patients with greater number of preoperative opioid prescriptions may not attain the same benefit from SCSs as patients with less opioid use. The most significant predictor of prolonged narcotic use was preoperative opioid use.
INTRODUCTION
Neuropathic pain is the sensation of discomfort in response to injury or dysfunction of the central or peripheral nervous system. It is very common, with an estimated prevalence between 6.9% and 10% of the general population, most commonly affects the legs and back, and is more likely to be chronic neuropathic than nociceptive pain [1]. In its chronic form, neuropathic pain can cause severe disability and plays a role in increasing both indirect and direct healthcare costs [2]. Furthermore, chronic neuropathic pain is notoriously difficult to manage, and is known to be less responsive than nociceptive pain to analgesic drugs like opioids [3]. As the United States faces an opioid epidemic and the use of opioid analgesics has been put under scrutiny, there is increased value in other treatment modalities that can potentially decrease the need for opioids. Persistent leg and back pain after spine surgery have been traditionally managed with reoperation or conservative medical management (CMM) but more recently, spinal cord stimulation (SCS) has emerged as a viable alternative [4]. SCS is indicated for management of disease entities that cause chronic neuropathic pain like postlaminectomy syndrome and complex regional pain syndrome (CRPS) [5,6]. Its efficacy in the treatment of pain caused by these conditions has been established. Kumar et al. [3], in a multicenter randomized clinical trial were able to conclude that addition of SCS provides greater relief from pain, reduced disability, and greater satisfaction when compared to CMM alone. The PROCESS (prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation) trial showed that patients receiving SCS for failed back surgery syndrome (FBSS) can expect their improvements to be sustained through 24 months [7]. In light of these and similar findings, SCS has become a more desirable option than prolonged opioid therapy.
While SCS has been shown to be a viable option for the treatment of chronic neuropathic pain, there is a paucity of literature describing opioid use in this patient population. Using frequency of opioid prescriptions, we assessed opioid use history before and after SCS placement to investigate effectiveness of SCS in the treatment of chronic neuropathic pain. The aim of this study was (1) to compare the trends and differences in preand postoperative oral prescription opioid usage in SCS patients and (2) to determine factors associated with prolonged postoperative opioid use in the same patient population.
MATERIALS AND METHODS
1. Data Source
A retrospective database review was conducted utilizing the commercially available PearlDiver Patient Records Database (www.pearldiverinc.com; PearlDiver Inc., Colorado Springs, CO, USA). This database contains private-payer insurance (Humana) patient records from 2008–2015, searchable by International Classification of Diseases, Current Procedural Terminology (CPT), Diagnosis Related Group codes among others. Institutional Review Board approval and need for informed consent were waived for this study as all queried data are de-identified and Health Insurance Portability and Accountability Act compliant.
2. Study Cohort
Our study includes all private-payer insurance beneficiaries who underwent a primary paddle lead SCS implantation via laminectomy (CPT-63655) between 2008 and 2015. Percutaneous cylindrical lead placements (CPT-63650) were not included because coding does not differentiate between trial and permanent cylindrical lead placement. Patients were included if they underwent primary placement of a paddle lead SCS with at least 6 months of active enrollment before and after placement. Patients with a history of spine infection, spine trauma, and primary malignancy or metastasis of the spine were excluded from our study cohort. Patient demographic parameters including age, sex, preoperative substance use, and comorbidities were identified.
3. Opioid Use History Before and After SCS Implantation
A query of commonly prescribed opioids, as described by Anciano Granadillo et al. [8], was made. Using National Drug Code, the following brand name and generic opioid formulations were included in our study: hydrocodone, hydromorphone, morphine, fentanyl, oxymorphone, propoxyphene, oxycodone, and oxycontin. Following the identification of oral prescription opioids, our resulting cohort was further arbitrarily stratified into 4 exclusive categories based on the number of filled narcotic prescriptions (1, 2, 3, and 4 or more) between 1 and 4 months prior to the date of SCS implantation. Patients with filled narcotic prescriptions only within the month leading up to surgery were excluded to reduce the risk of including patients who were preemptively filling postoperative opioids within days prior to their index operation. The same patient cohort was followed for up to 6 months postoperatively. Prolonged narcotic use was defined as opioids usage based on any filled narcotic prescription between 3 and 6 months following SCS placement. The 3- to 6-month window was selected as not to include narcotic prescriptions for subsequent unrelated surgeries requiring additional narcotic prescriptions. Our primary aim was to evaluate if differences in preoperative opioid use, stratified by the number of opioid prescriptions, affected prolonged opioid use following surgery. For that purpose, we analyzed the proportion of prolonged postoperative opioid use within each of our preoperative subgroups, included those with no preoperative opioid usage. To better establish patterns of perioperative opioid use, yearly trends of preoperative and prolonged postoperative opioids use were also evaluated.
4. Predictors of Prolonged Postoperative Opioid Use
Our study cohort was evaluated to identify factors associated with prolonged postoperative opioid use following SCS placement. We analyzed several robust variables such as pertinent demographic parameters (age and sex), comorbid diagnosis (obesity, morbid obesity, depression, FBSS, CRPS, lumbago/backache, neuritis/radiculitis, migraines, and fibromyalgia), substance abuse history (tobacco, alcohol, amphetamines, marijuana, and cocaine), and other preoperative pain medications (tramadol, methadone, anxiolytics, and muscle relaxants) in addition to the preoperative opioid use categories described above.
5. Data Analysis
We investigated the rate of SCS placement within various demographic parameters, including age range (< 65, 65–84 years), sex, comorbidity status, and preoperative substance use. Changes in annual rates of preoperative and prolonged postoperative narcotic use were compared using chi-square analysis. Patient’s postoperative opioid requirements were determined by analyzing the proportion of prolonged narcotic use within each preoperative opioid use subgroup; including those did not use preoperative opioids. Finally, multivariate logistic regression was used to identify the afore mentioned factors that were independently associated with prolonged postoperative opioid use, controlling for the pertinent demographic parameters, substance abuse history, preoperative pain medication use, diabetes mellitus, hyperlipidemia, hypertension, peripheral vascular disease, chronic liver disease, chronic kidney disease, congestive heart failure, coronary artery disease, and chronic obstructive pulmonary disease in addition to the afore mentioned comorbidities. R ver. 3.6.1 Project for Statistical Computing (Vienna, Austria) available through our database was used for statistical analysis. Factors were considered significant at p < 0.05.
RESULTS
1. Patient Demographics
A total of 2,374 patients who underwent paddle lead SCS implantation were included in this study. Of all subjects, 1,347 (56.7%) were aged 65 years or younger, while 1,136 (57.7%) were women. Prior to SCS implantation 1,819 patients (76.62%) had some degree of preoperative opioid use and 1,890 patients (79.61%) had prolonged use following SCS implantation (Table 1). Of those with preoperative opioid use 194 (10.67%) had 1 prescription, 188 (10.34%) had 2 prescriptions, 246 (13.52%) had 3 prescriptions, and 1,191 (65.48%) had 4 or more prescriptions between 1 and 4 months prior to SCS placement.
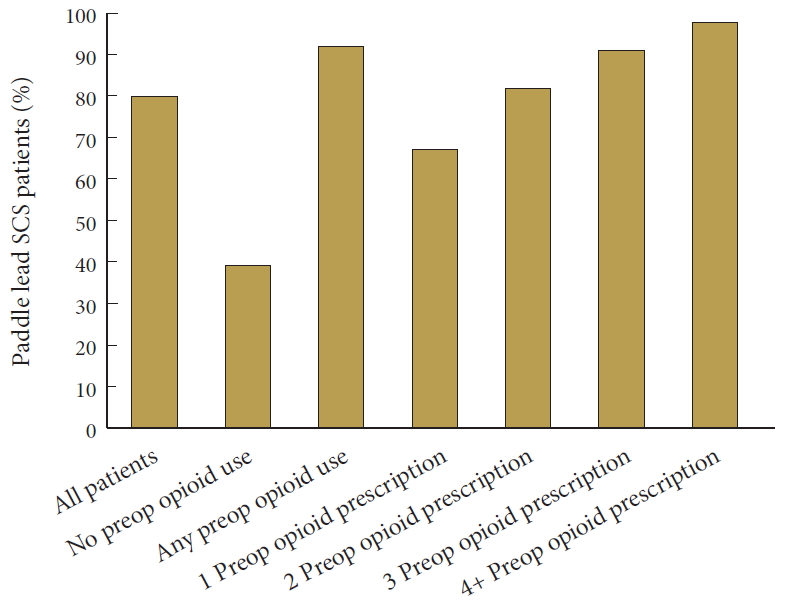
2. Trends in Preoperative and Prolonged Postoperative Opioid Use
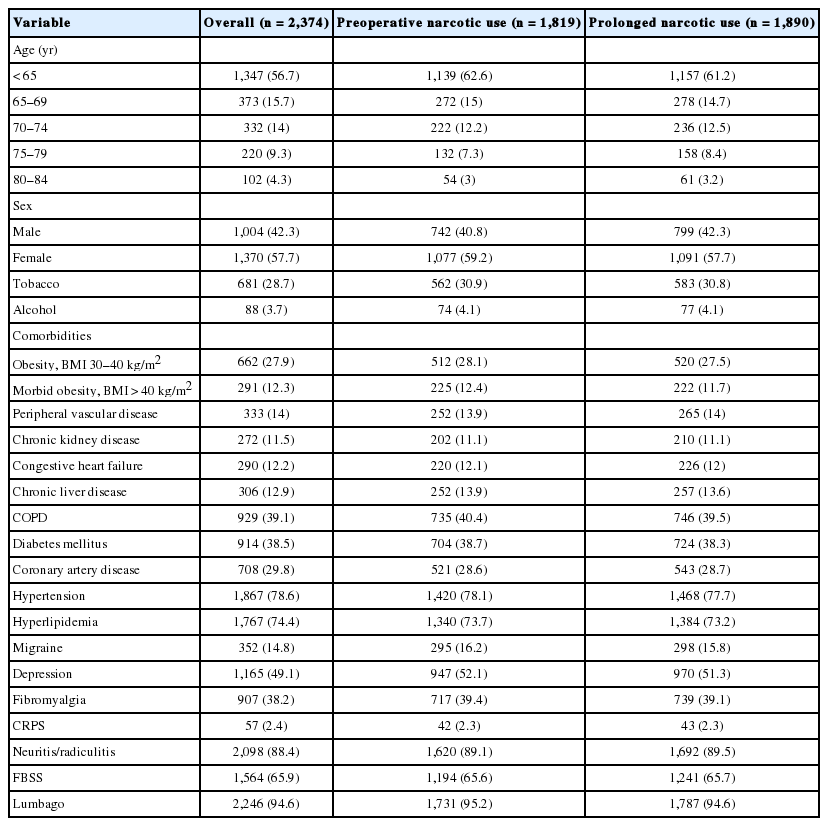
There was a significantly decreasing annual trend of preoperative opioid use (p = 0.023) between the years of 2008 to 2015, with peak values in 2010. Likewise, prolonged postoperative opioid use (p < 0.001) significantly decreased over the same time period, with rates peaking in 2009 (Fig. 1). Of those patients with no preoperative opioid use, 217 (39.10%) went on to have prolonged use. Among patients with any preoperative use, 1,673 (91.97%) had prolonged use, while within the preoperative opioid use subgroups, 130 patients (67.01%) with one preoperative prescription, 155 (82.54%), with 2 prescriptions, 223 (90.65%) with 3 prescriptions, and 1,165 (97.82%) with 4 or more prescriptions developed prolonged use (Fig. 2).
Trends in preoperative (preop) and prolonged postoperative (postop) opioid use in patients undergoing paddle lead spinal cord stimulator (SCS) from 2008 to 2015.
3. Predictors of Prolonged Postoperative Opioid Use
Several significant risk factors for prolonged opioid use following paddle lead SCS were identified (Table 2). Age < 65 years (odds ratio [OR], 1.52; 95% confidence interval [CI], 1.14–2.02; p = 0.004) and male sex (OR 1.33; 95% CI 1.02–1.73; p = 0.037) were the only demographic parameters significantly associated with prolonged postoperative opioid usage. Overall preoperative opioid use (OR, 15.04; 95% CI 11.69–19.46; p < 0.001) was the most significant risk factor for prolonged postoperative opioid use. As the number of preoperative opioid prescriptions increased, a trend of increasing odds of prolonged postoperative opioid use was observed –1 prescription (OR, 2.95; 95% CI, 2.07–4.24; p < 0.001), 2 prescriptions (OR, 6.83; 95% CI, 4.50–10.62; p < 0.001), 3 prescriptions (OR, 13.8; 95% CI, 8.76–22.67; p < 0.001), 4 or more prescriptions (OR, 60.64; 95% CI, 39.87–95.93; p < 0.001). In addition, preoperative anxiolytic (OR, 1.55; 95% CI, 1.15–2.10; p = 0.004) and muscle relaxant use (OR, 1.42; 95% CI, 1.03–1.97; p = 0.033) was found to be significantly associated with prolonged postoperative opioid use (Table 2).
DISCUSSION
Prescription opioid use amongst spine patients is an area of clinical interest as it relates to postoperative opioid use and surgical outcomes in spine surgery [9-13]. Upon examination of 2,374 patients who underwent implantation of a paddle lead SCS via a laminectomy, we observed that patients with greater number of preoperative opioid prescriptions were more likely have prolonged opioid use postoperatively. Approximately 39.1% of patients with no opioid use preoperatively went on to have prolonged postoperative narcotic use. This is in contrast to SCS patients with any preoperative opioid use, where 91.97% of patients developed prolonged postoperative opioid use. Subgroup analysis of this population found the highest rates of prolonged postoperative narcotic use was among patients with 4 or more preoperative narcotic prescriptions (97.82%). Furthermore, our multivariate analysis showed that age (less than 65 years), male sex, and preoperative anxiolytic and muscle relaxant use were additional significant independent predictors of prolonged opioid use following SCS placement. However, overall preoperative opioid use was the most significant predictor for prolonged opioid use postoperatively.
A number of studies in orthopaedic surgery have reported similar findings regarding predictors for prolonged postoperative opioid use. In a review of 8,377 adult patients who underwent lumbar fusion, Connolly et al. [14] reported that preoperative opioid use was significantly associated with an increased risk of long-term (> 365 days of opioid prescription filled) opioid use postoperatively. As the category of preoperative opioid demand increased, odds of long-term opioid use following lumbar fusion also increased. This is similar to our results on the predictors of prolonged postoperative opioid use. Likewise, Anciano Granadillo et al. [8] reported that one of the most significant predictors for prolonged postoperative opioid use following hip arthroscopy was having 4 or more opioid prescriptions filled within 4-months preoperatively (OR, 19.55; p < 0.001), while Agarwalla et al. [15] found similar results among patients requiring hip arthroplasty for osteoarthrosis (OR, 12.43; p < 0.001) and hip fracture (OR, 51.18; p < 0.001). Anderson et al. [16] also demonstrated that chronic opioid therapy before lumbar fusion was significantly associated with chronic opioid therapy after fusion (OR, 6.15; p < 0.001) in a review of 1,002 workers’ compensation patients.
Multiple studies have demonstrated that SCS is not only efficacious but also a superior option for the management of chronic neuropathic pain caused by syndromes like FBSS and CRPS when compared to other currently available management strategies [3-6]. Some of its advantages include the fact that it is a single, reversible intervention, which has been proven to have continued benefits years after implantation [7]. The specter of opioid epidemic that has plagued the U.S. in recent years only serves to magnify the lure of nonpharmacologic options for the management of chronic neuropathic pain. A number of studies have supported that the success of SCS is contingent on time from chronic pain diagnosis to SCS implantation, as well as the level of opioid use prior to SCS implantation [17-21]. The effects of opioids on SCS outcomes have been highlighted in a few studies. Using device explant as a marker of SCS failure, Sharan et al. [17] found that patients who failed SCS were more likely to be prescribed high opioid doses (at least 90 mg/day). Khan et al. [19] found that patients whose pain was severe enough to cause disability, as well as those who required opioids preoperatively were less likely to have pain remission in the year following SCS implantation. Using prolonged postoperative opioid use as a marker for patient’s pain status, in an attempt to measure SCS success, the present study similarly found that a greater proportion of patients in our preoperative 4 or more prescription opioid use subgroup were more likely to have prolonged opioid use after SCS placement compared to opioid naïve and the remaining opioid use subgroups. Interestingly, rates of prolonged postoperative use were higher than preoperative use, despite a reduction in all preoperative use subcategories. This stems from the no opioid use group, of which 39% developed prolonged opioid use. This could be reflective of natural disease progression and failure of alternative options, including SCS. Subgroup analysis of preoperative opioid use revealed that increasing number of preoperative prescriptions correlated with a stepwise rise in odds of prolonged postoperative narcotic use. Sharan et al. [17] further reported that leading up to SCS implantation, patients tended to have high levels of opioid usage. They hypothesized that this trend was partly due to worsening pain experienced by patients and the current dogma of increase in pharmacologic therapy before consideration of device implantation in the management of chronic back pain. Lad et al. [18] and Kumar et al. [20] both identified that a delay in time from the onset of chronic pain to SCS implantation may have a negative impact on the success of SCS therapy. Unfortunately, this study was limited in the ability to track time from diagnosis to time of SCS implantation. However, it is reassuring that trends among both opioid use categories showed significant decreases by the conclusion of the study period, likely representing a paradigm shift away from chronic opioid analgesia and towards alternative pain management modalities. Inversely, throughout the study period the annual rate SCS implantations rose on a near yearly basis reflecting the growing popularity of this treatment option for the management of chronic neuropathic pain.
To our knowledge, this is the first study describing the difference in preoperative and postoperative opioid use following SCS implantation; however, our study has a number of limitations. The use of a claims database relies on the coding habits of physicians, and any errors made may influence our findings. Further, due to the limitations imposed by our database, we were unable to determine the actual dose of opioids prescribed in each prescription. We quantified narcotic use by number of prescriptions filled as opposed to calculating morphine equivalents as described by other similar studies [3,17]. However, this method of determining preoperative and prolonged postoperative narcotic use and the association between them has been proven reliable over multiple prior studies [8,15,22,23]. Our measure of prescriptions also does not account for outside sources of narcotics, nor is it able to quantify the amount of narcotic a patient actually ingested whenever they filled a prescription. Finally, we used prolonged postoperative opioid use as a proxy for pain control as opposed to another clinical measure like the numeric rating scale or visual analogue scale. However, we found this proxy to be appropriate given the evidence that patients with greater opioid requirements tend to have higher pain ratings than patients with lower or no opioid requirements [24,25].
CONCLUSION
Utilizing perioperative opioid prescription history as a marker for patient’s pain status, our results show that the effectiveness of SCS on patients with preoperative opioid use remains questionable. Our findings imply that patients with greater number of preoperative opioid prescriptions prior to SCS implantation may not attain the same benefit from the intervention as patients with less opioid use. These patients may benefit from additional counselling to set appropriate expectations prior to SCS placement.
Notes
The authors have nothing to disclose.