Recovery Potential of Spinal Meningioma Patients With Preoperative Loss of Walking Ability Following Surgery – A Retrospective Single-Center Study
Article information
Abstract
Objective
Spinal meningiomas are neurosurgical rarities that manifest with progressive paraor tetraparesis. The effect of timing of surgery on the recovery after the loss of walking ability is poorly known. We studied the effect of timing of surgery on restoring walking ability in surgically-treated spinal meningioma patients.
Methods
Using electronic health records, we retrospectively identified ≥ 18-year-old patients operated on during 2010–2020. The patients were followed until 30th September 2020, death or emigration.
Results
We identified 108 patients (81% women) with operated spinal meningiomas. The mean age of the patients was 64 years (range, 18–94 years). A gross total resection was achieved in 101 (94%), and 21 patients (19%) suffered from perioperative complications. Of the 108 patients operated on, 49 (45%) could not walk without assistance prior to surgery. At the time of first postoperative visit (mean, 3.1 months; range, 1.3–13.1 months), 14 out of 24 patients (58%) operated on within 29 days and 8 out of 20 patients (40%) operated on later than 29 days since the loss of walking ability without assistance, were able to walk without assistance. Also, 3 out of 5 paraplegic patients who underwent surgery later than 29 days after they lost the walking ability, were able to at least walk with assistance at first postoperative visit.
Conclusion
Early surgical treatment following the loss of walking ability restores walking ability in a substantial number of patients. However, even late surgery may restore walking ability.
INTRODUCTION
Spinal meningiomas are benign intradural but extramedullary tumors that are most often located in the thoracic spine [1,2]. In spite of a low incidence of 0.32–0.33/100,000 persons [3,4], spinal meningiomas account for over one-third of primary spinal tumors [4]. More than 90% of spinal meningiomas are classified as World Health Organization (WHO) grade I tumors [4,5]. Due to their relatively slow growth rate, these tumors can occupy almost the whole spinal canal prior to inducing the most common symptoms, namely para- or tetraparesis and sensory losses [2,6].
The literature about the surgical treatment of spinal meningiomas is limited to a relatively small number of retrospective series. One of the largest retrospective series of 173 operated spinal meningiomas reported that 87% of the patients experienced postoperative functional improvement [1]. However, there is limited literature about the recovery potential following surgery of spinal meningiomas that have caused a preoperative loss of walking ability, or especially, the effect of preoperative time delay from losing walking ability to surgery. Therefore, our aim was to assess the outcomes of specifically those spinal meningioma patients who had lost their walking ability prior to surgery. In other words, this study addresses the question “Will I ever walk again?”; which is almost invariably asked by these patients.
MATERIALS AND METHODS
1. Ethical Consideration
The Institutional Review Board of Helsinki University Hospital approved the data collection and study design (HUS/190/2021). According to Finnish legislation, obtaining informed consent from the patients was waived. We conducted the study in line with the Declaration of Helsinki [7].
2. Study Cohort
Eighteen-year-old and older spinal meningioma patients, operated on between January 2010 and September 2020, were retrospectively identified from electronic health records. The catchment area of the department was around 2 million people during the study period. Patients with a comorbidity that prevented walking preoperatively were excluded.
3. Meningioma Characteristics
Magnetic resonance images were reviewed by 2 study authors (VV, RH). We calculated the number of spinal meningiomas, and defined (based on gadolinium-enhanced T1-weighted images) the craniocaudal (cervical, thoracic, lumbar) and anteroposterior (anterior, lateral, posterior to spinal cord) location, and the maximum tumor diameter of the operated meningioma. The final diagnosis was based on the histology of the tumor.
4. Functional Status
The walking ability was classified (by surgeon) as normal (not assisted), para-/tetraparesis (capable of assisted walking/assisted walking with weakness in upper limbs), and para-/tetraplegia (not capable of assisted walking). The cause for the loss of walking ability was presumably muscle weakness, but due to the retrospective study design, this could not be confirmed. The time interval (days) from loss of walking ability to skin incision was calculated for these patients. Bladder function was divided into normal, mild dysfunction (difficulties in spontaneous urination), and severe dysfunction (including different urinary catheter types).
5. Surgery
Only the patients with first-ever spinal meningioma surgery were included. Surgery was divided into 2 urgency categories: urgent (not booked in advance) and elective (booked in advance). The extent of the meningioma resection was classified according to its Simpson grade [8].
6. Follow-up
We collected data on walking ability and motor deficits at discharge, and at the first and last postoperative outpatient clinic visits. We also assessed the postoperative mortality rates and tumor recurrence rates during follow-up. Patient records were reviewed until the end of September 2020.
RESULTS
1. Patient Characteristics
One hundred eight patients with spinal meningiomas requiring surgical treatment were identified (Table 1). One patient with paraplegia due to a traumatic spine injury years prior to the meningioma surgery was excluded. The mean age of the patients was 64 years (median, 65 years; range, 18–94 years) at the time of the surgery and 87 patients (81%) were women.
2. Meningioma Characteristics
Preoperative spinal magnetic resonance imaging (MRI) studies were available for all patients. The meningiomas were most often located in the thoracic region (78%, Table 1). In the axial plane, the most common meningioma location was lateral to spinal cord (47%). Only 3 patients had multiple spinal meningiomas (2 patients with 2, and 1 with 3 meningiomas).
3. Preoperative Functional Status
The vast majority (91%) of the meningiomas were diagnosed after becoming symptomatic (Tables 2, 3). The most common preoperative symptoms (reported by the patients) were motor (n=78, 72%) and sensory deficits (n=72, 67%). Bladder dysfunction (n=27, 25%) was identified only in patients with motor deficits. Only 10 patients (9%) were preoperatively asymptomatic. Of the 78 patients with preoperative self-reported motor deficits, 36 (46%) were not able to walk without assistance and 13 patients (17%) could not walk at all when assessed by a surgeon. The inability to walk was more common among the patients with thoracic (81% women; mean age, 69 years; 21% anterior to spinal cord) than cervical (60% women; mean age, 67 years; 50% anterior to spinal cord) meningiomas. Only 5 patients (5%) needed preoperative urinary catheter (all women with thoracic meningiomas) and 3 of them were unable to walk at all.

The characteristics of the patients capable of independent and assisted walking, and not able to walk
4. Surgical Indications of Asymptomatic Patients
Of the 10 asymptomatic patients (9%) (Table 3), 2 were operated on because of tumor progression on follow-up magnetic resonance images and 8 to prevent possible tumor progression.
5. Time From Symptom Onset to Surgery
Of the 49 patients who could not walk without assistance, 24 (49%) were operated on urgently (all with thoracic meningiomas). The median interval from the preoperative loss of walking ability to surgery were 25 days (mean, 65 days; range, 1–365 days) for patients with thoracic meningiomas and 60 days (mean, 325 days; range, 18–1,440 days) for those with cervical. The median interval between the loss of ability to walk at all (13 patients with thoracic meningiomas) and surgery was 17 days (mean, 72 days; range, 3–365 days).
6. Surgery
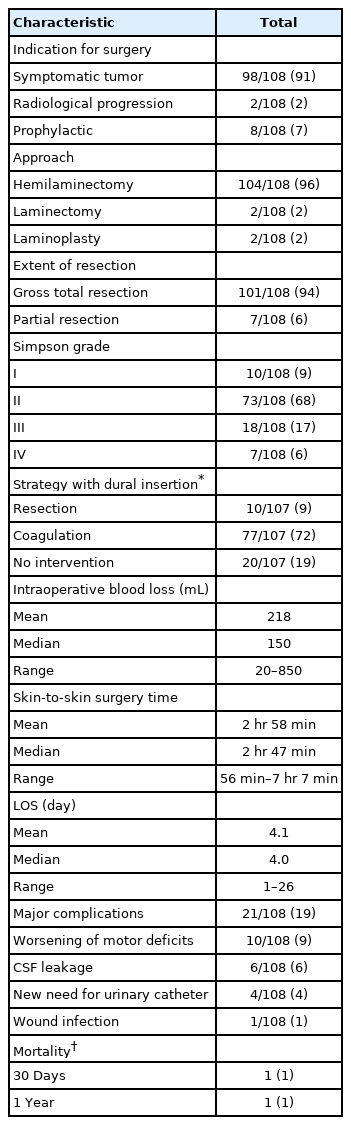
The surgeries were performed by 18 consultant neurosurgeons. Hemilaminectomy was the approach used in 104 surgeries (96%) (Table 3). Gross total resection was achieved in 101 cases (94%). Simpson grade I and II tumor removal was achieved in 10 (9%) and 73 surgeries (68%), respectively. Overall, 21 patients (19%) suffered from major complications, including 10 cases (9%) of worsening of preoperative motor deficits. None suffered from postoperative hematomas and no one died during the hospital stay. No major complications were observed among the asymptomatic patients.
7. Functional Status at Discharge
Of the 10 patients whose motor function decreased perioperatively, 6 had been able to walk without assistance preoperatively but needed assistance with walking on discharge. In addition, 4 were capable of assisted walking preoperatively, but were not able to walk at all on discharge. Furthermore, 3 preoperatively paraparetic/-plegic patients and 1 independently walking patient, none of whom had a preoperative urinary catheter, needed catheters when they were transferred to other healthcare facilities. Of the 13 patients with preoperative complete loss of ability to walk, 5 were capable of assisted walking on discharge, whereas 3 had urinary catheters prior the surgery and 4 after.
8. First Postoperative Visit
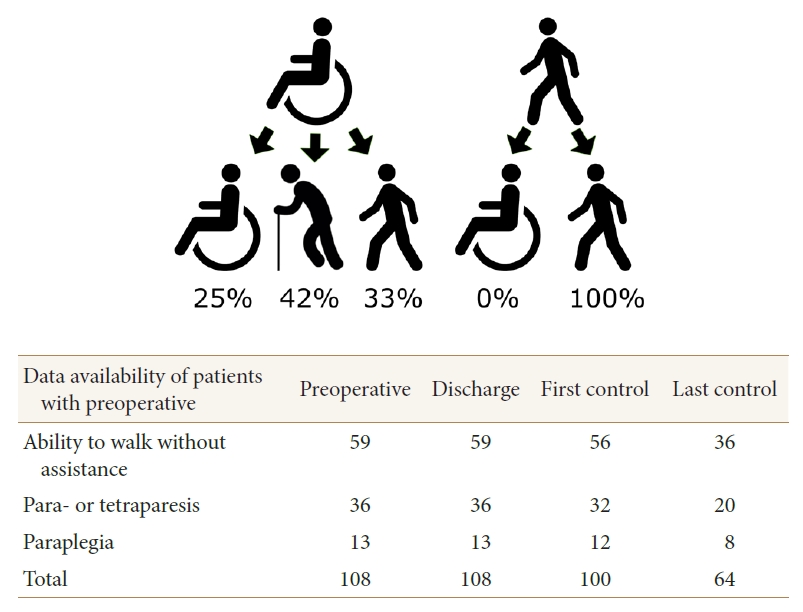
Data on the first postoperative visit were available for 100 out of 108 patients (93%) (Table 2). Of the 8 missing patients, 3 were capable of independent walking and 4 of assisted walking preoperatively, whereas 1 was not able to walk at all. The mean interval between the surgery and the first postoperative visit was 3.1 months (median, 2.8 months; range, 1.3–13.1 months). All 6 patients who deteriorated following the surgery (could not walk without assistance) recovered and walked independently. Similarly, all 4 patients who deteriorated following the surgery (could not walk at all), recovered and became capable of assisted walking. Of the 5 preoperatively tetraparetic, 4 (80%) were able to walk without assistance at the first postoperative visit, and of the 27 preoperatively paraparetic patients, 14 (52%) could do so. Of the 12 paraplegic patients (not able to walk preoperatively), 9 (75%) were able to walk assisted (Fig. 1). None of the patients who had a urinary catheter on discharge needed a catheter at the first postoperative visit.
9. Last Postoperative Visit
Data on the last (other than the first) postoperative visit were available for 64 patients (59%) (Table 2). Of the 44 missing patients, 23 were capable of independent walking and 16 of assisted walking preoperatively, and 5 were not able to walk at all. The mean time interval between the surgery and last postoperative visit was 33.0 months (median, 26.7 months; range, 4.3–108.4 months). The mean overall follow-up time was 57.1 months (median, 45.6 months; range, 0.9–129 months). The overall mortality rate was 6% and unrelated malignancies were the leading causes of death. A recurrent meningioma (mean maximal diameter, 7.8 mm) was diagnosed in 4 patients. Three of the recurrent tumors were operated on.
10. Surgical Delay and Short-term Outcome
The data on the first postoperative visit were available for 44 of the 49 patients (90%) with preoperative loss of walking ability (Table 2). Six of the 7 paraplegic patients operated on within 29 days, and 3 of the 5 patients operated on later than 29 days from the preoperative loss of ability to walk, were able to walk at the first postoperative visit (Fig. 1). Each paraplegic patient (n=4) who was operated on within 8 days from the loss of ability to walk, was capable of at least assisted walking at the first postoperative visit. Nine of the 15 paraparetic patients operated on within 29 days, and 5 of the 12 paraparetic patients operated on later than 29 days since the preoperative loss of ability to walk without assistance, were capable of independent walking at the first postoperative visit. Two tetraparetic patients (100%) operated on within 29 days, and 2 of the 3 tetraparetic patients operated on later than 29 days since the preoperative loss of ability to walk without assistance, were capable of independent walking at the first postoperative visit.
DISCUSSION
In this retrospective study of 108 patients with spinal meningiomas, we found that 49% of the patients with preoperative inability to walk without assistance, were able to walk independently at the first postoperative visit. Even 4 out of 12 preoperatively paraplegic patients (duration of paraplegia, 3–60 days) were able to walk without assistance. All 4 paraplegic patients who were operated within 8 days of the onset of paraplegia regained their capability for at least assisted walking at first postoperative visit. However, 3 out of 5 paraplegic patients who underwent surgery later than 29 days since the onset of paraplegia were capable of at least assisted walking at first postoperative visit. The longest interval between preoperative paraplegia and surgery, in a 77-year-old woman who regained the capability for assisted walking, was 365 days, suggesting that even late surgery for elderly paraplegic meningioma patients may be beneficial. A preoperative bladder dysfunction requiring urinary catheter was observed in 2 paraparetic and 3 paraplegic patients. All these patients got rid of the catheter at the first postoperative visit. Interestingly, bladder dysfunction also recovered among the paraplegic patients, whereas 3 of the 12 paraplegic patients did not regain their capability for assisted walking. This may suggest that bladder dysfunction has a better likelihood of recovery even after late surgery. Even though 6 patients had lost their walking ability and 4 patients their capability for spontaneous urination at surgery, they all fully recovered at the first postoperative visit. The preoperative percentual spinal canal obturation caused by meningioma was not associated with the outcome (results not shown).
Following surgery, an improvement of preoperative neurological deficits has been reported among the majority (85%–90%) of spinal meningioma and schwannoma patients in previous studies [1,2,9-11]. However, only a few studies have also assessed the association between surgical treatment delay and/or the recovery of the patients with preoperative neurological deficits [1,2,10]. Morandi et al. [10] retrospectively studied the effect of surgical treatment (perioperative complication rate 3%) of spinal meningiomas (93% thoracic) among 30 elderly patients (83% women; mean age, 77 years; 83% with preoperative para- or tetraparesis). Their average duration from symptom onset to surgery was 13.4 months. Opposing this study, the length of the preoperative delay was not associated with neurological improvement (also including other than motor deficits). Raco et al. [1] reported the factors associated with poor outcomes in a retrospective cohort of 173 operated (perioperative complication rate 4%) spinal meningioma patients (80% female; mean age, 56 years; 32% with motor deficits), 70% with thoracic meningioma). The average duration from symptoms to surgery was 20 months. Supporting the results of the present study, a longer duration from the onset of symptoms (also including other than motor deficits) to surgery was reported to associate negatively with good functional outcomes. Sacko et al. [2] retrospectively studied the results of surgery (perioperative complication rate 9%) on spinal meningiomas (85% thoracic) in 102 patients (85% women; mean age, 75 years) with preoperative paraparesis or -plegia. The average duration from symptom onset to surgery was 9.5 months. Unlike the findings of the present study, the delay from symptom onset (also including other symptoms than loss of walking ability) to surgery was not associated with the functional outcome. Ashry et al. [12] retrospectively studied the results of surgery on thoracic meningiomas in a sample of 20 paraplegic patients (75% women; median age, 49 years). Opposing the findings of this study, during the postoperative follow-up, 70% of the paraplegic patients recovered to at least assisted walking of whom all had their surgery within 2 weeks after the onset of paraplegia. When compared to this study, the sample size was smaller, the median age younger and only paraplegic patients were included.
Our study has numerous limitations. Inherent to retrospective studies, reporting of neurological findings and surgical outcomes was not standardized and consistent. For similar reasons, comorbidities and minor perioperative complications were not systematically recorded. Given these shortcomings in reporting, we decided to focus on walking ability, which was well reported in the electronic health records. Moreover, this surrogate outcome measure is a meaningful outcome measure for patients. However, it may be that the loss of walking ability in some patients was caused by the loss of proprioception rather than muscle strength. Unfortunately, the duration in hours from the development of paresis/plegia to surgery could not be confirmed. However, it might be impossible to report the exact durations, since not even a prospective design is feasible for this patient group. In addition, no information about histopathological findings, e.g., WHO Grading, was collected as these were not consistently reported in the patient records. Postoperative MRI was not arranged routinely for all patients, which is why some asymptomatic tumor recurrencies may not have been diagnosed. Furthermore, this also prevented the analysis of T2 hyperintensity since the spinal cord is often flattened by meningioma in preoperative MRI studies. Finally, the mean overall follow-up of 57.1 months may be too short to report true recurrence rates.
Our study may also have a few strengths in comparison to previous studies [1,2,6,11]. To our knowledge, we tried to define the role of time delay from the loss of walking ability to surgery in the recovery of walking ability within the first reports. The inclusion of a few asymptomatic patients in the cohort also provided some new information. Furthermore, the meningiomas were operated on by 18 different neurosurgeons, whereas some of the previous studies presumably included only the results of a few surgeons. Finally, the study population of the present study was one of the largest cohorts of spinal meningioma patients reported.
CONCLUSION
As the spinal cord compression in spinal meningioma patients develops slowly, even somewhat delayed surgery may lead to significant functional improvements. New paraparesis or -plegia are rare events following spinal meningioma surgery.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
An author (VV) received research funding for the preparation of this manuscript from Neurocenter, Helsinki University Hospital.
Author Contribution
Conceptualization: VV, MN, MK; Data curation: VV, RH; Formal analysis: VV, MK; Funding acquisition: VV; Methodology: VV, MN, MK; Project administration: VV, MN, MK; Visualization: VV, RH; Writing - original draft: VV; Writing - review & editing: VV, RH, MN, MK.