Description of the Diversity in Surgical Indication and Surgical Strategies for Primary Spinal Cord Tumors: A Nationwide Survey by the Neurospinal Society of Japan
Article information
Abstract
Objective
To assess the current management of primary spinal cord tumors (PSCTs) and determine whether and to what extent there are differences in surgical strategies for PSCTs.
Methods
The Neurospinal Society of Japan conducted a survey between April 1 and 30, 2021. Certified spine surgeons were requested for information on the frequency of surgeries in 2020 and the surgical strategies adopted for each PSCTs. The following tumor histologies were focused: schwannoma, meningioma, and cauda equina tumor as extramedullary tumors; and ependymoma, hemangioblastoma, astrocytoma, and cavernoma as intramedullary tumors. The participants were divided according to their response as follows: experts, who had experienced ≥ 100 surgeries for PSCTs, and nonexperts.
Results
Among 308 participants (63%), 35 (11%) were experts. The total number of PSCTs in 2020 was 802 of which 564 tumors were extramedullary and 223 were intramedullary. Schwannoma accounted for 53% of the extramedullary tumors, and ependymoma accounted for 39% of the intramedullary tumors. Surgical strategies significantly differed among both the experts and nonexperts groups. Some discrepancies in the adopted surgical strategies were observed between groups. Some of the nonexperts, and none of the experts, ruled out surgery for schwannomas (Eden type 4), astrocytomas, or cavernomas. Five nonexperts (2.2%), and none of the experts, resected the entire dura for meningiomas.
Conclusion
A nationwide survey revealed that a sufficient consensus did not exist regarding surgical strategies for PSCTs. A disease-specific registry for PSCTs is necessary in academic societies.
INTRODUCTION
Generally, primary spinal cord tumors (PSCTs) are uncommon and are approximately 5% of all the diagnosed primary central nervous system tumors [1]. PSCTs arise from the meninges, spinal cord, and cauda equina, with annual incidence of 0.35, 0.59, and 0.03 per 100,000 persons, respectively [2]. Approximately 70% of PSCTs are reported to be benign or unknown [3], suggesting that most PSCTs can be cured by total resection [4].
Postoperative worsening of neurological symptoms is not common in PSCTs, which has been reported to be 2.2% [5]. However, the removal of PSCTs, especially intramedullary tumors that originate in the spinal cord, inevitably involves invasion of normal nerve tissues, and the procedure requires a sensitive technique based on extensive experience. Nevertheless, accumulating sufficient experience in PSCT removal can be difficult as PSCTs are rare and extremely diverse in histology [2]. Some reports have suggested that conservative treatment is better than surgery [6], and when physicians should recommend resection of benign PSCTs with slow progression is controversial [7]. Since the pathogenesis and prognosis of PSCTs are different in each histology and genotype, generating evidence to support the strategy for each PSCTs can be difficult. Clinical decision may largely depend on the individual experience of each physician, and the existence of divergence regarding indications for surgery and extent of resection for PSCTs in actual clinical practice. However, no reports have demonstrated the actual degree of divergence to date.
If indeed a divergence in treatment strategy is observed, 2 issues become apparent. The first is the necessity to promote shared decision making [8] in which decisions are made based on a bidirectional exchange of information, including patients’ values and preferences. The second is an urgency of accumulating information for shared decision-making, regarding prognosis associated with surgical strategies. Generating evidence to address these issues in rare diseases such as PSCTs, consolidating and sharing treatment experiences are essential, and establishing an academic society-initiated disease-specific registry would be highly desirable.
Thus, this study aimed to assess the current management of PSCTs by neurosurgeons in Japan and to determine whether and to what extent there are differences in surgical strategies for PSCTs.
MATERIALS AND METHODS
This study was conducted as a survey of the Neurospinal Society of Japan (NSJ), an academic society that consists mainly of neurosurgeons involved in the treatment of spinal diseases and has approximately 1,300 members and 500 certified spine surgeons [9]. The survey was launched on April 1, 2021, and responses were collected until April 30, 2021. All certified spine surgeons listed in the NSJ membership directory with a valid email address were eligible to participate in the study and were asked to complete the web-based questionnaire. Those who refused to participate in the survey were excluded. This study was approved by certified local Institutional Review Boards of Katano Hospital (20210215-1), and all participants provided electronic informed consent.
1. Data Collection
The questionnaire consisted of 3 topics that included the characteristics of participants, surgical experience in PSCTs, and treatment strategies for each tumor histology. The characteristics of participants included age, sex, clinical experience as a spine surgeon, and type of the institution. For surgical experience in PSCTs, the information on the total cases and the cases for each tumor histology in lifetime and 2020 were collected. The participants were reminded to register only the surgical cases that they had performed as the primary surgeon. The focus was on 7 types of tumor histology as representative of PSCTs for this study: schwannomas, meningiomas, and cauda equina tumors as intradural extramedullary tumors, whereas ependymomas, hemangioblastomas, astrocytomas, and cavernomas as intramedullary tumors. Although cavernoma is classified as a vascular malformation, it was included in this study because its treatment was comparable to that of intramedullary tumors.
Additionally, information was sought on surgical strategies, such as indication for surgery and extent of resection. To investigate the indication for surgery, both the experts (with ≥ 100 cases of experience) and the nonexperts were asked to select one of the following 6 items that was close to their preference, assuming the patient to be a healthy 50-year-old without any comorbidities: (1) at the time of diagnosis, (2) worsening imaging findings, (3) mild neurological symptoms (e.g., mild numbness), (4) moderate or more neurological symptoms (e.g., motor paralysis), (5) progressive neurological symptoms, and (6) do not recommend surgery. For cavernoma, an additional question was asked, “At what time point do you think resection should be indicated for a bleeding-onset cavernoma?” To investigate the extent of resection, a set of questions with 3 to 4 items specific to each tumor histology was prepared. Regarding the extent of resection, information only on intradural extramedullary tumors was asked, and the responses were limited to those who were performing surgery for each tumor at the institution. For schwannomas and cauda equina tumors, no background disease such as neurofibromatosis was assumed. An English translation of the questionnaire is presented in Supplementary Table 1.
2. Statistical Analysis
The background characteristics of the participants were first described. Continuous variables were summarized by median and interquartile range, and dichotomous or categorical variables by absolute and relative frequencies. Second, the number of surgeries performed for each tumor histology in 2020 was described. Finally, the distribution of responses to each question by the experts and the nonexperts was illustrated. Data were managed using Stata 17 (StataCorp LLC; College Station, TX, USA).
RESULTS
Altogether, 321 participants (66%) responded to the questionnaire, of which 13 refused to participate, primarily because they do did not treat for PSCTs (Supplementary Fig. 1). For 308 participants, the median age was 50 years, median clinical experience as a spine surgeon was 15 years, and 46% were physicians working in university or public hospitals (Table 1). Of the included participants, 35 (11%) who had performed ≥ 100 surgeries for PSCTs were in the experts group; they were older, and many of them worked at university or public hospitals.
1. Surgical Experience in PSCTs
The total frequency of PSCTs cases among the study participants in 2020 was 802 cases, which included 564 cases of intradural extramedullary tumors, 223 cases of intramedullary tumors, and 15 cases of others. Among intradural extramedullary tumors, schwannomas accounted for half of the cases, and meningiomas and cauda equina tumors accounted for one quarter each. Among intramedullary tumors, ependymomas accounted for 39%, hemangioblastomas and cavernomas were almost equal in number and accounted for > 20%, and astrocytomas accounted for < 20% (Table 2).
2. Treatment Strategies for Each Tumor Histology
1) Intradural extramedullary tumors
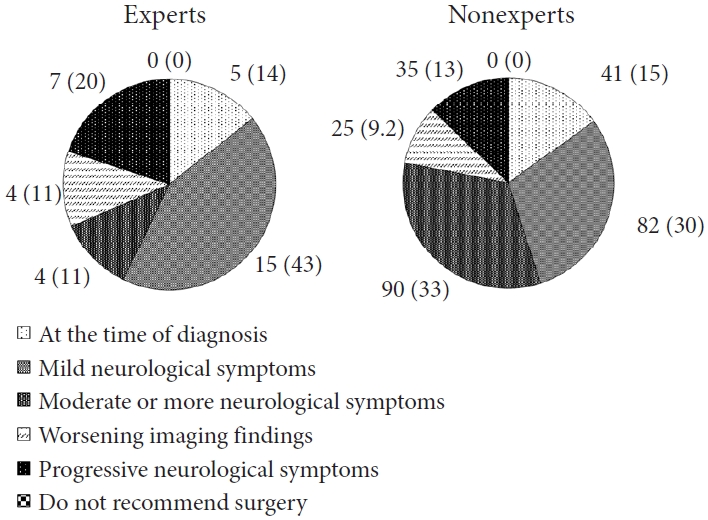
Indication for surgery for schwannomas (Eden types 1–3) are presented in Fig. 1. Although the experts were more aggressive than the nonexperts (57% vs. 45% for surgery at the time of diagnosis or mild neurological symptom), a large divergence in indication for surgery was observed in both groups. Similar trends were observed in schwannomas (Eden type 4; Supplementary Fig. 2), meningiomas (Supplementary Fig. 3), and cauda equina tumors (Supplementary Fig. 4). For schwannomas (Eden type 4), 12 of 273 (4.4%) of the nonexperts answered, “Do not recommend surgery,” while none of the experts selected that answer. The answers to the questions on the extent of resection for intradural extramedullary tumors are presented in Table 3. The extent of resection and indication for surgery varied in both the groups. None of the expert answered, “Resection of the entire dura” or “Preserve the dura, no coagulation” in the response regarding the extent of resection for meningiomas.
Indication for surgery of the experts (≥100 cases) and the nonexperts (<100 cases) for healthy 50-year-old patients with schwannomas (Eden types 1–3) without any comorbidities. Data are expressed as absolute and relative frequencies (%).
2) Intramedullary tumors
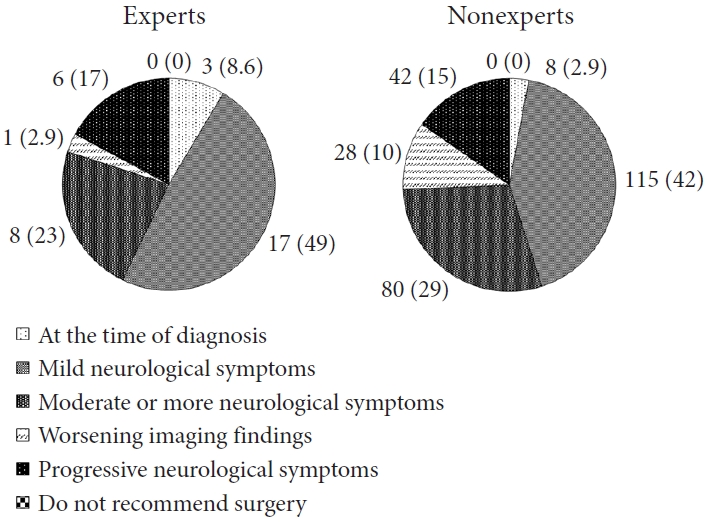
Indication for surgery for ependymomas are illustrated in Fig. 2. In comparison to intradural extramedullary tumors, both the groups had a higher percentage of indication for surgery at the time of diagnosis (14% of the experts and 15% of the nonexperts). As in the case of intradural extramedullary tumors, the experts were more aggressive than the nonexperts, and differences in the indication for surgery were observed among both groups. Similar trends were observed in hemangioblastomas (Supplementary Fig. 5), astrocytomas (Supplementary Fig. 6), and cavernomas (Supplementary Fig. 7). Compared to other tumor histologies, a high rate of surgical recommendations at diagnosis was noted for astrocytomas (23% of the experts and 28% of the nonexperts), while the rate was lower for cavernomas (5.7% of the experts and 4% of the nonexperts). Some of the nonexperts did not recommend surgery for astrocytomas and cavernomas (3 of 273 [1.1%] and 3 of 273 [1.1%], respectively). In an additional question for cavernoma, the majority of participants in both the groups refrained from surgery at initial bleeding (54% of the experts and 53% of the nonexperts; Supplementary Table 2).
DISCUSSION
A survey of NSJ certified neurosurgeons revealed the frequency of surgery for each PSCT in 2020, providing a roadmap for tumor histology to prioritize for generating evidence in the field of neurosurgery. Furthermore, a divergence in the surgical strategy for PSCTs (i.e., surgical indication and extent of resection) was observed among both the experts and the nonexperts, and the experts and the nonexperts had different preferences in some areas. Duong et al. [2] have reported that the number of new diagnoses of PSCTs in the United States (US) was 0.97 per 100,000. It should be noted that their study included only 3.3% of Asian/Pacific Islanders. However, if this rate is applied to the Japanese population [10], the estimated number of people diagnosed with PSCT per year will be 1,217. The Japan Neurosurgery Registry, which covers 74.2% of the annual predicted number of surgical cases, registered 1,200 (400 cases/yr) surgeries for extramedullary tumors, 798 (266 cases/yr) surgeries for intramedullary tumors, and 713 (238 cases/yr) surgeries for extramedullary tumors with extradural or paravertebral extension, from 2015 to 2017, that is 904 cases on average per year [11]. Considering these estimated incidence rates and the number of registered surgeries, the present data in this study (802 cases in total) has the potential to be representative. Therefore, this study would provide valuable information on how evidence should be accumulated and what kind of education should be implemented on an academic society basis.
1. Indications for Surgery
In a study including 430 PSCTs in the US, meningiomas, ependymomas, and schwannomas were reported to be the most commonly treated PSCTs, each accounting for approximately 20% of all PSCTs [5]. Another study including 2,355 PSCTs conducted in China in 1982 showed a different distribution, with meningiomas accounting for 14%, ependymomas for 4%, and schwannomas for 47% [12]. The 151 cases reported in Korea had the largest proportion of schwannomas (37%), followed by meningiomas (24%) and ependymomas (12%) [13]. In this study, the percentage of surgeries per tumor histology according to all surgeries performed (802) was 129 (16%) for meningiomas, 86 (11%) for ependymomas, and 297 (37%) for schwannomas. This shows that surgical rates in Japan for meningiomas and ependymomas are lower and those for schwannomas are higher than those in the US, and these rates are similar to those in other Asian countries. While this discrepancy may be due to racial differences in tumor prevalence, it also suggests that indications for surgery, which are defined by various factors, including the rate of tumor growth, impact of symptoms, curability, and difficulty and invasiveness of surgery, may differ between Asia and US. Furthermore, this survey revealed that there is no consistency in the indications for surgery according to tumor histology even within the same country, regardless of surgeon’s experience.
Regarding cavernoma as a specific example, 35% (108 of 305) of the participants recommended surgery at the initial bleeding, 53% (163 of 305) forwent surgery, and the rest did not consider the presence of bleeding as an indication for surgery (Supplementary Table 2). One of the key studies on the treatment of cavernoma was reported by Badhiwala in 2014, a meta-analysis of 632 patients, including approximately 10% conserved cases [14]. They have reported valuable findings, such as an annual bleeding rate of 2.1% and an association between resection within 3 months of symptom onset and improved neurological outcome (odds ratio, 2.1). However, meta-analyses of small retrospective studies have limitations, such as the restricted precision and number of variables that can be used for analysis, and the small number of conserved cases. Existing evidence does not resolve the specialized clinical question of “Should we recommend resection at initial bleeding?,” and clinical decisions are based on individual beliefs and experience, leading to divergence in indication for surgery.
There were some discrepancies in the indications for surgery between the experts and the nonexperts. Some of the nonexperts responded that they would not recommend surgery for schwannomas (Eden type 4), astrocytomas, and cavernomas, but none of the experts responded similarly. To establish whether surgery should be recommended for these tumors is challenging because it requires rigorous comparative studies with nonoperative groups. Expert opinion is meaningful considering the difficulty in generating high-quality evidence. Thus, we should raise awareness of the fact that these tumors may be indicated for surgery on the academic society basis.
2. Extent of Resection
Nakamura et al. [15] have reported on 75 surgical cases of cervical schwannomas, of which 13 involved subtotal resections and 5 involved partial resections, with 2 recurrences each; further, 23% had postoperative denervation symptoms and 8% had residual symptoms. Kuo et al. [16] noted that for acoustic neurinoma, the tumor is encapsulated, although the capsule is not sufficiently thick for the surgeon to recognize. These studies have suggested a trade-off between denervation and recurrence in the extent of tumor resection. Complete amputation of the schwannoma with anatomical nerve preservation should be the ideal surgical goal [17]. Although nerve stimulation has been proposed as an adjunct device that could solve this problem [18], it is not sufficiently trusted, as 32% of the experts in this study did not refer to the results of nerve stimulation when determining the extent of schwannoma resection (Table 3). Electromyography has been reported to be useful for predicting denervation after total resection [19,20], and there is a need to establish a method for predicting denervation using nerve stimulation or a combination of these methods. Careful dissection of the origin of schwannomas is also crucial for surgeons.
Regarding the extent of resection for meningiomas, 2.2% of the nonexperts answered that they would resect the entire dura, while none of the experts provided the same answer. As reported by Naito et al. [21] and Saiwai et al. [22], meticulous Simpson grade II resection may be the first choice in actual clinical practice. A case of spinal cord herniation secondary to entire dura resection for meningioma has been reported [23]. However, a previous study has reported that in approximately 30% of patients who underwent grade II resection, the tumor recurred after approximately 12 years [24]. Thus, accumulating evidence from long-term observation of a larger number of cases that are appropriately treated by the meticulous Simpson grade II resection is necessary.
3. Generation of High-Quality Evidence
Data from large population-based sources have provided accurate descriptive statistics on benign and malignant central nervous system tumors [25]. However, the establishment of an academic society-initiated disease-specific registry is essential to generate evidence to support specialized clinical decisions around PSCTs, such as the appropriate indication for surgery and the optimal extent of resection. Furthermore, there is an urgent need to train researchers who can manage the registry, design scientifically valid studies, and conduct valid analysis and interpretation.
Ideally, a randomized controlled trial is the best approach to examine the causal relationship between each clinical decision and prognosis. However, in PSCTs, the rarity and ethical considerations make it impractical to conduct randomized controlled trials. Therefore, the establishment of a comprehensive registry and high-quality observational studies using the data are required. Recently, target trial emulation, a method of causal inference using observational data, has been proposed [26], and high-quality observational studies using this method have been reported [27]. A registry of all surgical cases would enable generating evidence on the extent of resection using these modern epidemiological methods. If a registry of all cases, including conservative cases, could be established, to generating evidence for surgical indication would be possible.
4. Study Limitations
This study had several limitations. First, the survey collected data for the year 2020, when coronavirus disease 2019 began to threaten the world. Hence, surgeries for patients with mild symptoms may have been counted in the following year, resulting in fewer surgeries than in previous years. Second, since it is a self-reported questionnaire survey, the accuracy of the information obtained is not guaranteed. Third, because the survey did not include case presentations with imaging information indicating localization and size of the tumor, the severity of the PSCTs evoked by the participants may have differed, leading to variability in the responses. Therefore, the observed divergence in surgical strategy may have been overestimated. Finally, there is the issue of generalization. This survey was intended for neurosurgeons, and many orthopedic surgeons did not respond. Additionally, although the response rate was high for a web-based survey, 34% of the participants did not respond. However, most of the experienced surgeons in Japan are likely to have participated in this study, so the negative impact might be minimal.
CONCLUSION
In various PSCTs, the decision-making regarding the surgical indication and extent of tumor resection differed even among the experts. The least consensus was on the surgical issues such as the extent of resection of schwannomas, meningiomas, and cauda equina tumors, as well as the timing of cavernoma resection. These results indicate that the consensus on indications for surgery and extent of resection for each PSCT is insufficient and an evidence-based consensus is urgently needed. It is necessary to establish a detailed disease-specific registry of PSCTs managed by academic societies and high-quality observational studies conducted using data from these registries.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1-2 and Figs. 1-7 can be found via https://doi.org/10.14245/ns.2244686.343.
English translation of the questionnaire with excerpts from the section on the results of this study (original in Japanese)
Indication for surgery in bleeding-onset cavernomas
Flowchart of the study participants. NSJ, Neurospinal Society of Japan.
Indication for surgery of the experts (≥100 cases) and the nonexperts (<100 cases) for healthy 50-year-old patients with schwannomas (Eden type 4) without any comorbidities. Data are expressed as absolute and relative frequencies (%).
Indication for surgery of the experts (≥100 cases) and the nonexperts (<100 cases) for healthy 50-year-old patients with meningiomas without any comorbidities. Data are expressed as absolute and relative frequencies (%).
Indication for surgery of the experts (≥100 cases) and the nonexperts (<100 cases) for healthy 50-year-old patients with cauda equina tumors without any comorbidities. Data are expressed as absolute and relative frequencies (%).
Indication for surgery of the experts (≥100 cases) and the nonexperts (<100 cases) for healthy 50-year-old patients with hemangioblastomas without any comorbidities. Data are expressed as absolute and relative frequencies (%).
Indication for surgery of the experts (≥100 cases) and the nonexperts (<100 cases) for healthy 50-year-old patients with astrocytomas without any comorbidities. Data are expressed as absolute and relative frequencies (%).
Indication for surgery of the experts (≥100 cases) and the nonexperts (<100 cases) for healthy 50-year-old patients with cavernomas without any comorbidities. Data are expressed as absolute and relative frequencies (%).
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study was financially supported by the Neurospinal Society of Japan.
Author Contribution
Conceptualization: YH, SU, TY, DU, TT, MM, MH; Data curation: YH, SU, TY, DU, TE, TT, MM, MH; Formal analysis: YH; Methodology: YH, SU, TY, DU; Project administration: TT, MM, KH, MH; Visualization: YH, SU, MH; Writing - original draft: YH; Writing - review & editing: SU, TY, DU, TE, TT, MM, KH, MH.
Acknowledgements
A summary of the results of this study was presented as a video presentation (unpublished) at “The 36th Annual Meeting of the Neurospinal Society of Japan” in Kyoto (Japan), from June 3 to June 5, 2021. Available from: https://www.cs-oto.com/nsj2021/index.html. The Neurospinal Society of Japan (NSJ) covered the costs of English editing. We thank all members of the NSJ who responded to the questionnaire.