Risk Factors of Postoperative Cerebrospinal Fluid Leak After Craniovertebral Junction Anomalies Surgery: A Case-Control Study
Article information
Abstract
Objective
To identify potential risk factors for cerebrospinal fluid (CSF) leakage after craniovertebral junction (CVJ) anomaly surgery and to provide a reference for clinical practice.
Methods
Sixty-six patients who underwent elective CVJ anomaly surgery during a 6-year period (April 2013 to September 2019) were retrospectively included. Research data were collected from the patients’ medical records and imaging systems. Patients were divided into CSF leak and no CSF leak groups. Univariate tests were performed to identify potential risk factors. For statistically significant variables in the univariate tests, a logistic regression test was used to identify independent risk factors for CSF leakage.
Results
The overall prevalence of CSF leakage was 13.64%. Univariate tests showed that a basion-dental interval (BDI) > 10 mm and occipitalized atlas had significant intergroup differences (p < 0.05). Multivariate analysis indicated that a BDI > 10 mm was an independent risk factor for CSF leakage, and patients with CVJ anomalies with a BDI > 10 mm were more likely to have postoperative CSF leaks (odds ratio, 14.67; 95% confidence interval, 1.48–30.88; p = 0.004).
Conclusion
It is necessary to maintain vigilance during CVJ anomaly surgery in patients with a preoperative BDI > 10 mm to avoid postoperative CSF leaks.
INTRODUCTION
Craniovertebral junction (CVJ) anomalies are pathological changes of the anatomical and functional complex surrounding the foramen magnum, including the occipital bone, atlas, axis, related ligaments, and other tissues, which often cause nerve and vascular damage and change cerebrospinal fluid (CSF) dynamics [1]. CVJ anomalies include basilar invagination (BI), Chiari malformation, atlantoaxial dislocation (AAD), occipitalized atlas, atlanto-occipital dislocation (AOD), and platybasia, and may include a single deformity or multiple deformities coexisting [2-6]. At present, surgical treatment is adopted for patients with obvious neurological symptoms and ataxia [3-5]. The therapeutic principle is to relieve the compression on the brainstem, spinal cord, and nerve roots, maintain or reconstruct the stability of the CVJ, and restore normal CSF circulation [7]. Currently, there is no clear definition and diagnostic standard for CVJ instability, and it is difficult to correctly diagnose CVJ instability because of the complex anatomical structure of the CVJ and the diverse clinical manifestations and imaging [3,8,9]. However, it is clear that the anatomical structure and stability of the CVJ are destroyed during surgical decompression; therefore, rigid fixation is needed to reconstruct and maintain its stability.
The complicated anatomical structure of the CVJ, narrow operative space, and large individual differences among patients increase the difficulty of the operation and the probability of various postoperative complications. CSF leakage is a feared complication after CVJ anomaly surgery. According to our clinical experience, postoperative CSF leak occurs more frequently in patients with preoperative CVJ anomalies than in those with other spinal problems, which often affects the recovery and curative effect and is more difficult to handle. There is limited information about the risk factors for postoperative CSF leak. Therefore, the purpose of this study was to explore the potential risk factors for CSF leak after CVJ anomaly surgery and to provide a reference for clinical practice.
MATERIALS AND METHODS
1. Study Design
This retrospective analysis was conducted after obtaining approval from the Institutional Review Board (IRB) of the First Affiliated Hospital of Kunming Medical University (IRB No. 20211019). Data were collected from the medical records and imaging systems of our hospital. We reviewed the records of 77 patients who underwent an elective CVJ anomaly surgical procedure performed by the third author with the assistance of orthopedic surgery residents and spine fellows from April 2013 to September 2019. Sixty-six patients met the following inclusion criteria: (1) patients with congenital developmental CVJ anomalies who underwent CVJ surgery by anterior transoropharyngeal combined with a posterior approach or simple posterior approach; and (2) patients with complete clinical data. The exclusion criteria were as follows: (1) patients who underwent posterior fossa decompression with duraplasty; (2) CVJ anomalies caused by inflammation, tumor, trauma, tuberculosis, and rheumatoid immune diseases; and (3) revision surgery.
The information recorded for each patient included sex, age, disease course (DC), anterior atlantodental interval (AADI), Chamberlain’s line, basion-dental interval (BDI), basion-posterior axial line interval (BAI), occipitalized atlas, cranial base angle (CBA), clivus-canal angle (CCA), upper cervical stenosis, cerebellar tonsillar herniation (CTH), syringomyelia, surgical approach, surgical segment, and surgery duration.
2. Recognition of Postoperative CSF Leak
Postoperative CSF leak was recognized by the following: (1) A dural tear or clear fluid extravasation from the dura during surgery; (2) a large amount of clear drainage after surgery, with clear liquid flowing out of the incision, and the presence of tinnitus, blurred vision, orthostatic headache, nausea, and vomiting [10]; (3) some evidence of fluid leakage on postoperative MRI with magnetic resonance myelography [10,11].
An artificial dura mater or dural suture was used for patients with an intraoperative dural tear, and drainage was closely monitored after the operation.
3. Measurement Parameters
Important bone markers were mainly obtained by CT image measurement because bony landmarks are easily identifiable and consistently reproducible on CT images [12], and important nervous system markers were obtained by MRI measurement. The measurement parameters were as follows:
(1) AADI is the horizontal distance between the anterior arch of the atlas and the dens of the axis. Adults with an AADI > 3 mm can be considered to have AAD [13] (Fig. 1A).

Measurement parameters. (A) Anterior atlantodental interval (red line). (B) Chamberlain line (white line), the distance between the tip of the dens and Chamberlain line (red line). (C) Basion-dental interval (red line). (D) Basion-posterior axial line interval (red line), the superior extension of the posterior cortical margin of the body of the axis in the median plane (white line). (E) Cranial base angle, the line joining the nasion with the center of the pituitary fossa (left red line), the line joining the anterior border of the foramen magnum with the center of the pituitary fossa (right red line). (F) Wackenheim line (upper red line), the line constructed along the posterior surface of the axis body and odontoid process (lower red line). (G) Cerebellar tonsillar herniation, basion-opisthion line (white line), the distance of the cerebellar tonsils downward beyond the basion-opisthion (red line). CBA, cranial base angle; CCA, clivus-canal angle.
(2) Chamberlain line is a line joining the back of the hard palate with the opisthion on a lateral view of the CVJ [14]. Chamberlain line helps to recognize BI, which is said to be present if the tip of the dens is > 3 mm above the line [15] (Fig. 1B).
(3) The BDI is the distance from the most inferior portion of the basion to the closest point of the superior aspect of the dens in the median plane. The BDI was > 10 mm in the median plane, indicating AOD [16,17] (Fig. 1C).
(4) The BAI is the distance between the basion and superior extension of the posterior cortical margin of the body of the axis in the median plane. If the basion is in front of the superior extension of the posterior cortical margin of the body of the axis and the BAI is > 12 mm, it indicates AOD. If the basion is located behind the superior extension of the posterior cortical margin of the body of the axis and the BAI is > 4 mm, it indicates AOD [16,17] (Fig. 1D).
Almost all patients with a BDI of > 10 mm and BAI of > 12 mm or 4 mm were combined with occipitalized atlas; if they are described as AOD, there may be conceptual contradictions. The original atlanto-occipital joint was replaced by an abnormal atlantoaxial (or occipitoaxial) joint, which belongs to the CVJ. The instability of this abnormal joint should be classified as CVJ instability. Therefore, we regard a BDI of > 10 mm and BAI of > 12 mm or 4 mm as CVJ instabilities (CVJI [BDI] and CVJI [BAI]) in this study.
(5) The CBA is formed by the line joining the nasion with the center of the pituitary fossa and the line joining the anterior border of the foramen magnum with the center of the pituitary fossa; this indicates platybasia if the CBA is > 143° [18] (Fig. 1E).
(6) Wackenheim line is formed by drawing a line along the clivus and extending it inferiorly to the upper cervical canal [16] (Fig. 1F).
(7) The CCA is formed at the intersection of Wackenheim line with a line constructed along the posterior surface of the axis body and odontoid process, which normally ranges between 150° and 180°. Brain stem and spinal cord compression often occurs when the CCA is ≤ 150º [19] (Fig. 1F).
(8) CTH: If CTH is > 5 mm, the distance of the cerebellar tonsils downward beyond the basion-opisthion line indicates CTH [20,21]. (Fig. 1G).
The measurement was performed by third-party radiologists, who were unaware of the patients’ condition and grouping. Each imaging data was measured 3 times and averaged.
4. Statistical Analysis
The prevalence of CSF leakage was calculated as the proportion of CSF leakage to the total number of individuals undergoing CVJ anomaly surgery. To identify factors associated with the CSF leaks, we used Student t-tests to determine the relationship between CSF leaks and age, DC, and surgery duration. Chi-square tests were used to determine the relationship between CSF leaks and CVJ instability (BDI), CVJ instability (BAI), AAD, BI, brainstem and spinal cord compression, platybasia, CTH, syringomyelia, surgical approach, and sex. Because the overall number of CSF leaks was small, the Yates correction factor was used to calculate the chi-square statistic if the expected frequency in any one cell was ≥ 1 and < 5. We used Fisher exact test to identify the effect of the occipitalized atlas, upper cervical stenosis, and surgical segment on the risk of CSF leak because the expected frequency in one cell was < 1. We also calculated the odds ratio (OR) and 95% confidence interval (CI) for each risk factor of CSF leakage. The a priori alpha level for all statistical tests was set at 0.05. All statistical analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Demographic Data
Seventy-seven patients chose surgical treatment, among whom, 66 met the inclusion criteria. Of the 66 patients, 21 were male (31.82%) and 45 were female (68.18%), with an average age of 41.42 ± 12.68 years old. Fourteen patients underwent surgery using the anterior transoropharyngeal combined with a posterior approach, and 52 patients underwent surgery using a simple posterior approach.
CSF leakage occurred in 9 patients, and 3 dural tears were diagnosed intraoperatively by visualizing clear fluid extravasation from the dura. The remaining 6 patients were diagnosed with postoperative CSF leaks due to complications of CSF leak, incision, drainage fluid, and auxiliary examination. The overall prevalence of CSF leaks was 13.64%.
Dural tears occurred during resection of the posterior arch of the atlas and decompression of the foramen magnum (2 patients), and while the posterior atlanto-occipital membrane was incised (1 patient). The other 6 patients with CSF leak had obvious adhesions between the posterior atlanto-occipital membrane (or ligamentum flavum) and dura, which were carefully separated and protected. No visible dural tear occurred during the operation, but clear drainage appeared 2 days after the operation. Of the 19 patients with a BDI > 10 mm, 2 had a dural tear during the resection of the posterior arch of the atlas and decompression of the foramen magnum, and 4 had no visible dural tear but presented with CSF leak postoperatively.
2. Treatment
All dural tears were repaired primarily by tight sutures and the cover of an artificial dura mater (TianXinFu biological membrane 30× 40 mm, TianXinFu Medical Appliance Co., Ltd., Beijing, China). A submuscular drain was placed for posterior wounds and a drainage bag was placed on the patient’s bed so that it would not hold suction. For patients with CSF leakage, we appropriately extended the drainage time depending on the drainage volume. Submuscular drains were discontinued on postoperative day 4 and 11 (average) in patients without and with CSF leak, respectively. All patients with CSF leak were confined to bed rest with the head elevated at 30° for at least 1 day. We performed debridement, suturing, and pressure bandaging of the incision to close the CSF cutaneous fistula in a patient with clear liquid outflow from the incision in the operating room. Antibiotics were also administered in an attempt to avoid central nervous system infections.
3. Outcome
The neurological symptoms of the 66 patients who received surgical treatment were relieved. Three patients with dural tears showed resolution of all signs and symptoms of the leak within 1 day. Of the 9 patients with CSF leak, 3, 2, and 1 patients had resolution of all signs and symptoms within 2, 3, and 6 days, respectively.
Three patients with dural tears still had CSF leaks after dural repair. Of the 3 patients with a dural tear, a lumbar CSF drain was inserted and left in place for 7 days for 1 patient with a persistently draining wound and a large amount of clear drainage. We extended the drainage time for the other 2 patients; however, 1 patient died of a severe central nervous system infection.
With the exception of the patient who died, the other 8 patients with CSF leak healed wounds within 2 to 4 weeks after timely treatment. No patient had wound infection, central nervous system infection, sinus tract formation, or sequelae of CSF leak during the follow-up period of more than 2 years after discharge (Fig. 2).
4. Univariate Analysis
Univariate analysis of potential risk factors showed no significant difference between the CSF leak group and the no CSF leak group in terms of sex (p = 0.294), age (p = 0.119), DC (p = 0.559), AAD (p = 1.000), BI (p = 0.516), CVJ instability (BAI) (p= 0.125), platybasia (p= 1.000), CCA (p= 0.233), upper cervical stenosis (p = 0.585), CTH (p = 0.535), syringomyelia (p = 1.000), surgical approach (p = 0.604), surgical segment (p= 0.376), and surgery duration (p= 0.497). However, the occipitalized atlas (p= 0.041) and CVJ instability (BDI) (p= 0.021) showed significant differences between the CSF leak group and no CSF leak group (Tables 1–4).
5. Multivariate Analysis
Multivariate logistic regression analysis was used to analyze the occipitalized atlas and CVJ instability (BDI). The OR for the development of CSF leak was calculated for nonoverlapping subsets of patients. The OR for the development of a CSF leak for a normal atlas compared to an occipitalized atlas was not statistically significant (p≥ 0.05) (Table 5). CVJ instability (BDI) was an independent risk factor for CSF leakage (OR, 14.67; 95% CI, 2.418–89.059; p= 0.004) (Table 6).
Without controlling for bias, patients with CVJ anomalies and CVJ instability (BDI > 10 mm) were 6.769 times more likely to develop CSF leaks than those without CVJ instability (BDI > 10 mm). After simultaneously correcting the “age and sex” biases on the basis of the crude OR value, the COR minus the adjusted value (AORage & sex, 14.674; 95% CI, 2.42–89.06; p= 0.004) was negative, indicating that the influence of preoperative CVJ instability (BDI > 10 mm) on the likelihood of postoperative CSF leakage may be underestimated, while age and sex were not considered. The calculation of confounding bias was 53.871%, indicating that the possibility of postoperative CSF leakage was underestimated by 53.871%, regardless of age and sex.
Patients with CVJ instability (BDI > 10 mm) were 14.67 (95% CI, 2.42–89.06) times more likely to have a CSF leak than patients without this condition (31.58% vs. 6.38%, p< 0.05) (Fig. 3).
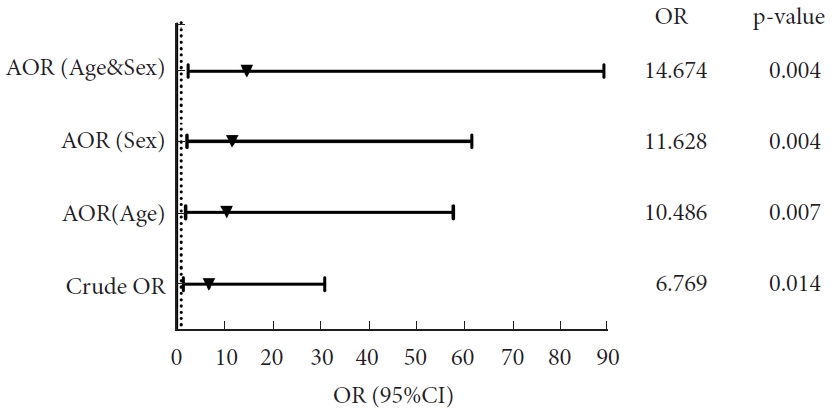
Adjusted odds ratio (AOR). Crude OR: logistic regression analysis of the relationship between craniovertebral junction instability (basion-dental interval [BDI]<10 mm) and cerebrospinal fluid leak without adjustment; AOR (age): OR value corrected for “age” bias based on “Crude OR”; AOR (sex): OR value corrected for “sex” bias based on “Crude OR”; AOR (age & sex): OR value corrected for “age and sex” biases based on “Crude OR.” Calculation of confounding bias: (COR-AOR)/AOR×100.
DISCUSSION
The overall prevalence of CSF leakage after CVJ surgery in our series was 13.64% (9 of 66 patients), which is comparable to the 10% for CVJ surgery and the 13% and 17% for posterior fossa surgery mentioned in other studies [22-24]. Therefore, the prevalence of CSF leak after CVJ surgery is higher than that of other spine surgeries (generally < 10%) [25-27]. Few studies have examined the correlation analysis of CSF leak and its risk factors after congenital developmental CVJ anomalies surgery, especially simple posterior cranial fossa decompression, CVJ reduction, and fixation without duraplasty. Previous studies on the risk factors of CSF leak after spine surgery have mainly focused on the cervical spine [28]. To the best of our knowledge, few relevant studies have specifically targeted CVJ anomaly surgery. The incidence of postoperative complications, including postoperative CSF leak, has been mentioned in some studies on CVJ surgery [22-24]; however, the risk factors have not been analyzed. The risk factor of “BDI > 10 mm” identified in this study has not been found in other studies of CSF leak after CVJ anomalies or upper cervical spine surgery. The BDI, as an imaging parameter of the CVJ, can be easily obtained by preoperative imaging examination in the diagnosis and treatment of patients with congenital developmental CVJ anomalies. The BDI has a unique guiding significance for the prevention of CSF leakage after CVJ anomaly surgery.
Among the 66 patients, 45 had occipitalized atlas and 9 had CSF leak, with a prevalence of 20%, which was higher than the overall prevalence. In other words, all patients who developed postoperative CSF leakage had an occipitalized atlas. Although occipitalized atlas is not an independent risk factor for CSF leak after CVJ anomaly surgery, it does increase the difficulty of decompression and has a greater possibility of damaging the dura. This may be because the posterior arch of the atlas in such patients usually protrudes forward into the spinal canal, making surgical decompression difficult. The dura mater is closely adhered to the periosteum in the region of the foramen magnum, and the abnormal bony structure computes the space between the dura mater and the posterior arch of the atlas, which not only increases the difficulty of bone decompression but also makes the posterior atlanto-occipital membrane (ligamentum flavum) more likely to adhere to the dura mater, increasing the possibility of dural and arachnoid injuries when the posterior atlanto-occipital membrane (ligamentum flavum) is released for decompression. Additionally, ligamentous laxity, weakened “holding ligaments,” and weight-bearing and age-related degenerative changes may precipitate joint instabilities [12,29,30]. With the stimulation of abnormal joint activities and inflammatory reactions, the soft tissue around the joint is hyperplasia and adhesion, making it more difficult to separate the soft tissue and the dura during the operation.
The presence of a preoperative BDI of > 10 mm was an independent risk factor for the development of CSF leak in our series. Patients with CVJ anomalies who have a preoperative BDI > 10 mm are 14.67 times more likely to have postoperative CSF leak than those who do not (p < 0.05). Of the 66 patients included in our study, 19 had a preoperative BDI > 10 mm and 6 of them developed CSF leak (31.58%), which was significantly higher than the overall prevalence. This may be because weightbearing over time weakened the “holding ligaments” and increased the obliquity of joints and ligamentous laxity. Moreover, age-related degenerative changes may precipitate progressive telescoping of the cervical spine into the skull base, resulting in an increased distance from the basion to the odontoid tip (BDI) and joint instability [12,29,30]. With aging, joint stability worsens, abnormal joint movement increases, minor local damage accumulates, and repeated inflammatory reactions lead to tissue hyperplasia. In the long-term, the bone structure and surrounding soft tissue easily adhere to the dura mater, which is difficult to separate during the operation. Additionally, the complex anatomical structure and narrow operation space in the CVJ often necessitates the use of a Kerrison rongeur to resect the posterior arch of the atlas, posterior edge of the foramen magnum, and posterior atlanto-occipital membrane during the operation, which may cause visible or undiscovered dural tear during the operation, eventually leading to postoperative CSF leak (Fig. 4).

Cause analysis. BDI, basion-dental interval; CVJ, craniovertebral junction; CSF, cerebrospinal fluid.
For the patients whose occipitalized posterior arch of the atlas or posterior margin of the foramen magnum protrates forward into the spinal canal, it is difficult to contact the deep bone through the Kerrison rongeur and to remove the bone from the posterior arch of the atlas or posterior margin of the foramen magnum. If the Kerrison rongeur is pushed deep, the dura mater can be easily teared when the bony structure is removed. In such patients, the “invagination” of the bony structure can be detected by imaging examination before surgery. Our experience is to combine preoperative computed tomography and magnetic resonance imaging to determine the extent of surgical decompression, and to perform “concentric decompression” from the edge. Ultrasonic osteotome, grinding drill, and Kerrison rongeur can be used to make the operation safer and more effective. For some patients with an occipitalized atlas, the boundary between the ligamentum flavum and the dura mater is unclear, which may be due to the reasons mentioned above. When the ligamentum flavum is cut and separated between the atlas and axis, it can easily cause dural tears or microtears not found during the operation, which eventually leads to postoperative CSF leakage. For such patients, our experience is to progressively remove the fascial tissue by searching for a weak spot between the ligamentum flavum and dura. Rather than blindly pursuing complete resection of the fascia or ligamentum flavum, more attention should be paid to decompression of the bone, release of membranous structures, and reduction of abnormal anatomical relationships. However, excessive reduction should be avoided due to the possibility of causing dural tears or new spinal cord or nerve damage (Fig. 5).
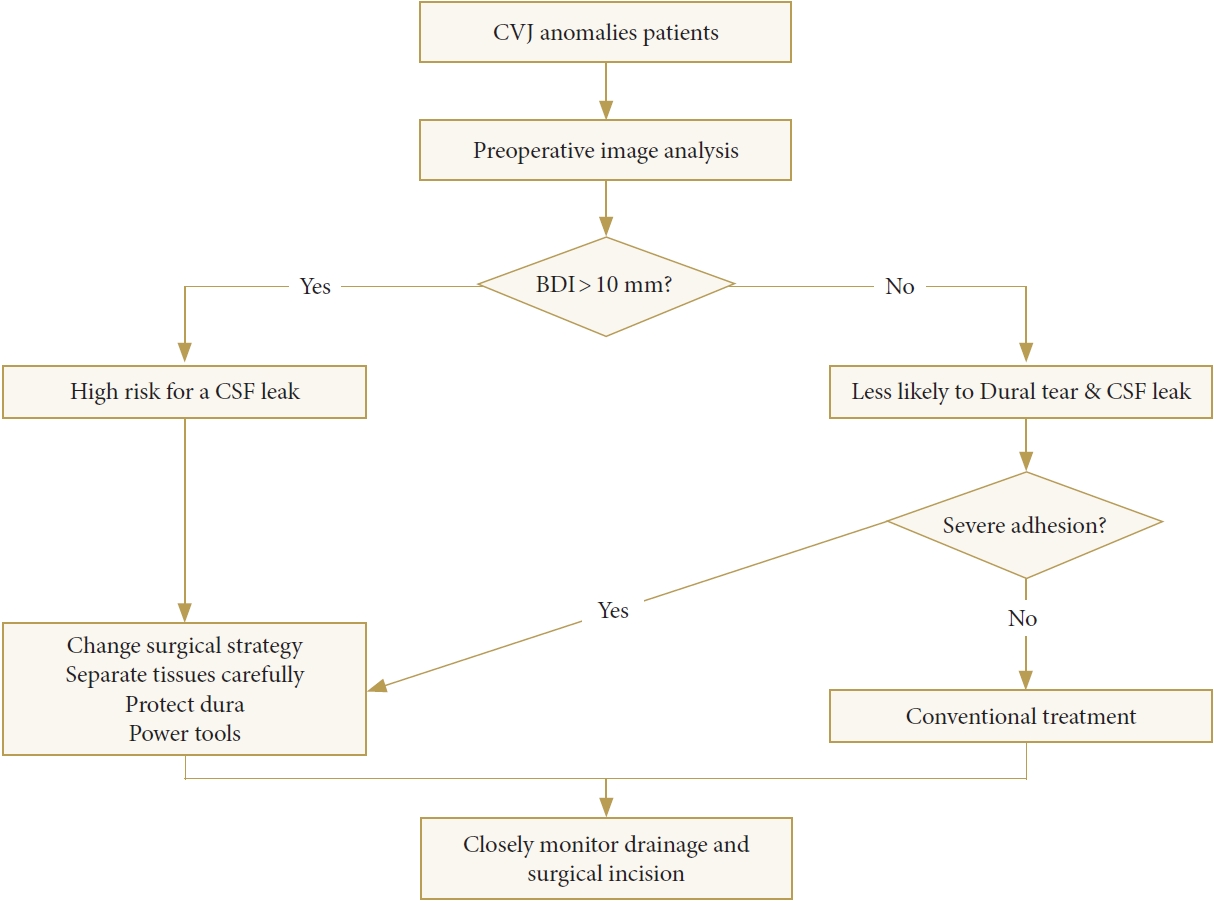
Algorithm for the treatment of CVJ anomalies. CVJ, craniovertebral junction; BDI, basion-dental interval; CSF, cerebrospinal fluid.
This study has a few limitations. First, as a single-surgeon or single-group case series, it may have to do with the technique used by our group. Second, the small sample size may have resulted in missing true risk factors due to inadequate power. Third, there may be additional risk factors that were not analyzed.
CONCLUSION
In the treatment of patients with CVJ anomalies with a preoperative BDI > 10 mm, it is necessary to maintain vigilance during surgery to avoid postoperative CSF leaks. For such patients, the surgical strategy should be adjusted appropriately, and careful separation of tissue and protection of the dura are warranted during surgery. When necessary, power devices can be combined to improve the efficiency and safety of the operation.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study was supported by the Major Science and Technology Project of the Yunnan Provincial Department of Science and Technology, Yunnan Provincial Orthopedic and Sports Rehabilitation Clinical Medicine Research Center (202102AA310068).
Author Contribution
Conceptualization: YX, BW; Data curation: YX, LC, ZG; Formal analysis: YX, YC; Funding acquisition: BW; Methodology: BW; Project administration: BW; Visualization: YX; Writing - original draft: YX; Writing - review & editing: BW, ZL







