Complications and Management of Endoscopic Spinal Surgery
Article information
Abstract
In the past, the use of endoscopic spine surgery was limited to intervertebral discectomy; however, it has recently become possible to treat various spinal degenerative diseases, such as spinal stenosis and foraminal stenosis, and the treatment range has also expanded from the lumbar spine to the cervical and thoracic regions. However, as endoscopic spine surgery develops and its indications widen, more diverse and advanced surgical techniques are being introduced, and the complications of endoscopic spine surgery are also increasing accordingly. We searched the PubMed/MEDLINE databases to identify articles on endoscopic spinal surgery, and key words were set as “endoscopic spinal surgery,” “endoscopic cervical foramoinotomy,” “PECD,” “percutaneous transforaminal discectomy,” “percutaneous endoscopic interlaminar discectomy,” “PELD,” “PETD,” “PEID,” “YESS” and “TESSYS.” We analyzed the evidence level and classified the prescribed complications according to the literature. Endoscopic lumbar surgery was divided into full endoscopic interlaminar and transforaminal approaches and a unilateral biportal approach. We performed a comprehensive review of available literature on complications of endoscopic spinal surgery. This study particularly focused on the prevention of complications. Regardless of the surgical methods, the most common complications related to endoscopic spinal surgery include dural tears and perioperative hematoma. transient dysesthesia, nerve root injury and recurrence. However, Endoscopic spinal surgery, including full endoscopic transforaminal and interlaminar and unilateral biportal approaches, is a safe and effective a treatment for lumbar as well as cervical and thoracic spinal diseases such as disc herniation, lumbar spinal stenosis, foraminal stenosis and recurrent disc herniation.
INTRODUCTION
As life expectancy increases, the number of patients with degenerative spinal diseases is increasing worldwide [1]. As patients age increased, surgeons have to manage patients with increased medical comorbidities such as liver, lung, heart and kidney dysfunction, along with the increased risk of general anesthesia. Accordingly, recently, many elderly patients have preferred minimally invasive spinal surgery over conventional surgical methods, and endoscopy-based spinal surgery is being performed. Endoscopic surgery is also a subset of minimally invasive spinal surgery, which is rapidly and continuously evolving to help manage older patients at high risk for general anesthesia [2,3]. Endoscopic surgery has advantages such as less muscle and bone damage, less pain, early rehabilitation, shorter hospitalization and an early return to work [4-6].
In the past, the use of endoscopic spine surgery was limited to intervertebral discectomy, however, it has recently become possible to treat various lumbar degenerative diseases such as lumbar spinal stenosis and foraminal stenosis, and the treatment range has expanded from the lumbar spine to the cervical and thoracic regions. However, as endoscopic spine surgery develops and its indications widen, more diverse and advanced surgical techniques are being introduced, and the complications of endoscopic spine surgery are also increasing accordingly.
Still now, literatures on the complications of endoscopic spinal surgery are very rare. Therefore, this study aimed to conduct a literature review of the complications of endoscopic spinal surgery and to predict the prognosis for the incidence of complications, and solutions to complications related to endoscopic spinal surgery.
MATERIALS AND METHODS
The PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines were used as templates for this systematic review. These guidelines are an evidence-based minimum set of items aimed at helping authors improve the reporting of systematic reviews and meta-analyses. The review process started with a search of the PubMed and Cochrane databases to identify articles on spinal stenosis and endoscopic decompression protocol. A reviewer assessed all articles and references and agreed on which articles should be included. To prevent selection bias during the review, abstracts from the search were numbered and pasted onto a document after deleting the publication journal, author, and institution. The initial search included the keywords “endoscopic spinal surgery,” “endoscopic cervical discectomy,” “endoscopic cervical foraminotomy,” “endoscopic lumbar discectomy,” and “endoscopic lumbar decompression,” which yielded 494 results. After duplicates were identified and removed, 421 articles were obtained.
The search also included the exact surgical technique term “endoscopic spinal surgery” and returned 188 articles published between 1980 and 2021. The exclusion criteria included no reported complication results (57 articles), microendoscopic surgery (23 articles), metastasis (7 articles), and studies not in English (7 articles). A total of 94 articles that met our inclusion criteria were identified through the search process and analyzed (Fig. 1). Additionally, we included 9 case studies and technical notes dealing with the complications of endoscopic spinal surgery. After excluding articles that met the inclusion criteria, 103 articles were included.
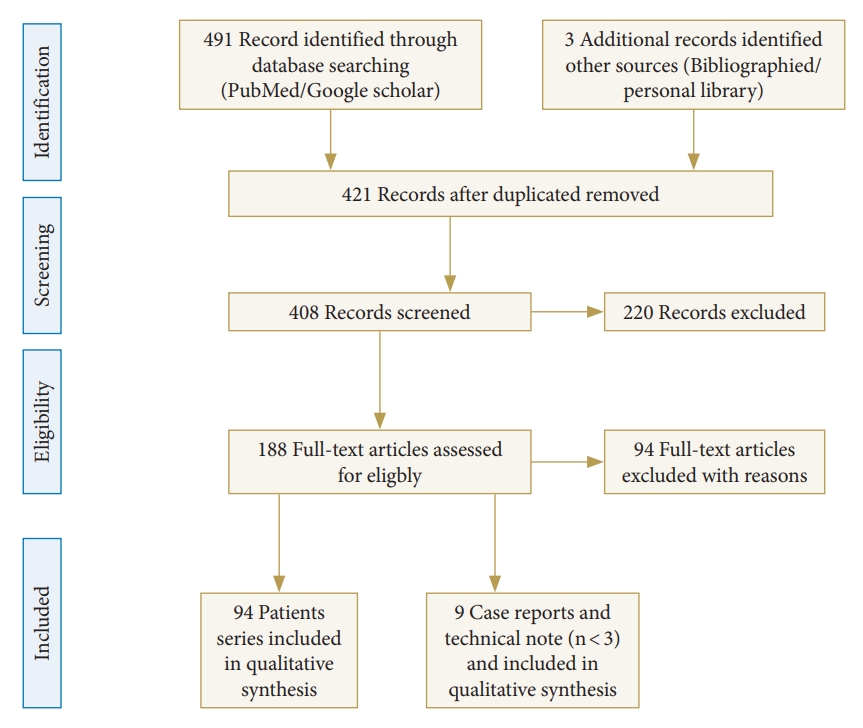
Flow diagram (PRISMA format) of the screening and selection process of full endoscopic spinal surgery.
To date, randomized controlled trials (RCTs) on full endoscopic surgery have been scarce. There were only 3 RCTs for 2 full endoscopic interlaminar lumbar decompressions and full endoscopic cervical approaches. No RCT has compared full endoscopic transforaminal and interlaminar approaches and unilateral biportal approaches with complications. Therefore, direct meta-analysis was not possible for either method, and only a narrative analysis was performed.
RESULTS
A total of 103 articles related to complications of endoscopic spinal surgery were reviewed and analyzed.
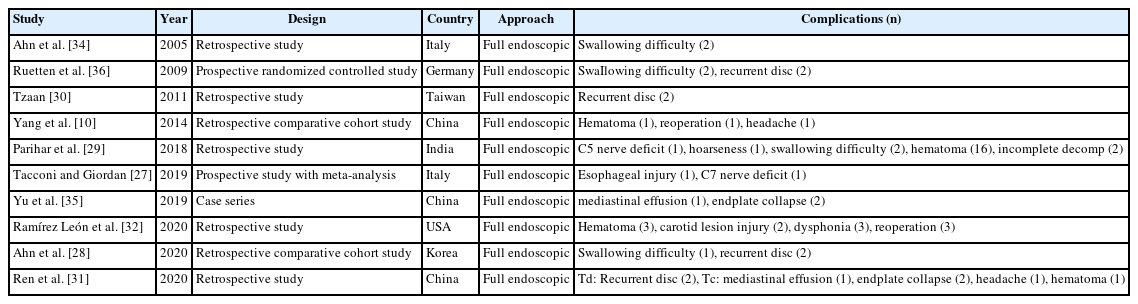
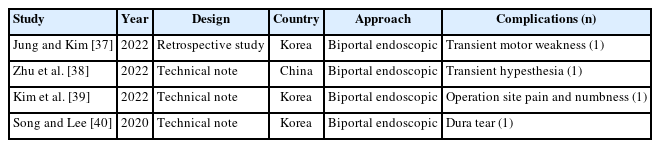
Complications of full endoscopic surgery were reported in 38 articles in cervical spinal disease, 4 on complications of full endoscopic cervical surgery (Table 1), and 11 on full endoscopic anterior cervical approach (Table 2), 19 on full endoscopic posterior cervical approach (Table 3), and 4 on biportal endoscopic cervical surgery (Table 4) [7-44].
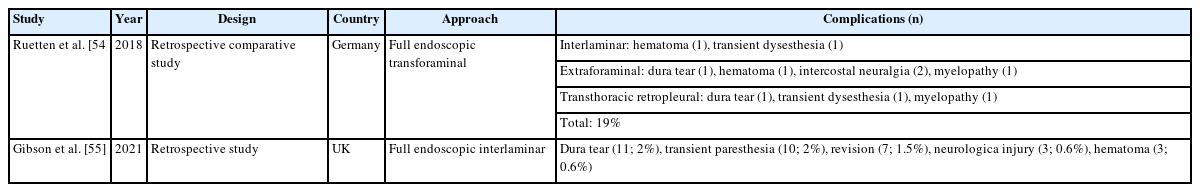
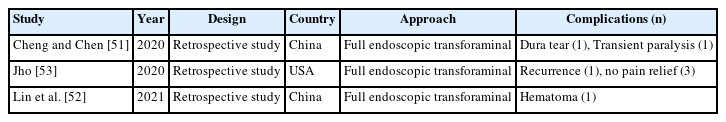
Twelve articles on complications of endoscopic thoracic spinal surgery were reported, 2 on review articles of full endoscopic thoracic surgery (Table 5), 7 on full endoscopic transforaminal thoracic approach (Table 6), and 3 on full endoscopic interlaminar thoracic approach (Table 7) including complications. However, there is no articles on biportal endoscopic thoracic surgery including complications [45-55].
Complications of lumbar endoscopic surgery were reported in 53 articles on full endoscopic lumbar decompression. Regardless of the transforaminal or interlaminar approach method, complications of full endoscopic lumbar decompression were reported in a total of 5 studies (Table 8).
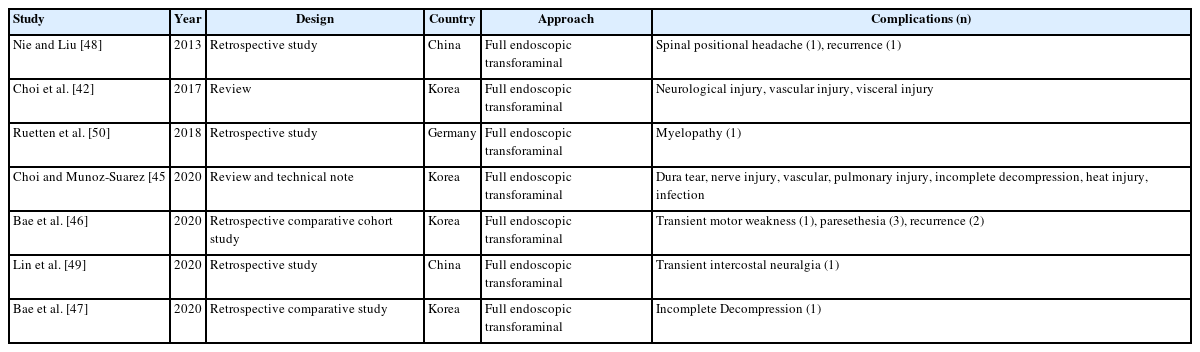
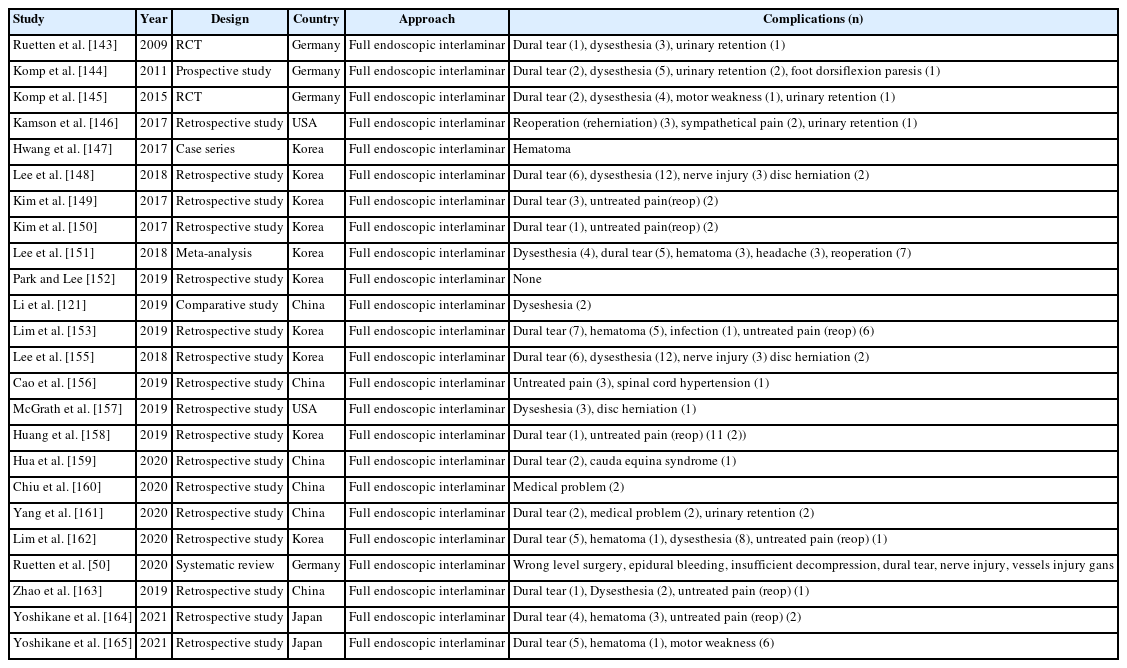
A total of 24 studies reported complications for full endoscopic transforaminal lumbar decompression (Table 9) and total 24 articles describing interlaminar approach contained complications (Table 10).
The overall incidence of clinically symptomatic complications is below 10%. Most complications were minor, and life-threatening complications, such as thromboembolism, sepsis, severe bleeding, or pulmonary complications are less frequent than open surgery. The complications of endoscopic cervical surgery at approximately 5% with both anterior and posterior approaches had an incidence equivalent to that expected from open cervical surgery.
According to the complication analysis of endoscopic spinal surgery, regardless of the cervical, thoracic or lumbar spine, regardless of the uniportal or biportal approach, main complications such as dural tears, postoperative hematoma, neurological irritation (dysesthesia), untreated pain are commonly reported.
1. Complications of Endoscopic Cervical Spinal Surgery
Regardless of the cervical anterior or posterior approach method, several literatures on the complications of full endoscopic cervical surgery have already been reported. According to the analysis of complications of endoscopic spinal surgery, Guo et al. [41] reported total complication rate of 4.7% and a reoperation rate of 1.1% in cervical endoscopic surgery. In anterior full endoscopic cervical surgery, recurrent laryngeal nerve injury and swallowing dysfunction are unique complications of this method. In addition, numbness, hematoma, discitis, vascular injury, and persistent pain were reported as complications.
In posterior endoscopic cervical surgery, transient dysesthesia, neck pain, and nerve (spinal cord and dura) injuries are comparatively common complications.
2. Complications of Endoscopic Thoracic Spinal Surgery
Gibson et al. [55] reported complications of endoscopic thoracic surgery, in this study, dura tear (2%) and transient paresthesia (2%) were common complications, and revision (1.5%), neurological injury (0.6%), and hematoma (0.6%) were reported as complications.
In endoscopic transforaminal thoracic surgery, intercostal neuralgia is unique complication. Other common complications include neurological injury, vascular injury, visceral injury, recurrence, dysesthesia, and incomplete decompression. In endoscopic interlaminar thoracic surgery, dural tears, transient paralysis, and dysesthesia were relative common complications.
In thoracic spine endoscopic surgery, motor weakness due to the deterioration of myelopathy has been reported as a complication, requiring careful and meticulous techniques.
3. Complications of Endoscopic Lumbar Spinal Surgery
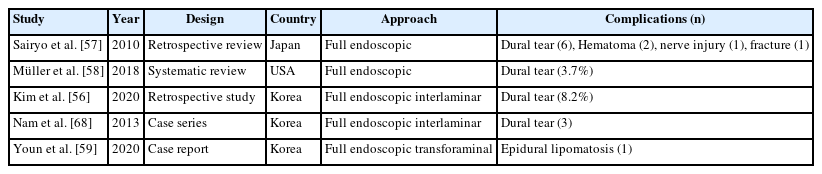
Regardless of the lumbar transforaminal or interlaminar approach method, literatures on the complications of full endoscopic surgery have already been reported [56-59]. According to the analysis of the complications of endoscopic spinal surgery, dural tears, postoperative hematomas, neurological complications, lower condyle fractures, and epidural lipomatosis were reported.
Lee et al. [60] reported a meta-analysis that compared the transforaminal decompression versus the interlaminar approach for lumbar lateral recess stenosis, in which the transforaminal approach had 3 times more complications (9.1%) than interlaminar decompression (3.4%).
Lin et al. [61] reported a systematic review of unilateral biportal endoscopic spinal surgery (UBESS), reporting a mean incidence of complications of 6.7%. The most common complication was a dural tear. The total mean incidence of dural tears was 4.1% after the UBESS procedure in 6 studies (range, 2.9%–5.8%).
Fan et al. [62] reported complications and risk factors of percutaneous endoscopic transforaminal discectomy (PETD). In this study, the incidence of different types of complications was 9.76% (72 of 738). The complications and occurrence rates were as follows: 2.30% (17 of 738) of recurrence, 3.79% (28 of 738) of persistent lumbosacral or lower extremity pain, 1.90% (14 of 738) of dural tear, 0.81% (6 of 738) of incomplete decompression, 0.41% (3 of 738) of surgical site infection, 0.27% (2 of 738) of epidural hematoma and 0.27% (2 of 738) of intraoperative posterior neck pain.
Ju et al. [63] reported a study of narrative analysis, comparing the complications of the transforaminal and interlaminar approaches. They found that dural tears are overwhelmingly common (2.19%) in interlaminar decompression followed by epidural hematoma (0.76%) and transient dysesthesia, whereas in transforaminal decompression, dysesthesia (1.46%) was the most common, followed by untreated pain (1.20%) and dural tearing.
In UBESS, Liang et al. [64] reported the overall complication rate was 5% and dural tears were the most frequent complications at 2%, followed by epidural hematoma with an incidence of 1%. The remaining complications included nerve root injury, inadequate decompression, and postoperative headache.
In addition, Wang et al. [65] reported the results of a single-arm rate meta-analysis, which showed that the overall complication rate of unilateral biportal endoscopic treatment of lumbar spinal stenosis was 6.27%, the incidence of dural tear was 2.49%, the incidence of transient paresthesia was 0.14%, postoperative spinal epidural hematoma was 0.27%, and postop headache, inadequate decompression, root injury and infection were 0%.
Summing up several papers on complications of endoscopic spine surgery, the most common complications of endoscopic spine surgery are dural tears, epidural hematoma, transient dysesthesia, and incomplete decompression.
This study also discusses the treatment method for each complication, along with the thesis review.
4. Management of Complications of Endoscopic Spinal Surgery
1) Dural tear
Dural damage is the most common complication of endoscopic spinal surgery, and it can lead to serious complications if an accurate diagnosis and appropriate treatment are not performed. The overall rate of dural tears in endoscopic spinal surgery was 2.7%, range from 0% to 8.6% [58]. The incidence of a dural tears was much greater in cases with lumbar stenosis (3.7%) than in lumbar discherniation (2.1%). The risk of dural tears is greater in bilateral decompression procedures than unilateral decpmpression.
Pan et al. [66] reported that the incidence of dural rupture increased to 1.1% when percutaneous endoscopic lumbar discectomy (PELD) was switched from an “inside-out” technique to an “outside-in” technique. Dural injury by instruments or radiofrequency, spinal canal adhesions, large disc fragments, and a loose dura are risk factors for dural tears.
However, Klingler et al. [67] reported that the occurrence of complications after durotomy in minimal invasive surgery is lower than after open surgery because of the preservation of the paraspinal musculature. The paraspinal musculature is not dissected during minimally invasive surgery and slides back to its original position after removal of the tubular retractor.
In UBESS, Liang et al. [64] reported dural tears were the most frequent complication at 2%. Wang et al. [65] reported that the incidence of dural teara was 2.49%. There are several main reasons for spinal dural tears caused by UBE spine endoscopic surgery. (1) Beginners easily make mistakes because the visual field under endoscopy is a 2-dimensional plane and is easily blurred. (2) UBE does not require retraction of the anatomical structure to expose the dura mater, which is quite different from other techniques. (3) Patients with complex conditions require operations of long duration, increasing the risk of spinal membrane tears. (4) During the operation, the injected saline squeezed both sides of the dura mater, causing the area to fold. The central area may be damaged during ligamentum flavum resection. (5) When using high-speed drills, the peripheral fibrous bands and vascular bundles of the dura may stretch around the drill neck, causing larger tears.
Several methods have been introduced for the treatment of incidental dural tears during endoscopic spinal surgery. An autologous muscle or fat graft in combination with fibrin glue or a fibrin-sealed collagen sponge seems to be a good and safe method for the management of dural tear in lumbar endoscopic spine surgery [9].
Kim et al. [56] reported the incidence of incidental durotomy was 8.2% and classified the incidental durotomy during endoscopic decompression according to lumbar levels, 40.7% occurred at L3–4, 44.4% at L4–5, and 14.8% at L5–S1. They also divided incidental durotomy into 4 types: 29.6% are type 1 (peripheral type), 70% are type 2 (central type), 7.4% are type 3 (complex type), and 3.7% are type 4 (unrecognized). They recommended the endoscopic patch blocking dura repair technique should be considered in type 1 to type 3A of dura tears with a good prognosis and clinical outcome. However, open surgical repair is recommended in types 3B, 3C. and 4 dura tears with fair to poor outcome.
Nam et al. [68] introduced double-Layer TachoSil packing technique for incidental durotomy in endoscopic surgery. A hemostatic agent, TachoSil (Nycomed, Linz, Austria), is used for control of local bleeding in several types of surgery, but its use in dural repair in endoscopic spinal surgery has not been described. When TachoSil packing is performed, the intradural TachoSil is inserted to avoid spilling, and the extradural TachoSil is sealed over the intradural TachoSil. Therefore, TachoSil did not cause a mass effect. Nevertheless, a thin layer of TachoSil must be applied; the application of larger quantities results in pooling that could lead to serious side effects such as compression of the spinal cord and nerve roots. If used incorrectly, excess TachoSil may cause additional iatrogenic dural tearing. In our experience, mild swelling of the TachoSil in the intradural space reinforces the dural repair site and prevents secondary rupture; it also ensures good adhesion of the edge of the TachoSil to the intact surrounding dura. Second, while maneuvering TachoSil intradurally, the cast side will dissolve in cerebrospinal fluid and adhere to nerve roots, which could be dangerous and difficult to reverse. Thirdly, thrombin is proinflammatory and could cause arachnoiditis and neuritis in the postoperative period if deployed intradurally.
2) Postoperative epidural hematoma
An epidural hematoma occurs mainly after an interlaminar approach. Recently, as endoscopic surgery for spinal stenosis and intervertebral discectomy has increased, the complication rate has also increased. It is also one of the most common complications in biportal endoscopic surgery.
The incidence of postoperative epidural hematoma is approximately 0.27%. Continuous saline irrigation is necessary during biportal endoscopic spine surgery [69-71]. The use of an infusion pump during surgery may be an unavoidable risk factor. However, it may increase the epidural pressure and, subsequently, result in meningeal irritation, indicated by neck pain or headache [69-71]. When the outflow of saline solution is blocked, the pump continues to infuse saline to increase the pressure in the surgical field, cover up bleeding points, and cause intraoperative hemostasis. Lack of saline solution may cause postsurgical epidural hematoma [72].
There are 2 possible mechanisms of increased epidural and intracranial pressure by continuous saline irrigation [73]. The first is the direct pressure effect by continuous irrigation of saline. The second is direct cranial movement of irrigation fluid. Prolonged operating time or poor patency of the irrigation fluid can increase epidural pressure during biportal endoscopic surgery [72]. In the biportal endoscopic approach, continuous saline is passed from the endoscopic portal to the working portal. The patency of saline outflow and constant flow is important for maintaining epidural pressure. An infusion pump pressure >50 mmHg can increase the cervical epidural pressure in this surgery. Reducing the operation time and maintaining the pump pressure below 40 mmHg may be useful in reducing the complications caused by the increase in epidural pressure [74-76]. Additionally, postoperative epidural Hemovac insertion may help to drain excessive irrigation fluid. Neck pain or headache can be improved with bed rest and conservative treatments.
Although symptomatic postoperative epidural hematoma is relatively rare (the incidence rate is 0.02% to 4.6%) [74], it can lead to serious consequences such as cauda equina syndrome and even lower limb paralysis, which affects patients’ quality of life. Therefore, early detection and handling are important.
Ahn et al. [73] reported that postoperative epidural hematoma is one of the complications that are considered to develop more often in biportal endoscopic surgery than in conventional spine surgery. The radiological thecal sac compression by hematoma was 39.8% of grade 1 (thecal sac compression less than a quarter), 30.1% of grade 2 (between a quarter and a half), 26.5% of grade 3 (between a half and three quarters), and 3.6% % of grade 4 (over three quarters) in biportal endoscopic surgery.
Kim et al. [74] reported that the overall occurrence rate of postoperative hematoma was 23.6% after biportal endoscopic spinal surgery. Female sex, old age (> 70 years), preoperative anticoagulation medication, and usage of intraoperative water infusion pump were significantly correlated with the occurrence of postoperative hematoma. Although symptomatic postoperative hematoma was extremely rare (1.9%), radiologic hematoma confirmed by postoperative magnetic resonance imaging (MRI) was higher (23.6%). The perioperative risk factors of postoperative hematoma after biportal endoscopic spinal surgery include female sex, older age (> 70 years), preoperative anticoagulation medication, usage of intraoperative water infusion pump, and surgery requiring more bone work (laminectomy or interbody fusion).
Additionally, Kim et al. [75] reported that the total number of patients with hematoma was 39 (24.7%) according to T2-weighted axial postoperative MRI. The incidence of postoperative spinal epidural hematoma after biportal endoscopic spinal surgery according to postoperative MRI was higher than expected, regardless of the patients’ postoperative symptoms. Postoperative hematoma has a decisive influence on postoperative results, and revision surgery may be necessary if canal encroachment is >50% with concomitant symptoms.
Symptomatic postoperative spinal epidural hematoma is a devastating complication that could develop after biportal endoscopic spine surgery [76]. Gelatin-thrombin matrix sealant (GTMS) is commonly used to prevent postoperative spinal epidural hematoma. Intraoperative use of a GTMS during biportal endoscopic spine surgery may be related to a reduction in the occurrence rate of epidural hematoma. Specifically, patients treated with GTMS appliances showed a marked decrease in the occurrence of postoperative spinal epidural hematoma and had better clinical outcomes.
3) Retroperitoneal hematoma
Although rare, hematomas can occur as a result of vessel injury during the full endoscopic transforaminal approach. A small amount of bleeding is not a problem even with conservative treatment, however, if the segmental artery branch is damaged, a large retroperitoneal hematoma may occur and cause symptoms. To avoid blood vessel damage, it is important to carefully insert the needle into the relatively safe avascular area by touching the bone of the facet in the safety zone during the transforaminal approach [77-79].
In addition, because damaged blood vessels may not be seen well in the field of view of endoscopic surgery when the endoscope is removed after the surgery, it is necessary to check slowly that there is no bleeding in the surrounding tissue. Bleeding control during surgery is the most important thing, and when bleeding occurs, the method using the radiofrequency probe is mainly used, or another electrocautery or bone wax is used when there is bone bleeding. However, in cases of severe bleeding, it is often difficult to identify the bleeding site because it is difficult to secure a visual field. At this time, a hemostatic agent such as GTMS, can be helpful, and while the surgical field is secured for a while with the hemostatic agent, you must check the bleeding site and perform sufficient hemostasis with radiofrequency or other electrocautery before leaving. Additionally, if bleeding continues even after hemostasis, Hemovac drainage after surgery can be a good way to prevent hematoma.
4) Postoperative dysesthesia
Postoperative dysesthesia often occurs in the transforaminal approach, and this surgical method can be caused by direct irritation of the exiting nerve root that anatomically borders the safety zone.
Ju et al. [63] reported that dysesthesia and untreated pain are relatively common complications of the transforaminal approach after decompressing ipsilateral foraminal and lateral recess stenosis.
Silav et al. [80] reported that postoperative dysesthesia is caused by irritation of the instruments and improper operation. The dorsal root ganglion (DRG) lies in the intraforaminal region and is vulnerable to disc herniation, foraminal stenosis, and mechanical damage by operative instruments. Damage to the DRG brings symptoms different from those associated with primary pathology. As a unique complication of PETD, postoperative dysesthesia greatly affects recovery and the postoperative quality of life. Cho et al. [81] applied the floating retraction technique to prevent postoperative dysesthesia and revealed that this technique was effective in 154 patients. Fluoroscopy is essential to locate guiding wire and working cannula to avoid mechanical stretch or damage to the upper DRG.
For the prevention of postoperative dysesthesia, the foraminoplasty is performed to expand the safety zone without causing an exiting nerve root irritation [63,82-84]. Foraminoplasty is not always needed during the endoscopic transforaminal approach to prevent postoperative dysesthesia. However, it is an especially useful method for widening the safety zone in cases of narrowed intervertebral foramina, such as facet hypertrophy or superior articular process overriding. It is also a safe and effective technique for entering the epidural space without exiting nerve root injury, especially in cases of central disc herniation or a downward migrated disc herniation, which have a high risk of causing an exiting nerve root injury. To effectively remove herniated disc material, the insertion angle of the endoscope was modified depending on the type of disc herniation. Significantly, the closer the incidence angle is to that of the vertical axis, the wider the safety zone, thereby reducing the possibility of exiting nerve root injury; however, it is difficult to access the epidural space and to secure the field of view. On the other hand, if the angle of incidence is close to that of the horizontal axis, accessing the epidural space and securing the field of view is easier, but the safety zone is narrowed, which increases the possibility of exiting nerve root injury. Therefore it is important to determine the appropriate angulation based on the pattern of disc herniation. To reduce exiting nerve root irritation within the safety zone as much as possible. Keeping the endoscopic cannula steep and located in the inferior disc space rather than the superior disc space is important [82].
In biportal endoscopic surgery. the incidence of transient paresthesia is approximately 0.14% [65]. The main reason for transient paresthesia after surgery is that palsy and pain are both caused by sensory nerves. The pain is transmitted by small unmyelinated fibers, and the conductive palsy is thick [85-88]. The structure of unmyelinated fibers is relatively simple, and the postoperative recovery is faster, while myelin fibers need to undergo a longer and more complex repair process; In addition, pain is a more acute and uncomfortable sensation than palsy, thus, surgery is often covered up. After the postoperative pain is weakened or recovered, the palsy is exposed [87]. Most patients will relieve the palsy. However, because palsy is positively related to illness time and degree of stenosis, the recovery times of different patients are different [88].
5) Incomplete decompression
Whether resection of the herniated disc is complete depends on the position of the working cannula, type of disc herniation, and size of the herniated fragments. Incomplete discectomy is particularly common in downward migration or high canal compromised disc herniation. Choi et al. [89] retrospectively analyzed 10,228 patients treated by PETD, and found 283 cases of incomplete resection, among which 95 were caused by improper location. Regarding the type of herniation, there were 91 cases with central herniation (32.2%), 70 with migrated herniation (24.7%), 63 with axillary type herniation (22.3%), 18 with shoulder-type herniation (6.4%), and 12 with foraminal/extraforaminal herniation (4.2%). Lee et al. [90] found that herniations with high canal compromise and high-grade migration make it harder for PELD to efficiently remove herniated disc. Herniated disc fragments should be adequately released from the annulus before they are grasped and removed. Detailed planning of the puncture route is the key for complete removal. A careful check for residual fragments is necessary, and placing the bevel of the working cannula toward the fragments helps achieve sufficient removal of the herniated disc. On the other hand, excessive resection of the herniated disc may increase the risk of dural tears and damage to the nerve root, thus, surgeons need to restore the normal motion and pulsation of the nerve root [78.91].
In the transforaminal approach, the foraminoplastic technique is a safe and reliable method for discectomy, and the migrated disc can be easily removed using a curved probe or forceps. Because the field of view of endoscopic surgery is narrow, it may not be possible to check the lesion area, and dura free pulsation must be checked to ensure sufficient decompression.
During spinal stenosis decompression, unilateral and bilateral decompression should be performed to sufficiently decompress the superior articular process in the lateral recess area to confirm the traversing nerve root, and sufficient laminectomy is required for sufficient decompression.
Decompression is usually excellent in UBESS for lumbar spinal stenosis. However, decompression may be inadequate in patients with severe lumbar spinal stenosis. Deviations in the preoperative assessment and intraoperative decompression range have been the main reasons for inadequate decompression [92]. Choi et al. [93] showed that for early cases, postoperative MRI revealed inadequate resection of the proximal and contralateral ligamentum flavum. These patients’ acute neurologic symptoms were relieved, although they had complained of tiredness in the affected calf. Choi et al. [93] reported that angled curettes were more useful in performing adequate flavectomy than Kerrison punches. Angled curettes, but not straight curettes or Kerrison punches, might scrape the ligamentum flavum under the lamina without excessive laminectomy. To decompress the contralateral side, Liang et al. [64] reported that a wider interspinous gap should be created to allow for simultaneous insertion of an endoscope and an instrument into the small midline space, with partial resection of the upper and lower ends of the spinous processes using a high-speed burr.
Intraoperative irregularities and thermal injuries from radiofrequency ablation have been the main causes of nerve root injury. The use of an arthroscopic radiofrequency ablation tip in the spinal canal can cause significant thermal damage to the neural structures. Therefore, it is important to be gentle during the procedure, to identify nerve structures carefully, and to reduce the voltage of the radiofrequency device if necessary [64].
6) Recurrence of disc herniation
Recurrent lumbar disc herniation (LDH) is defined as a recurrence of disc herniation at the same site of a previous discectomy in a patient who has experienced a pain-free interval after surgery. However, the minimum length of the pain-free interval is debatable, ranging from any interval of pain resolution to 6 months [94-97]. Moreover, recurrent disc herniation should be discriminated from incomplete discectomy or endoscopic operative failure.
The purpose of PELD is not to remove nucleus pulposus totally but to remove partially the herniated disc fragments and decompress nerve root. Therefore, recurrence of LDH sometimes occurs with aging, inappropriate weight-bearing, and other factors like male gender, obesity (body mass index [BMI] ≥ 25 kg/m2), old age (≥ 50 years), trauma history, and central disc herniation. But PELD also has some unique risk factors for LDH recurrence, such as surgeons’ having less experience with PELD (≤ 200 cases) and performing operations in the early development stage of PELD [91,97]. Especially, early recurrence after PELD is associated with several risk factors such as BMI, degeneration scale, combined herniation nucleus pulposus, and early ambulation [98]. Preoperatively, surgeons should study imaging examinations and design the puncture route carefully. Postoperative instructions like lumbar muscle exercise, proper weight burden, and appropriate sitting posture are essential to decrease the possibility of LDH recurrence [63].
7) Increased epidural pressure
With UBESS via the interlaminar approach, the use of high intraoperative water pressure can increase cerebrospinal fluid pressure and intracranial pressure, leading to postoperative headache and can even induce seizures [85,86]. Therefore, we searched for the early symptoms of seizures after surgery, such as neck pain, headache, blurred vision, and drowsiness. To avoid the occurrence of postoperative headache, it is crucial to prevent high intraoperative water pressures. Rather than attempting to obtain a clear vision by increasing the infusion pressure, Kim et al. [99] reported that it would be preferable to improve the outflow by applying an extension or crosscut of the fascia incision via the working portal, which will allow for a clear view and prevent the occurrence of postoperative headache. Czigléczki et al. [100] reported that irrigation could lead to meningeal irritation and postoperative headache; however, reducing the operative time can avoid such complications. Choi [101] recommended that the irrigation pump pressure should be kept at < 30 mmHg when using the pump.
8) Intervertebral infection
The incidence of intervertebral infection after spine surgery ranges from about 0.1% to 4.5%, most cases are caused by bacterial infection [98-104]. However, because of the continuous saline irrigation and short operation time in endoscopic spinal surgery, postoperative infection is rare. Additionally, the low trauma of PELD makes intervertebral infection uncommon, but the risk still exists.
Gu et al. [105] reported an incidence being 0.47%, among 209 cases of LDH treated by PETD, they found only one infected patient recovered through intravenous antibiotics after 2 weeks. Pyogenic spondylodiscitis is a devastating complication after spinal surgery and causes severe spinal nerves dysfunction. Even if the infection is not suspected after endoscopic spinal surgery, early tests such as erythrocyte sedimentation rate and C-reactive protein should be performed. MRI is of little value in early diagnosis. Needle biopsy of the disc guided by fluoroscopy is diagnostic and helpful in identifying pathogenic bacteria. Once diagnosed, patients with mild symptoms need positive antibiotics and a braking system on the bed. As for patients with severe symptoms and signs, intervertebral washing and drainage should be performed. Open debridement and fusion are necessary if conservative therapy is of no benefit. Postoperative MRI alone is very difficult to make an early diagnosis of infection and is of little value. Disc needle biopsy by fluoroscopy allows for a definitive diagnosis and helps identify pathogenic bacteria. Once diagnosed, patients with mild symptoms require positive antibiotics and bed rest. In patients with severe symptoms and signs, intervertebral lavage and drainage should be performed. If conservative treatment does not help to control symptom, open debridement and fusion are required.
9) Postoperative instability and facet joint injury
Postoperative segmental instability or facet joint injury is another complication of biportal endoscopic laminotomy [106-108]. In addition, iatrogenic inferior articular process fractures can occur during laminotomy, and these complications are similar to those from conventional or microscopic surgery. Therefore, preoperative instability is a contraindication of biportal endoscopic lumbar decompression.
10) Cervical and thoracic endoscopic spinal surgery
Cervical and thoracic endoscopic spinal surgery is currently performed in hospital in the Far East, but is not popular in Europe or the United States. Attempts were made to remove cervical discs with minimally invasive anterior approaches in the 1990s, but the techniques used were not widely adopted because the inherent risks associated with the surgical approach and the lack of well-designed equipment [40].
Although it is well recognized that posterior cervical lamino-foraminotomy for discectomy and root decompression with foramen widening will minimize blood loss and enhance patient recovery compared to anterior cervical surgery, the benefits regarding clinical outcomes are less well established [40].
This is because a various posterior surgical methods have been used by surgeons, from microsurgery with tubular retractors to purely endoscopic techniques [109]. It is not clear endoscopic techniques leads to better surgical outcomes than the former.
Surgical complications of approximately 5% in both the anterior and posterior approaches were the same as expected in open cervical surgery, and there appeared to be a low rate of reoperation. The posterior approach may reflect the generally shorter clinical follow-up [7,110,111].
Choi et al. [42] reported the 3 main complications of cervical endoscopic spinal surgery: (1) neurological injury like damage to the cervical cord or nerve root due to inadvertent use of forceps or laser (transient with laser), (2) vascular injury like carotid vessels during percutaneous endoscopic cervical discectomy (PECD) and vertebral artery while foraminotomy. (3) visceral injury, mainly oesophagus because it is soft collapsible tube and highly prone to injury while needle insertion in PECD.
In the anterior cervical approach, the essential technical factor is the precise targeting of disc pathology. The surgeon should feel the carotid pulse and push the anterior neck down into the space between the carotid artery and tracheoesophagus until the fingertips touch the anterior surface of the vertebral body [112]. The tracheal air shadow on the fluoroscopic view may be a good indicator of the position of tracheoesophagus. For the patient with a short and thick neck, the shoulder shadow may interfere with C6–7 or lower level. An oblique fluoroscopic view can be useful to approach the C6–7 level. Regarding selective discectomy, direct fragment removal with small instruments is difficult because of tenacious annular anchorage. Careful release of fibrotic adhesion around the herniated fragment is mandatory before the removal of the freely movable herniation fragment [112-115].
In the posterior cervical approach, a definitive dissection of bony structures and identification of the laminofacet junction (so-called “Y-point”) is essential for a safe and precise cervical foraminotomy. To prevent postoperative instability, the extent of facetectomy should be limited to no more than 50% of the facet joint. After adequate foraminotomy, the herniated foraminal disc fragment should be removed while preventing a dural tear. The dissection between the herniated disc and the neural tissues can be performed with a blunt dissector. The exposure of herniated fragment with firm nerve retraction can be achieved by rotating the bevel ended tip of the working cannula. After adequate nerve retraction, the herniated piece can be removed by endoscopic forceps and supplementary radiofrequency or laser. Epidural bleeding may occur from flourishing venous plexus. A gentle tamponade with hemostatic agents or hydrostatic pressure may be useful with a bipolar coagulator [115].
In thoracic endoscopic spinal surgery, 3 main complications of thoracic endoscopic surgery were reported: (1) neurological injury like damage to the spinal cord and its nerve roots, (2) vascular injury like damage to the inferior vena cava or thoracic aorta can be life threatening, (3) visceral injury like damage to the lung or mediastinal viscera [42]. In endoscopic transforaminal thoracic surgery, intercorstal neuralgia is a unique complication of insertion of a working cannula between the intercostal spaces. Above all, since thoracic surgery can cause serious neurological damage such as myelopathy, the operation must be performed very carefully and safely.
DISCUSSION
Recently, endoscopic spinal surgery has expanded from lumbar discectomy to lumbar spinal stenosis decompression and foraminal stenosis decompression as the transforaminal and interlaminar approaches are advanced, respectively. In addition, endoscopic spinal surgeries have become possible from the lumbar spine to the cervical and thoracic spine, and various endoscopic surgical techniques are still being introduced and rapidly developing.
However, in spite of various advantages, endoscopic surgery does not yield good results for all spinal diseases, and it is important to select the appropriate surgical indications to obtain successful surgical results. The indications for endoscopic spinal surgery show slight differences depending on the interlaminar and transforaminal approach.
Choi et al. [42] reported a good indication of full endoscopic anterior cervical discectomy, in which disc herniation did not responding to conservative treatment and an annular tear with concordant pain on provocative discography. On the other hand, migrated disc herniation, calcified disc, collapsed disc space < 5 mm, instability, infection and past history of anterior cervical surgery were contraindications.
In full endoscopic posterior cervical foraminotomy, good indications were foraminal disc herniations (predominantly unilateral arm pain), single or multilevel foraminal stenosis (unilateral arm pain), persistent symptoms despite previous anterior cervical discectomy and fusion. On the other hand, axial neck pain, instability, and cervical kyphosis were contraindications [42].
Lewandrowski et al. [116] reported a systematic review of the contraindications for full endoscopic transforaminal decompression. In this article, more difficult central stenosis or complex foraminal stenotic lesions should be considered as alternative endoscopic approaches.
Also, Wagner et al. [117] reported calcified disc, severe stenosis, cauda equina syndrome, painless weakness, severe fibrotic adhesion, pyogenic spondylodiscitis, and severe spinal infection were contraindicated in full endoscopic interlaminar decompression.
Ju et al. [63] reported a systematic review article of contraindications and complications of full endoscopic lumbar decompression for lumbar spinal stenosis. In this study, considering the contraindications of transforaminal and interlaminar lumbar decompression, the transforaminal approach can be successful in the ipsilateral extraforaminal, foraminal, lateral recess, and central spinal canal. However, surgical access to the contralateral area is not possible owing to anatomical limitations. Therefore, in the case of the transforaminal approach, it is a major contraindication for multiple spinal stenosis, bilateral symptoms, and a high iliac crest. However, in the case of the interlaminar approach, access to the bilateral central stenosis and lateral recess is possible, but access to the foraminal or extraforaminal areas is difficult, thus, foraminal stenosis can be a contraindication. However, both the methods share similar contraindications [63].
Heo et al. [118] reported the contraindications of unilateral biportal endoscopic lumbar decompression to include trauma, infection, tumor, instability, high-grade spondylolisthesis, isthmic spondylolisthesis, and severe scoliosis.
For successful endoscopic spinal surgery, it is most important to understand the advantages and disadvantages of endoscopic approach methods and to select the most effective and convenient surgical approach for the disease.
The endoscopic spinal surgery has developed rapidly as new delicate techniques have been introduced. The transforaminal endoscopic surgery developed into foraminoplasty with the spread of endoscopic drills, enabling decompression of foraminal stenosis at the surgical site, which was previously limited to discectomy. In addition, with the development of an interlaminar approach to drill-assisted laminectomy, bilateral and contralateral decompression has become possible. However, as the scope of endoscopic surgery is widen, high-level surgical skills are required and difficult, and the complications of endoscopic surgery are also increasing.
According to our study, the incidence of complications was similar between the transforaminal and interlaminar approaches, regardless of cervical, thoracic and lumbar endoscopic surgery. However, the incidence of some complications depends on the surgical approach and method.
In cervical endoscopic spine surgery, the anterior approach and posterior approaches are used. Since the anterior approach is similar to open surgery, there is an anatomically high risk of damage to the anterior structures of the spine, which can cause swallowing difficulties and complications such as hematoma and hoarseness. On the other hand, in the posterior approach, many complications such as nerve root injury, hematoma, and dysesthesia occur as the nerve root is exposed and needs to be managed during foraminotomy.
The surgical approach for the thoracic spine is subdivided into various methods due to its complex anatomical structure, however, in endoscopic surgery, it can be largely divided into transforaminal and interlamiar approaches.
In the full endoscopic thoracic transforaminal approach, it is necessary to enter between the ribs and access the epidural space through foraminoplasty, which can cause intercostal nerve injury from the moment the endoscope is inserted. Stimulation of or damage to the spinal cord during performance can cause serious complications that exacerbate myelopathy.
The interlaminar thoracic approach requires decompression of the spinal cord using a curret and Gerison punch after sufficient laminectomy as open surgery. In a state where sufficient laminectomy is not performed, it is dangerous because it can compress and damage the spinal cord during the process of inserting a curret, drill, or punch.
In particular, lesions that compress the anterior spinal cord, such as ossification of posterior longitudinal ligament, can exacerbate myelopathy during surgery, therefore, endoscopic thoracic spine surgery is a very dangerous and difficult, and open surgery should be actively considered if there is insufficient experience.
In lumbar endoscopic surgery, the full endoscopic interlaminar approach had a higher incidence of dural tear than the transforaminal approach, which might have been caused by medical instruments when dealing with the ligamentum flavum or adhered disc. According to our study, comparing the complications of the 2 methods, transforaminal approach had a high incidence of exiting nerve root injury, so dysesthesia was the most common, followed by untreated pain due to a high probability of incomplete surgery, and incidental dural tears which was less common. In contrast, the interlaminar approach requires decompression of nerves on both sides in the epidural space. The possibility of a dural tear and the incidence of epidural hematoma are high during instrument manipulation. The incidence of other complications was similar between the 2 methods.
Dura tear is the most frequently reported complication of endoscopic surgery in works of various literatures. Since nerve root herniation causes serious symptoms and secondary nerve damage, it is important to prevent nerve root herniation in dura defects. Until now, the gold standard treatment for dural damage is open dural repair, but recently, sealing dura defect by using TachoSil (collagen fleece) has been widely performed a lot in endoscopic surgery without open surgery requiring general anesthesia.
Hematoma is another common complication of the endoscopic spinal surgery. Intraoperative bleeding not only obstructs the surgical field of vision and delays the surgical time, but also can cause serious postoperative complications by unintentionally damaging structures during blind surgery. In general, electrical coagulation using radiofrequency is performed, however, it can be very difficult to control bleeding under a narrow endoscopic view.
In this case, it is easy to temporarily secure the surgical field using a hemostatic agent such as floseal, finding the bleeding site and cauterizing bleeding point. Even at the end of surgery, it is important to insert a hemostatic agent to prevent undetected bleeding during surgery. In patients with massive intraoperative bleeding or bleeding tendencies, it is important to insert a Hemovac to prevent hematoma so that unexpected bleeding is well drained and nerves are not compressed.
Nerve damage is a complication that occurs during surgery, and once it occurs, it cannot be treated surgically. Therefore, prevention is the most effective treatment option. In order to prevent this, accurate anatomical knowledge of endoscopic surgery and the safety of the approach must be considered, and careful and delicate surgery must be performed to avoid injury to the nerve during surgery.
Once nerve damage occurs, it takes a lot of time to recover even if it is reversible, and various treatments such as drug treatment and rehabilitation treatment must be performed because the symptoms vary depending on the degree and site of nerve damage.
Prevention is the best treatment for many other complications mentioned in this text. Even in a narrow space with a narrow field of view, it is necessary to obtain the same results as open surgery, therefore, a lot of experience and a long learning curve cannot be avoided.
In the last 5 years, many literatures related to endoscopic spinal surgery have been published. However, retrospective studies (level 3 evidence) are the mainstream, and level 1 evidence papers such as RCTs are absolutely lacking. In addition, there are many papers on full (uniportal) endoscopic spine surgery, however, papers on biportal endoscopic surgery are very rare, especially on the cervical and thoracic spine. For future endoscopic spinal surgery to have the same basis as open surgery, which is still the gold standard, more high-quality evidence such as RCT is needed. Based on these literatures, it is expected that meta-analyses on various topics will be conducted.
CONCLUSION
According to literature analysis, the endoscopic spinal surgery in lumbar, dura tear, postoperative hematoma, transient dysesthesia and untreated pain are relatively common. Additionally, various complications such as urinary retention, motor weakness, cauda equine syndrome, wound infection may occur. On the other hand, endoscopic cervical surgery, swallowing difficulty, hoarseness are common complication in anterior approach, dura tear, postoperative hematoma, transient dysesthesia and weakness are common in posterior approach. In summary, it is most important to understand the advantages and disadvantages of various endoscopic approach methods and to select the most effective and convenient surgical approach for the spinal disease.
Notes
Conflict of Interest
The author has nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.