Polyetheretherketone Versus Titanium Cages for Posterior Lumbar Interbody Fusion: Meta-Analysis and Review of the Literature
Article information
Abstract
Objective
Lumbar fusion with implantation of interbody cage is a common procedure for treatment of lumbar degenerative disease. This study aims to compare the fusion and subsidence rates of titanium (Ti) versus polyetheretherketone (PEEK) interbody cages after posterior lumbar interbody fusion and investigate the effect of clinical and radiological outcomes following fusion on patient-reported outcomes.
Methods
A systematic search strategy of 4 electronic databases (MEDLINE, Embase, Web of Science, and Cochrane) was conducted using different MeSH (medical subject headings) terms until January 2020. Pooled odds ratios (ORs) with 95% confidence intervals (CI) were calculated using fixed and random-effect models based upon the heterogeneity (I2) to estimate the association between interbody cages and the measured outcomes.
Results
A total of 1,094 patients from 11 studies were reviewed. The final analysis included 421 patients (38.5%) who had lumbar surgery using a Ti and/or a Ti-coated interbody cage and 673 patient (61.5%) who had lumbar surgery using a PEEK cage. Overall, PEEK interbody devices were associated with a significantly lower fusion rate compared with Ti interbody devices (OR, 0.62; 95% CI, 0.41–0.93; p = 0.02). There was no difference in subsidence rates between Ti and PEEK groups (OR, 0.91; 95% CI, 0.54–1.52; p = 0.71). Also, there were no statistically significant differences in visual analogue scale (VAS)-low back pain (p = 0.14) and Japanese Orthopedic Association scale (p = 0.86) between the 2 groups. However, the PEEK group had lower odds of leg pain after surgery compared to the Ti group (OR [VAS-leg], 0.61; 95% CI, 0.28–0.94; p = 0.003).
Conclusion
Ti and Ti-coated PEEK cages used for posterior lumbar interbody fusion are associated with similar rates of subsidence, but a higher rate of fusion compared to PEEK interbody cages. Randomized controlled trials are needed to better assess the effect of cage materials and potential factors that could influence the outcomes of interbody lumbar fusion.
INTRODUCTION
Symptoms arising from lumbar degenerative disease are common and can be debilitating, leading to surgical intervention to alleviate pain and restore function. The prevalence of low back pain (LBP) due to lumbar spondylosis is estimated at 3.6% worldwide, and 4.5% in North America [1]. When indicated, the application of interbody techniques to posterior lumbar fusion surgery is performed in a way to promote circumferential fusion across the instrumented levels. The use of interbody fusion techniques has increased and is a technique often utilized in the treatment of spondylolisthesis and spinal deformity [2,3]. However, as rates of both disease and surgical treatment rise, the number of patients undergoing unsuccessful fusion operations has increased as well [4,5]. Hence, understanding factors and surgical techniques influencing achievement of bony fusion is becoming increasingly crucial for improving outcomes in treatment of lumbar spondylosis.
One of the most commonly employed instrumentation techniques for achieving fusion is implantation of an interbody cage. A study from the Nationwide Inpatient Sample database showed as many as 83% of surgeries for degenerative spondylolisthesis involve the use of an interbody cage [6]. The BAK titanium (Ti) cage (Spine-Tech, Minneapolis, MI, USA) was the first cage to be introduced and to be successfully implanted in humans using a posterior approach in 1992 [7]. Ti was used in interbody cages because it enhances cell adhesion and osseointegration favoring bone fusion, but at the same time, may have a higher rate of subsidence compared to polyetheretherketone (PEEK) due to differences in the modulus of elasticity [8,9]. Despite that, PEEK is chemically inert with limited cell adhesion and fixation to bone [10].
The characteristics and clinical outcomes of Ti and PEEK cages for lumbar spinal fusion were explored in several studies [11-20], but the findings were largely inconsistent regarding fusion and subsidence rates. Hence, this study presents a systematic review and meta-analysis to evaluate the clinical outcomes of interbody cages in posterior lumbar fusion surgery. In this study, we determined the effect of interbody cage materials (Ti vs. PEEK) on fusion rates, cage subsidence rates, and patient-reported outcomes (PROs) following posterior lumbar interbody fusion (PLIF).
MATERIALS AND METHODS
We performed a systematic review and meta-analysis in line with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines [21]. MEDLINE, Embase, Web of Science, and Cochrane were searched for peer-reviewed articles written in English and published from database inception to January 2020, that included retrospective and prospective assessments of outcomes following lumbar spinal fusion using Ti vs. PEEK interbody cages. We used the following search terms: polyetheretherketone, PEEK, Ti, cage, interbody, interbody fusion, and lumbar fusion.
1. Study Selection
Two of the authors (EM and NF) independently identified articles eligible for review with input by the senior author (JHS). Studies were selected for inclusion in the meta-analysis if they evaluated PEEK and Ti interbody cages in spinal lumbar fusion procedures for degenerative spinal disease, intervertebral disc herniation, spondylolisthesis, and spinal stenosis. Studies were included in the meta-analysis if they reported at least one of the following outcomes: (1) fusion rates, (2) subsidence rates, or (3) PROs for Ti and PEEK. Initial screenings of abstracts were performed, followed by full-text reviews. Covidence Systematic Review Software (Veritas Health Innovation, Melbourne, VIC, Australia) [22] was used to organize the screening of abstracts, fulltext articles, and the selection of studies that meet the inclusion criteria.
2. Outcomes
The primary outcome was fusion rate and we tested the null hypothesis that Ti and PEEK have the same fusion rate in PLIF. Ti and Ti-coated interbody cages were grouped because they bring together the bio-compatible characteristics of Ti important for fusion. We compared patient baseline characteristics (age, sex, and comorbidities) in studies reporting on the primary outcomes to examine if patient characteristics can affect fusion and subsidence rates and could therefore inform the interpretation of nondifferential results. Secondary outcomes included the rate of cage subsidence and PROs that were assessed in the included studies: (1) Oswestry Disability Index; (2) visual analogue scale (VAS) for low back or leg pain; (3) Japanese Orthopedic Association (JOA) score for LBP, and radiologic outcomes when available.
3. Data Extraction and Quality Assessment
Data were independently extracted by 2 of the authors (EM and NF) using a standardized protocol and reporting electronic sheet. Disagreements between the 2 authors were resolved by arbitration when consensus could not be reached after discussion. Two reviewers independently assessed the quality of the included studies using the Newcastle-Ottawa scale, which allocates each study a quality grade of maximum 9 points based on (1) selection of the study groups, (2) comparability of the groups, and (3) assessment of outcomes [23].
4. Statistical Analysis
Continuous data were analyzed by calculating the pooled weighted mean difference with 95% confidence interval (CI). The association between the type of interbody cages and the primary and secondary outcomes were reported using the odds ratio (OR) with 95% CI. Between-study heterogeneity was evaluated using the I2 statistic. A fixed-effect model was used for I2 < 50%, while for I2 > 50% a random-effect model was employed. Statistical tests were 2-sided and p-value < 0.05 was considered statistically significant. We inspected the symmetry of the funnel plots and performed the Egger test to assess publication bias. Also, we used a nonparametric trim-and-fill procedure to identify and correct for funnel plot asymmetry and re-estimate the aggregate results [24]. We used R ver. 3.5.3 (R Project for Statistical Computing, Vienna, Austria), with meta [25] and metafor packages for all analyses [26].
RESULTS
1. Study Characteristics
Eleven studies involving PLIF with Ti and PEEK cages were included in this meta-analysis. The results of our search strategy are summarized in the PRISMA chart (Fig. 1). Eight single-center studies were retrospective, observational, and 3 single-center studies were prospective studies of which, 1 study was a randomized clinical pilot study. Four studies were carried out in Germany [17,20,27,28], 4 studies in Japan [13,16,18,19], 1 study in Italy [12], 1 study in China [15], 1 study in the United Kingdom (Table 1) [14].
The data of 1,094 patients was analyzed in this meta-analysis, of which 673 (61.5%) had lumbar interbody fusion using a Ti or Ti-coated cage and 421 (38.5%) had lumbar interbody fusion using a PEEK cage. The mean follow-up time in the Ti and PEEK groups was 20.5 and 22.3 months respectively (range, 6–84 months). The Ottawa-Newcastle quality assessment tool showed that most studies carry a potential risk of bias (Supplementary Table 1).
2. Demographics
Demographic characteristics of Ti and PEEK patients extracted from each study are summarized in Table 2. There was no difference between the mean age of the Ti (59.23±3.89 years) and PEEK groups (58.44±3.43 years) (p = 0.89). Overall 49.5% and 48.9% were men in the Ti and PEEK groups, respectively. Body mass index (BMI) for Ti and PEEK patients was reported in 3 studies only [15-17]. Mean BMI for Ti and PEEK groups were similar (25.76±0.64 kg/m2 vs. 26.37±1.08 kg/m2; p = 0.93). Comorbidities such as diabetes mellitus, Parkinson disease, treatment with hemodialysis, long-term steroid use for rheumatoid arthritis or systemic lupus erythematosus, were reported only in one study (p = 0.64) [19].
3. Posterior Lumbar Fusion
Lumbar fusion was performed for a total of 1,094 patients (Ti421, PEEK-673). PLIF was performed in 5 studies [13,15,18,27,28] for 390 patients (35.64%) (Ti-165, PEEK-225) and transforaminal interbody fusion (TLIF) in 6 studies [12,14,16,17,19,20,29] for 704 patients (64.36%) (Ti-256, PEEK-448). The different types of interbody cages used were summarized in Table 1, revealing 3 studies using Ti-coated PEEK cages [13,17,18]. Only 3 studies reported local autograft in their surgical protocol [16-18]. One of these studies used autograft mixed with bone graft substitute [17]. Estimated blood loss and operative time were only reported in 1 study [16]. Mean blood loss in the Ti group was 386.4±128.8 mL vs. 360.8±145.0 mL (p = 0.53). Average operative time was 183.8±29.4 minutes vs. 174.7±32.9 minutes (p = 0.32).
4. Fusion Rate
Early fusion status was assessed most commonly at 12 months and 24 months but as early as 3 months in 1 study by dynamic plain radiographs, computed tomography (CT) scan, and multiplanar reformation (MPR)-CT scan (Table 3). The assessment of fusion was not uniform across the studies, although it was most commonly determined based on bone bridges inside and outside the cage (summary in Table 3). None of the studies reported using bone morphogenic protein. The fusion rates were reported in all studies at the last follow-up. The range of the reported fusion rates was 53%–100% in the Ti group and 32%–100% in the PEEK group. Pooled analysis of fusion rates showed a statistically significant difference with PEEK interbody devices demonstrating 38% lower odds of fusion compared with Ti interbody devices (OR, 0.62; 95% CI, 0.41–0.93; p =0.02; I2=25%) (Fig. 2).

Summary of the definitions of fusion and subsidence rates used in the included studies, the follow-up period, and the modality used for assessment of fusion and subsidence

Forest plot showing the effect sizes and 95% confidence intervals (CIs) of studies comparing the fusion rates of PEEK vs. Ti. PEEK shows less odds of fusion compared to titanium cage for lumbar interbody fusion (odds ratio, 0.62; 95% CI, 0.41–0.93; p=0.02). PEEK, polyetheretherketone; Ti, titanium; df, degrees of freedom.
5. Subsidence Rate
Subsidence rates were successfully extracted from 6 studies [12,15-18,27] at a follow-up range (6–24 months). Four studies used CT scans [12,15,18,27], 1 study used MPR-CT scan [16], and 1 study used X-ray [17]. The definition of subsidence used in the studies is summarized in Table 3. Two studies reported no subsidence in either groups and were excluded from the meta-analysis because they do not provide any indication of either the direction or the magnitude of the effect. The range of subsidence rates in the Ti and PEEK groups was 0%–36% and 0%–31%, respectively. Overall, there was no difference in the rate of subsidence between Ti and PEEK interbody cages (OR, 0.91; 95% CI, 0.54–1.52; p =0.71; I2= 0%) (Fig. 3).

Forest plot showing effect sizes and 95% confidence intervals (CIs) of studies comparing subsidence rates for titanium and PEEK interbody cages. Titanium and PEEK have similar odds of subsidence (odds ratio, 0.91; 95% CI, 0.54–1.52; p=0.71). PEEK, polyetheretherketone; Ti, titanium; df, degrees of freedom.
6. Patient-Reported Outcomes
PROs were not reported in most the included studies. VAS-leg and VAS-LBP were reported in only 2 studies [16,17], and JOA scale for LBP was only reported in 2 studies [16,18]. There were no statistically significant differences in VAS-LBP (p = 0.14) and JOA scale (p = 0.86) between the Ti and PEEK groups. However, the PEEK group had 39% lower odds of leg pain after surgery compared to the Ti group (OR [VAS-leg], 0.61; 95% CI, 0.28–0.94; p = 0.0003; I2= 88%) (Fig. 4).
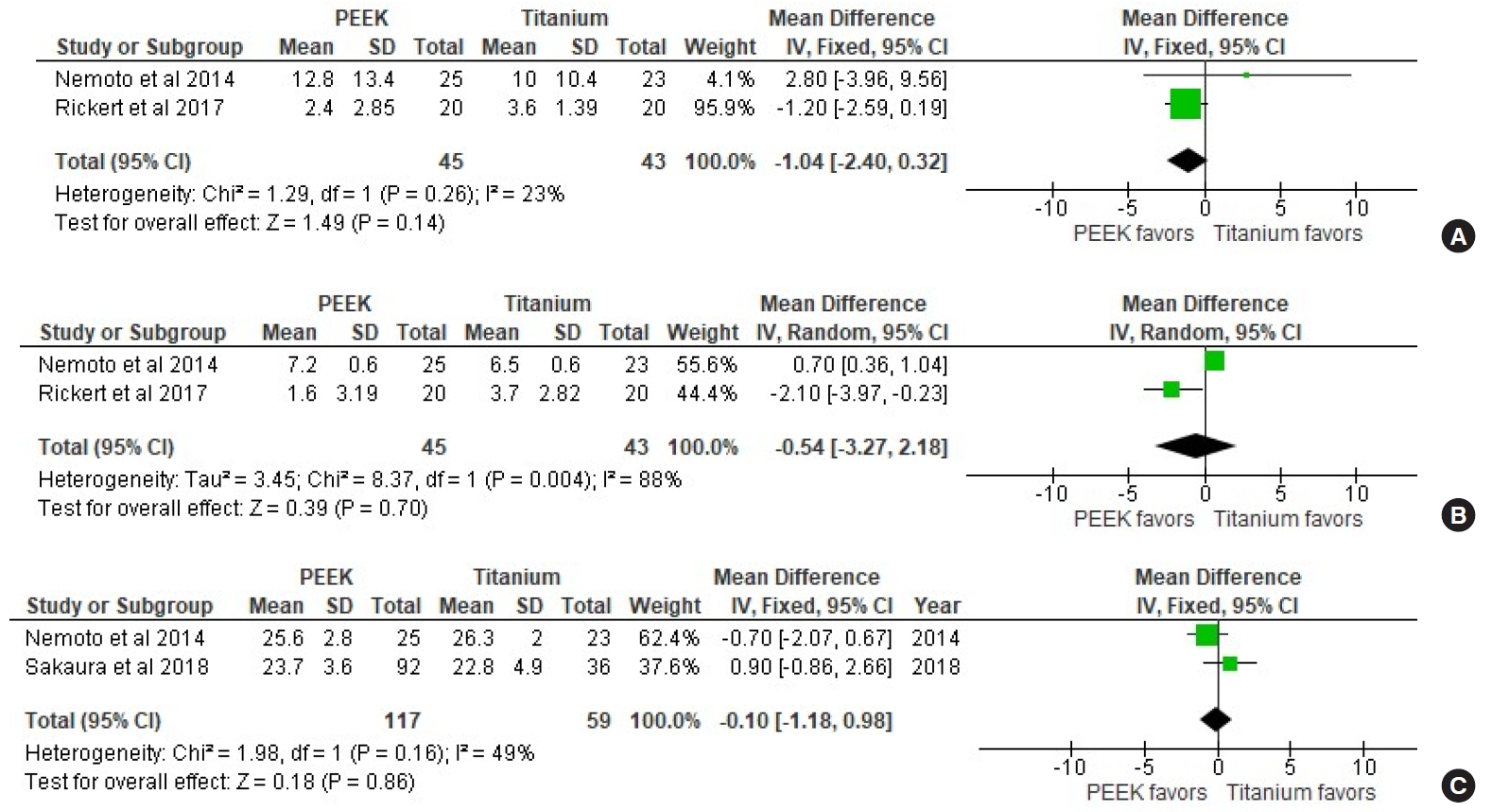
Forest plot showing effect sizes and 95% confidence intervals (CIs) of studies comparing visual analogue scale (VAS) scores for low back pain (A) and leg pain (B), and the Japanese Orthopedic Association (JOA) score for low back pain (C) for titanium and PEEK interbody cages. PEEK, polyetheretherketone; df, degrees of freedom.
7. Publication Bias
We found some evidence of publication bias, as suggested by slight asymmetry of the funnel plot (Egger test, z=-3.367; p = 0.009) and association between effect sizes and corresponding sampling variances (Begg test, z=-2.415; p = 0.01). According to the trim-and-fill method to correct for publication bias (Fig. 5), the association between type of cage and fusion rate was not significant after imputing 3 possible missing studies (adjusted OR, 0.90; 95% CI, 0.60–1.35; p = 0.61), suggesting a potential role for small-study effects or publication bias on the meta-analysis results.
DISCUSSION
This systematic review and meta-analysis of 11 studies involving 1,094 patients who underwent posterior lumbar fusion demonstrated increased odds of bony fusion with use of Ti and Ti-coated interbody cages in comparison to PEEK interbody cages for posterior lumbar fusion (p = 0.02). Demographic characteristics including age, sex, and BMI were similar between the 2 groups. However, important factors such as smoking status, osteoporosis and bone mineral density (BMD) were not reported in the included studies. Studies investigating posterior lumbar fusion identified low BMI, diabetes mellitus, osteoporosis, loosening of posterior instrumentation, and pear-shaped disc as potential risk factors for subsidence [14,30]. In addition to that, posterior screw fixation, the size and shape of the interbody cage, and the number of spinal levels fused, could impact the biomechanics of the lumbar spine following posterior fusion [31,32]. However, none of the studies included in this meta-analysis presented a comparative analysis of PEEK vs. Ti to study the effect of potential risk factors across the types of interbody cages.
Ti and PEEK are the most common materials used for interbody cages. In fact, PEEK implants are widely used for different applications because of their mechanical properties and good chemical resistance. In addition to that, their radiolucent property allows for better assessment of fusion by imaging [33]. However, the application of PEEK has been limited by the formation of a biofilm layer around its surface that potentially affects fusion to cortical bone [34]. This limitation of PEEK could be avoided by the application of Ti which has a microscopic rough surface that increases osteogenic cell differentiation factors. To further explore the properties of Ti, several studies investigated PEEK interbody cages with electron beam coating of Ti onto the surfaces showing some benefits compared to PEEK alone [35]. In fact, Ti promotes an inflammatory cellular response in its environment affecting bone remodeling [36]. In this instance, bioactive substances, in particular microstructured Ti, have been shown to improve the biocompatibility of PEEK interbody cages and to increase the rate of bone fusion [37].
Unlike PEEK that has an elastic modulus similar to bone, Ti material has an elastic mismatch that can lead to stress shielding and bone remodeling around the implant [38,39]. In this study, there was no difference in subsidence rates between Ti and PEEK cages. However, Seaman et al. [40], reviewed 4 cervical studies and 2 lumbar studies and showed that the rate subsidence for Ti was greater in the cervical and lumbar spine. This meta-analysis included exclusively 11 lumbar studies that give our results more power, but also included Ti-coated PEEK cages, a composite that overcomes the modulus of elasticity of Ti that leads to subsidence and provides effective osseointegration. Ti-coated PEEK cages may benefit from the properties of both materials to allow early osseointegration and fusion, at the same time, maintaining ideal disc heights and alignments for degenerative lumbar disease [41].
In addition to that, this meta-analysis included 5 PLIF studies and 6 TLIF studies. Previous comparative studies revealed that PLIF with bilateral cage placement was shown to be equivalent in fusion to TLIF with a unilateral interbody device. Intraoperative and postoperative complications were shown to be lower in TLIF compared to PLIF procedures [42,43]. So far, only 1 randomized control study reported radiological and clinical outcomes using Ti-coated and uncoated PEEK cages for TLIF but did not demonstrate a superiority for Ti-coated PEEK. Høy et al. [44], noted several problems in their study could affect fusion, such as endplate preparation, severity of osteopenia, osteoporosis, and fixation stability. Nevertheless, several experienced centers have reported improved clinical outcomes with minimal subsidence if the endplates are prepared appropriately [45,46], but these clinical studies have small sample sizes and factors associated with poor clinical and radiological outcomes for lumbar interbody fusion have not been extensively explored yet.
This meta-analysis is adherent to PRISMA guidelines and includes all relevant articles identified by an extensive literature search to assess the outcomes of interbody fusion in posterior lumbar surgery. According to the Ottawa-Newcastle quality assessment tool the quality of the included studies is low. A heterogeneity between the studies for subsidence rate and PROs was identified in the statistical analysis. The definition criteria, follow-up period and modalities used for assessment of fusion and subsidence were different across studies as shown in Table 3. The effect of important factors such as smoking, osteoporosis, and BMD were not reported in these studies. Many studies did not report the type and size of interbody cages used (Table 1). Also, a small-study effect was demonstrated in the setting of low-quality evidence, indicating that the results should be carefully interpreted. While Seaman et al. [40] published a meta-analysis including 4 anterior cervical discectomy and fusion studies and 2 additional TLIF studies using Ti and PEEK, this is the first study that evaluates the outcomes of Ti and PEEK in posterior lumbar fusion procedures. However, the results of this meta-analysis need to be investigated in a randomized controlled trial (RCT) that takes into account all the possible factors that could be associated with the clinical outcomes of interbody lumbar fusion.
CONCLUSION
As detailed in this review, the comparison of cage materials between PEEK and Ti revealed a competitive advantage of Ti and PEEK on high fusion and low subsidence respectively. In this meta-analysis, Ti interbody cages demonstrated a significantly higher fusion rate than that of PEEK in posterior spinal fusion. However, PEEK did not show significant superiority to subsidence in posterior spinal fusion. Although our understanding of indications and outcomes is steadily increasing, rigorous evaluation of indications and characterization of risks and outcomes is still required. So far, one pilot RCT compared Ti and PEEK interbody cages for posterior lumbar fusion. Future RCTs are needed to better investigate the implants and the associated factors that influence the outcomes of interbody fusion.
Notes
The authors have nothing to disclose.
Acknowledgements
Elie Massaad, MD research work, and education at Harvard Medical School are supported by a scholarship from the Dubai-Harvard Foundation for Medical Research (DHFMR).
SUPPLEMENTARY MATERIALS
Supplementary Table 1 can be found via https://doi.org/10.14245/ns.2040058.029.
Detailed Newcastle-Ottawa Scale of each included cohort study




