INTRODUCTION
Spinal metastases may result in neurological deficits due to metastatic epidural spinal cord compression (MESCC). The evidence-based treatment for patients with MESCC with neurological deficit is urgent surgical decompression and stabilization of the affected spinal level [
1-
3]. Prior to surgery, it is generally recommended to administer high doses of corticosteroids to any patient with (suspected) MESCC to prevent (further) neurological deterioration while the patient is awaiting surgery, and during surgery [
4].
Animal studies have demonstrated that steroids decrease edema and inflammation around the spinal cord by decreasing the water content of the cord at the level of the tumor [
5,
6]. This in turn is thought to decrease spinal cord compression and thereby stabilize or reverse neurological deficits until definitive treatment with surgery. Previous
in vivo studies investigated the effect of steroids in patients with MESCC who underwent radiotherapy [
7]. These studies demonstrated inconclusive results regarding the effects of steroids on ambulation and pain, interpretation of the results was furthermore compromised by the short time interval between the administration of steroids and the start of radiotherapy [
7]. However, high doses of corticosteroids are demonstrated to be associated with an increased risk of serious adverse events [
7,
8]. Despite the recommendation for the use of steroids in patients with MESCC, the clinical evidence is still limited [
4,
9].
The purpose of this study was to describe the effect of corticosteroids on preoperative neurological function in patients with (symptomatic) MESCC who underwent surgical intervention.
MATERIALS AND METHODS
1. Design
This study was part of an international multicenter prospective cohort study including patients with spinal metastases requiring surgery and/or radiotherapy was conducted (EPOSO, Clinical trials.gov identifier: NCT01825161). Patients between 18 and 75 years of age who underwent treatment of spinal metastases were eligible for inclusion in the observational study. For this analysis, patients were included if they underwent surgery between August 2013 and February 2017 for the treatment of spinal metastases, received steroids for (prevention of) neurological deterioration in case of MESCC, and reached at least 3 months of follow-up or dropped out/died before this time point. Patients with a primary spinal nervous system tumor, primary spinal bone tumor, and patients who received steroids for an indication other than neurological deficits (e.g., part of systemic therapy) were excluded from the analysis. The ethics board of each participating spine center approved the research protocol.
2. Intervention
Administration of steroids was based on discretion of the treating surgeon. In all cases, the corticosteroid used was Decadron (dexamethasone), either administered orally or intravenously. Usually, a loading dose of 10 mg, followed by 2 to 4 mg every 6 hours was prescribed. Surgery was carried out by fellowship-trained orthopaedic or neurosurgical spine surgeons.
3. Patient Outcomes
Data regarding demographics, diagnosis, treatment, neurological function, adverse events, and quality of life was prospectively collected. Steroid use, the effect, and duration of effect on neurological function were also prospectively recorded. Neurological function was measured using the American Spinal Injury Association (ASIA) scale [
10]. Quality of life was evaluated at baseline, 6, 12, and 26 weeks or until death using the Spine Oncology Study Group Outcomes Questionnaire (SOSGOQ2.0) [
11], EuroQol 5 dimensions (EQ-5D [3L]), and the Numerical Rating Scale (NRS) pain score. Governmental databases were accessed to retrieve information regarding survival. Data was stored in a secure web-based application (REDCap, Vanderbilt University, Nashville, TN, USA).
4. Statistical Analysis
Standard descriptive statistics were used to present demographic data. A mixed-effect model was used to test for differences in clinical outcomes over time. Kaplan-Meier survival curves were drawn with patients who did not reach the 3-month follow-up time point censored. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The significance level was set at p<0.05.
RESULTS
A total of 101 patients receiving steroids at baseline were identified. Of these 101 patients, only 30 patients were treated surgically and received steroids for preservation or prevention of deterioration of neurological function and were included in the final analysis. Four of these 30 patients received adjuvant radiotherapy.
The patients included in the final analysis had a mean age of 58.2 years (standard deviation, 11.2 years) at the time of surgery and included 14 females (46.7%) and 16 males (53.3%). The most common primary tumor was renal cell followed by breast cancer. Nine patients had a solitary spinal metastasis; in 9 patients, 2 vertebral levels were affected by spinal metastases and in the remaining 12, more than 2 levels were affected. The majority of patients had metastases in the thoracic spine (90%) followed by metastases in the lumbar spine (33%). The most common ASIA score at baseline was D in 18 patients (60%) followed by E in 9 patients (30%), and 1 patient in each of the ASIA A, B, and C categories (
Table 1).
A posterior surgical approach was used in 29 patients with the anterior approach being used in only 1 patient. A median of 5 (interquartile range [IQR], 4ŌĆō6) vertebral levels were bridged with a median operating time of 268 minutes (IQR, 220ŌĆō351 minutes).
Most of the patients were treated with steroids for 1 to 7 days (56.7%) before surgery, whereas 30% were treated for 8 to 14 days. In half of the patients, neurological function continued to deteriorate whereas in 30% of the patients neurological function stabilized, and in 20%, neurological function improved before surgery (
Table 2). The effect on neurological function lasted more than 48 hours in 53% of the patients that showed improvement or stabilization of neurological status. In 40% of the patients, the effect lasted between 12 and 48 hours, and in 1 patient (7%), the effect lasted less than 12 hours. The duration of steroid use did not correlate with the effect on neurological function.
Table 3 displays the ASIA impairment scales over time.
1. Adverse Events
Three patients experienced intraoperative adverse events (2 dural tears and new-onset atrial fibrillation). In addition, 44 postoperative adverse events were observed in 18 patients (60%; 95% CI, 41%ŌĆō70%). The most common postoperative adverse events were medical adverse events (including electrolyte imbalances and anemia) followed by excessive pain, neurological deterioration, and urinary tract infection (
Table 4).
2. Health-Related Quality of Life & Survival
Compared to baseline, the mean NRS pain scores reduced by 3.2 points (95% CI, -4.7 to -1.7; p<0.001) at 6-week postsurgery and sustained over time. The EQ-5D (3L) score showed significant improvements up to 12-week postsurgery (+0.28; 95% CI, 0.10ŌĆō0.46; p=0.002) with a slight decline at 26-week posttreatment (+0.22; 95% CI, 0.01ŌĆō0.43; p = 0.042). Patients demonstrated significant improvements in the SOSGOQ2.0 at 12-week postsurgery (+18.2; 95% CI, 5.9ŌĆō30.3; p=0.003) compared to baseline (
Table 5).
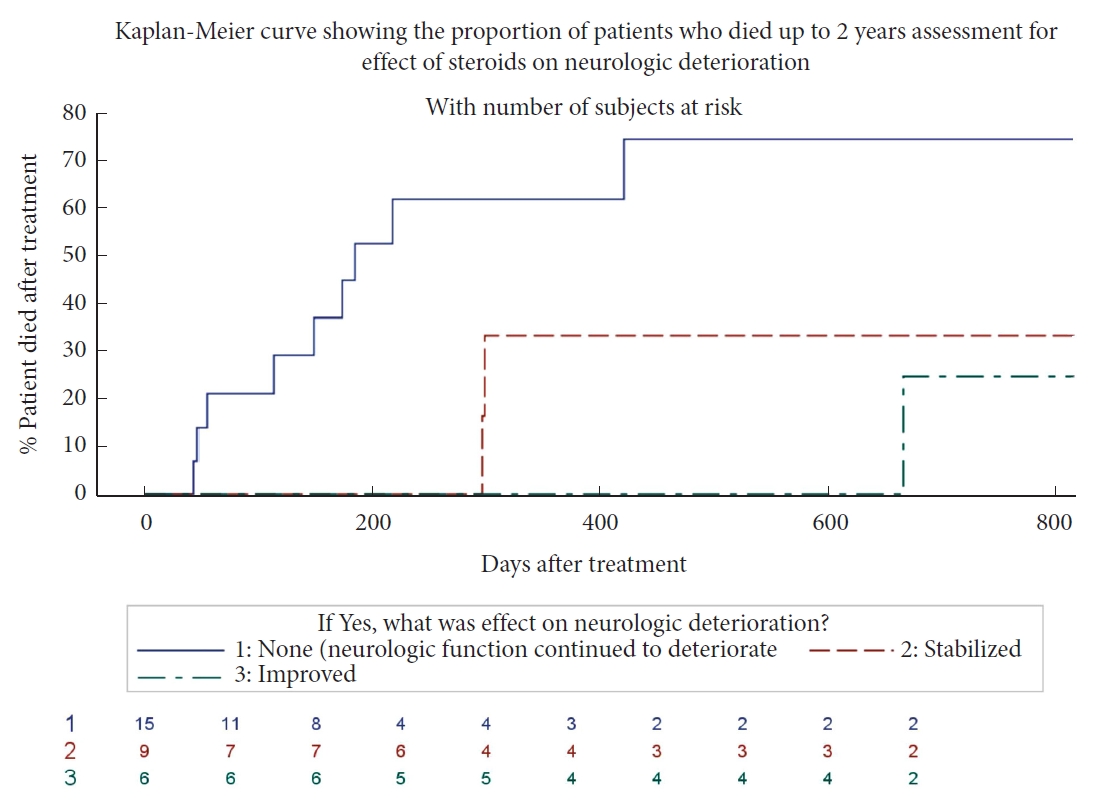
Patients who stabilized or improved in neurological function after steroid use showed a trend for improved survival within the first 3-month posttreatment compared to patients who continued to deteriorate in neurological function. A similar trend was seen in survival during the 2-year posttreatment (
Fig. 1).
DISCUSSION
Steroids are generally administered to patients with MESCC to preserve or maintain neurological function, often to create time to prepare for surgical intervention. Patchell et al. [
3] showed that surgery for patients with MESCC is beneficial but the evidence to support the use of corticosteroids is however limited [
8,
9]. In our study population, 50% of the patients who received steroids for MESCC continued to deteriorate in neurological function, in 30% of the patientsŌĆÖ neurological function stabilized, and in 20% of the patients, neurological function improved. Additionally, 18 patients (60%) experienced at least 1 postoperative adverse event.
Only a few clinical studies have been conducted to investigate the effect of corticosteroids in patients with MESCC [
7]. These studies were conducted in the 1980ŌĆÖs and beginning of the 1990ŌĆÖs when patients with MESCC were treated with radiotherapy instead of surgical decompression and stabilization, which is nowadays the evidence-based treatment algorithm. These studies demonstrated inconclusive results regarding ambulation and pain. Greenberg et al. [
12] demonstrated regain of ambulatory function in 28% of their patients, and S├Ėrensen et al. [
13] preservation or restoration of ambulation in 81% of patients treated with corticosteroids compared to 63% patients who did not receive corticosteroids. Yet, studies conducted by Heimdal et al. [
8] and Vecht et al. [
14] did not demonstrate a significant effect of steroids on ambulatory function. Furthermore, interpretation of the results of these studies is compromised by the fact that radiotherapy treatment was started soon after the administration of steroids.
In our study, patients who experienced continued neurological deterioration after the administration of steroids showed a trend of impaired survival at 3-months and 2-year posttreatment compared to patients who demonstrated stabilization or improvement of neurological function. In fact, 100% of patients that stabilized or had neurological improvement were still alive at 3-month posttreatment compared to 80% for those who showed deterioration. Although no definitive conclusions can be drawn from this observational data, these results are promising for patients that show improvement or stabilization of preoperative neurological status after steroid therapy. Whether this improved survival is related to improved neurological function or simply a predictive characteristic of favourable outcome is unclear and warrants further investigation.
Despite the widespread use of corticosteroids for patients with MESCC, consensus regarding the optimal regimen is still unclear [
8,
13-
15]. Many different regimens have been proposed and used in the literature with varying results. Kumar et al. [
15] conducted a systematic review on this topic and only found a limited number of studies. Based on the paucity of data, they recommended an initial 10 mg intravenous bolus of dexamethasone followed by 16 mg taken orally daily, as this was associated with a decreased adverse event rate compared to higher dose regimes [
15]. LŌĆÖEsp├®rance et al. [
16] recommended no loading dose and 16 mg of dexamethasone per day as soon as cord compression is diagnosed or suspected. This was also based on the high rate of adverse events observed after high-dose corticosteroids. In our observational study, the dexamethasone regimen was not standardized, inherent to the observational study design, but the 16 mg per day dosage was given most often.
Some of the most common side effects of corticosteroids used in neurooncological patients include diabetes, myopathy, osteoporosis, gastritis, Cushing syndrome, thromboembolic events, immunosuppression, and psychiatric disorders [
17]. Moreover, corticosteroids are known to increase the risk of infection and can have a negative effect on wound healing. Despite these drawbacks, corticosteroids are commonly used in the treatment of neuro-oncological patients including patients with spinal metastases and neurological deterioration [
4,
15]. Heimdal et al. [
8] showed that increased side effects were present in patients who received higher doses of corticosteroids. In our cohort, 18 patients (60%) had postoperative complications including systemic infection, wound infection, urinary tract infection, thromboembolism, and wound dehiscence that may be related to the surgical procedure and/or corticosteroids use. Although the rate of adverse events in our cohort of patients receiving corticosteroids was high, no significant difference in the incidence of adverse events could be determined when compared to patients not receiving corticosteroids within the complete population of our observational study. Yet, this could be attributed to the limited number of patients included in this study resulting in statistical constraints.
The major limitation in this paper is the lack of a control group in which steroids have not been administered. Thus, no direct comparison can be made in for the use of steroids in patients with spinal metastasis undergoing surgical intervention. Another limitation is that it was not possible to delineate the time from steroid prescription to surgery; specifically, we were not able to extract the timing from initiation of steroids to surgical intervention. Aside from resulting in statistical limitations the number of patients also resulted in a limited range of neurological impairments. The observational study nature resulted in the lack of a standardized steroid regimen for all patients, which can confound the results. In addition, we were unable to differentiate between the mechanism of cord compression, epidural tumor compression versus bony compression, due to lack of radiological review of the observational data. Steroids may have a different effect on neurological improvement depending on the type of compression. Moreover, the location of compression in the spine was not studied and may play a role in the different neurological outcomes of the study, although most of the metastases were limited to the thoracic spine.
Despite these limitations, this is the first study to explore and report on the effect of steroids in a surgical cohort of metastatic spine tumor patients. Despite lack of statistically significant, 50% of patients continued to deteriorate when given steroids; this is in line with the inconclusive results of previous studies regarding the effect of steroids on pretreatment physical function yet with a high incidence of adverse events. This study underscores the importance of future studies to investigate the effect steroids on pretreatment ambulation and adverse events including evaluation of the effect of type of spinal cord compression and tumor histology.